以废咖啡渣为原料,采用水热提取液,以商业酿酒酵母菌株D254为原料制备酒精饮料。采用感官描述、德国AIRSENSE电子鼻、日本INSENT电子舌、气相色谱-质谱(GC-MS)对SCG酒精饮料的感官特性进行分析。
方案详情

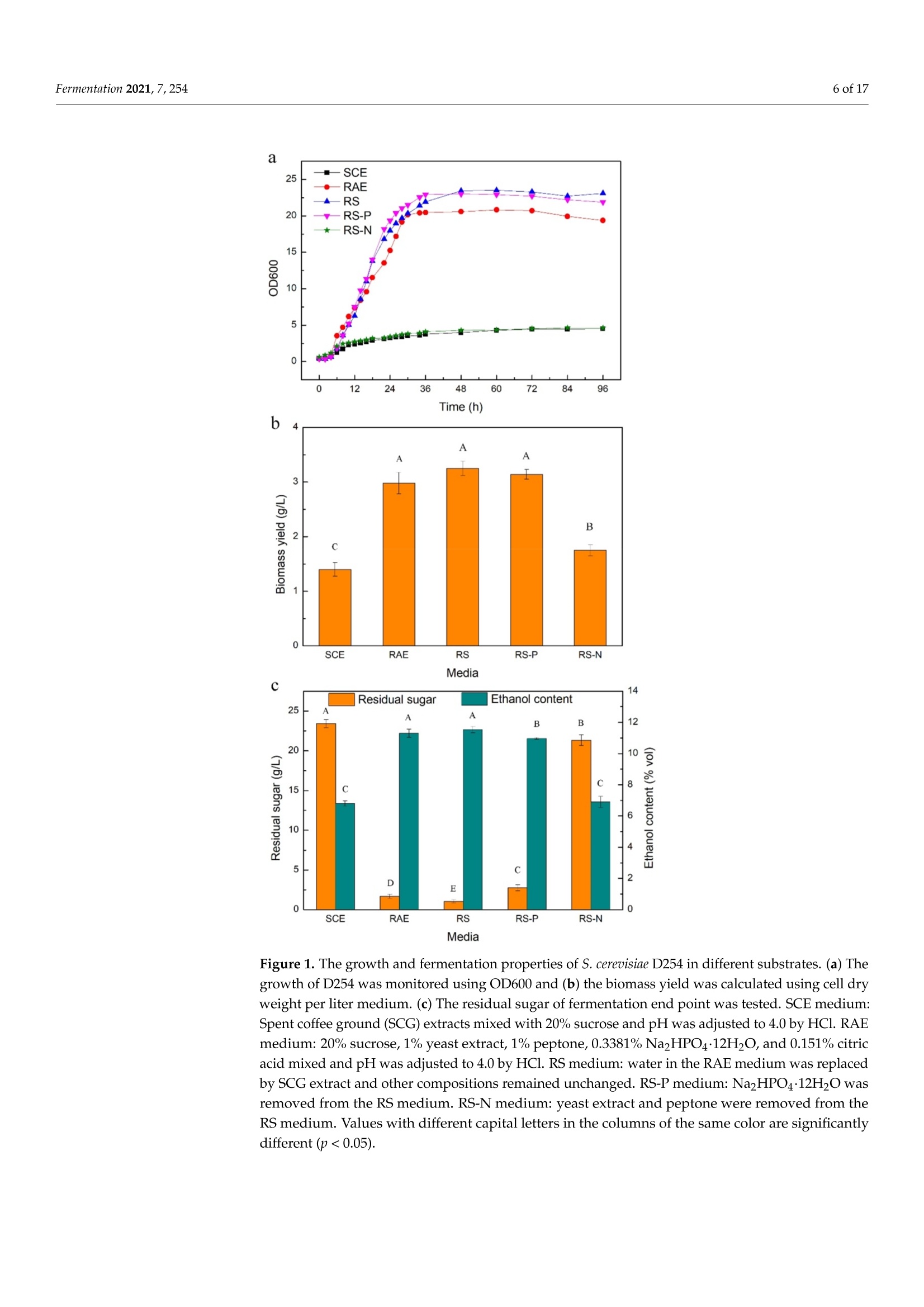
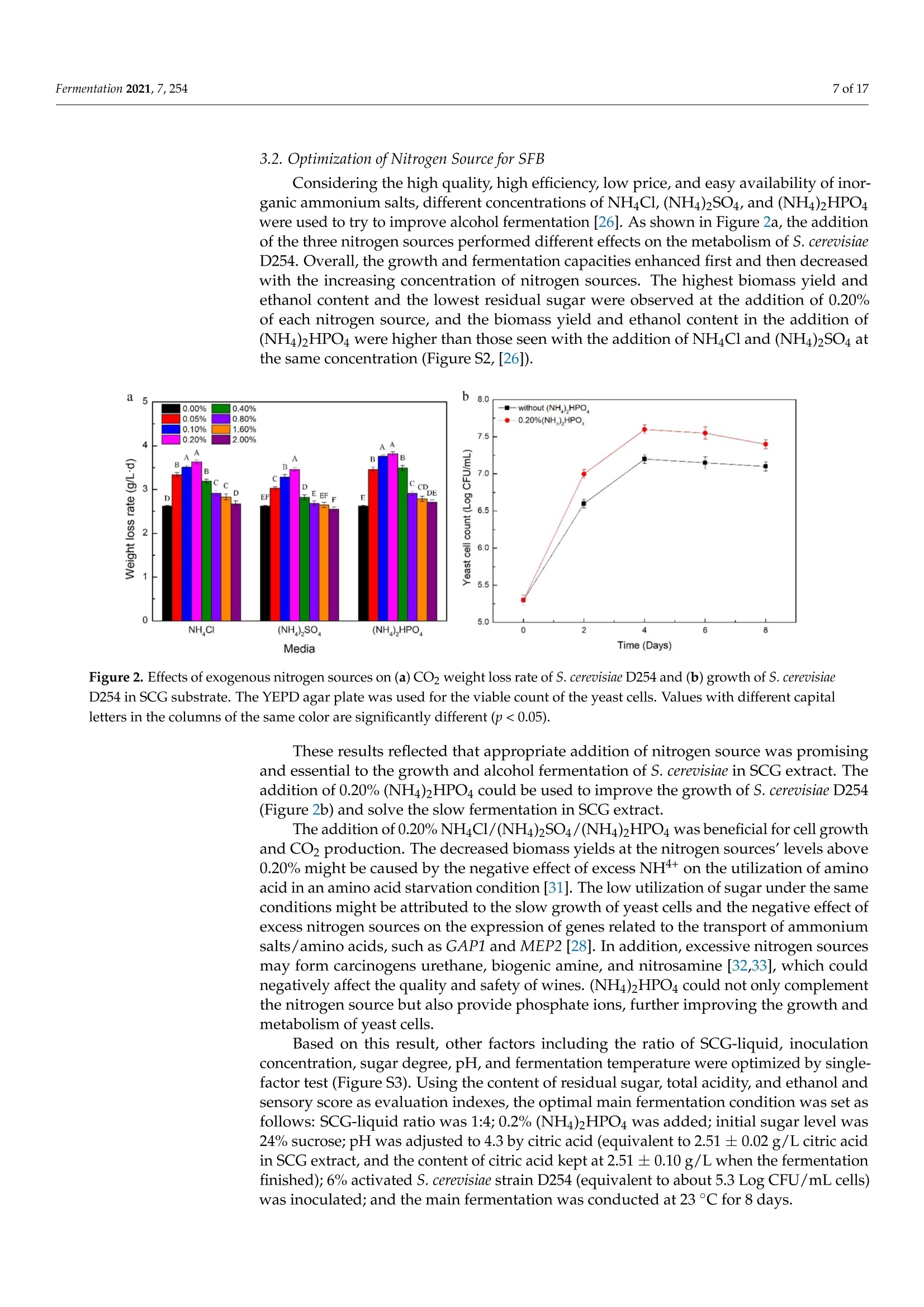
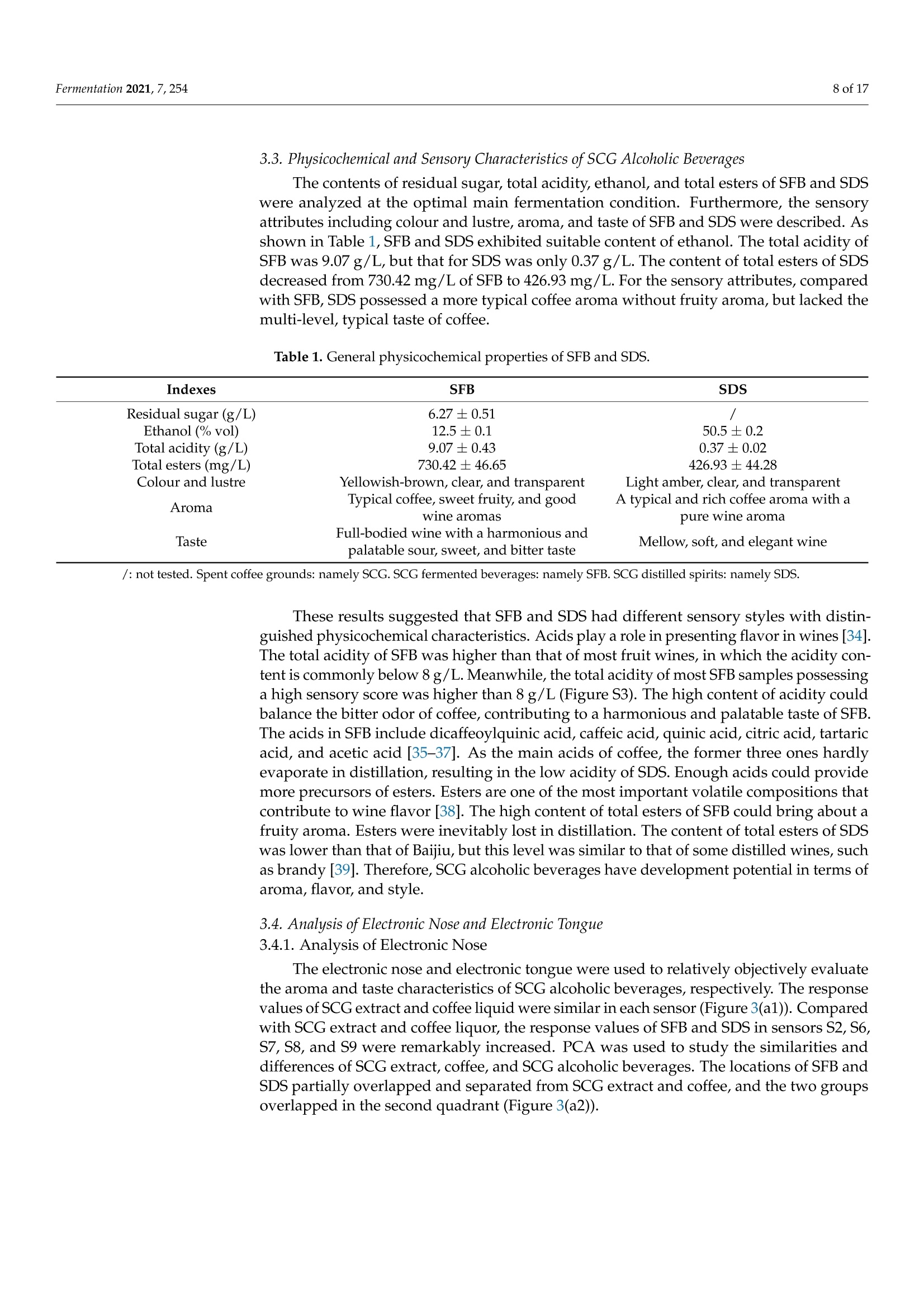
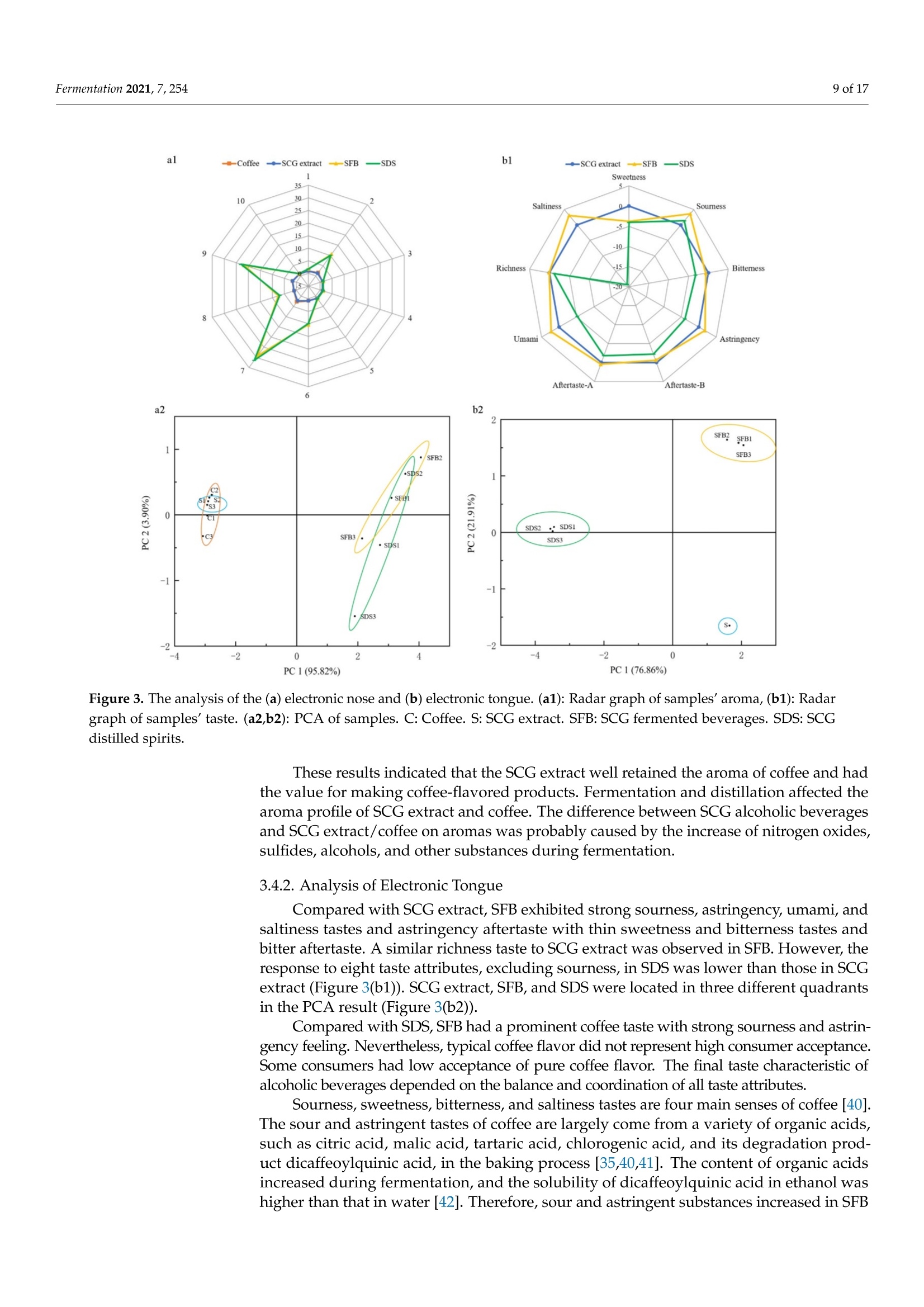
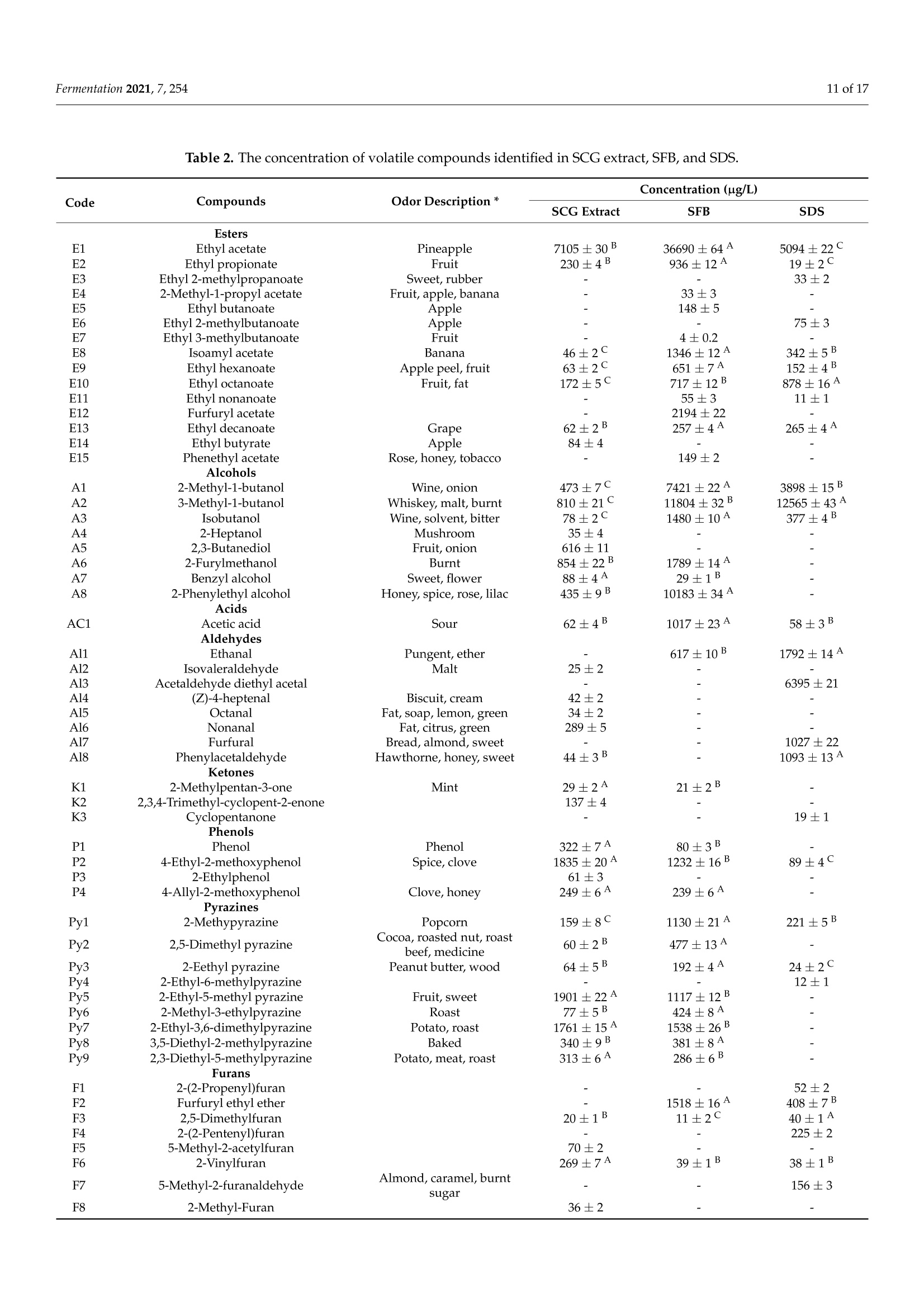
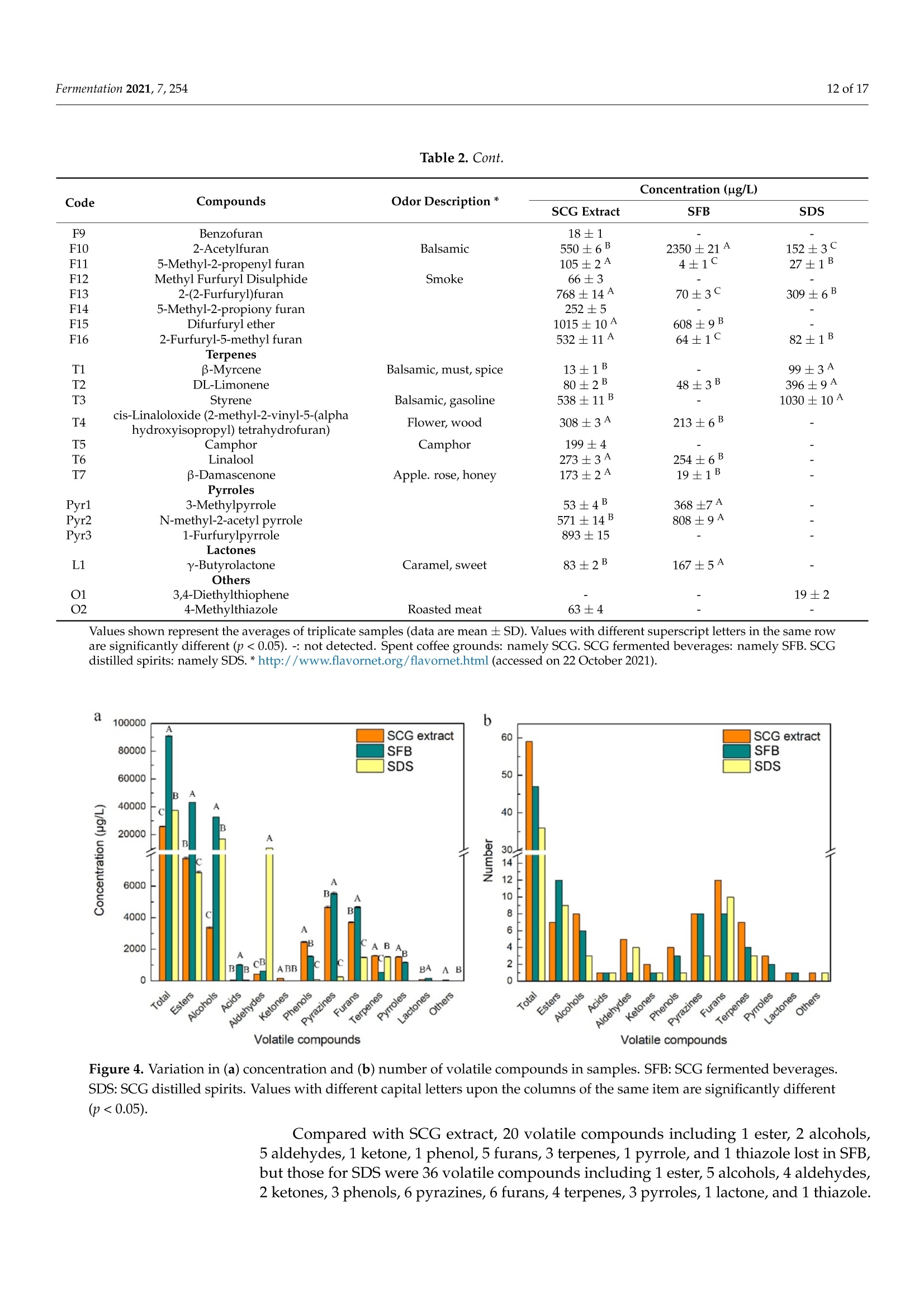
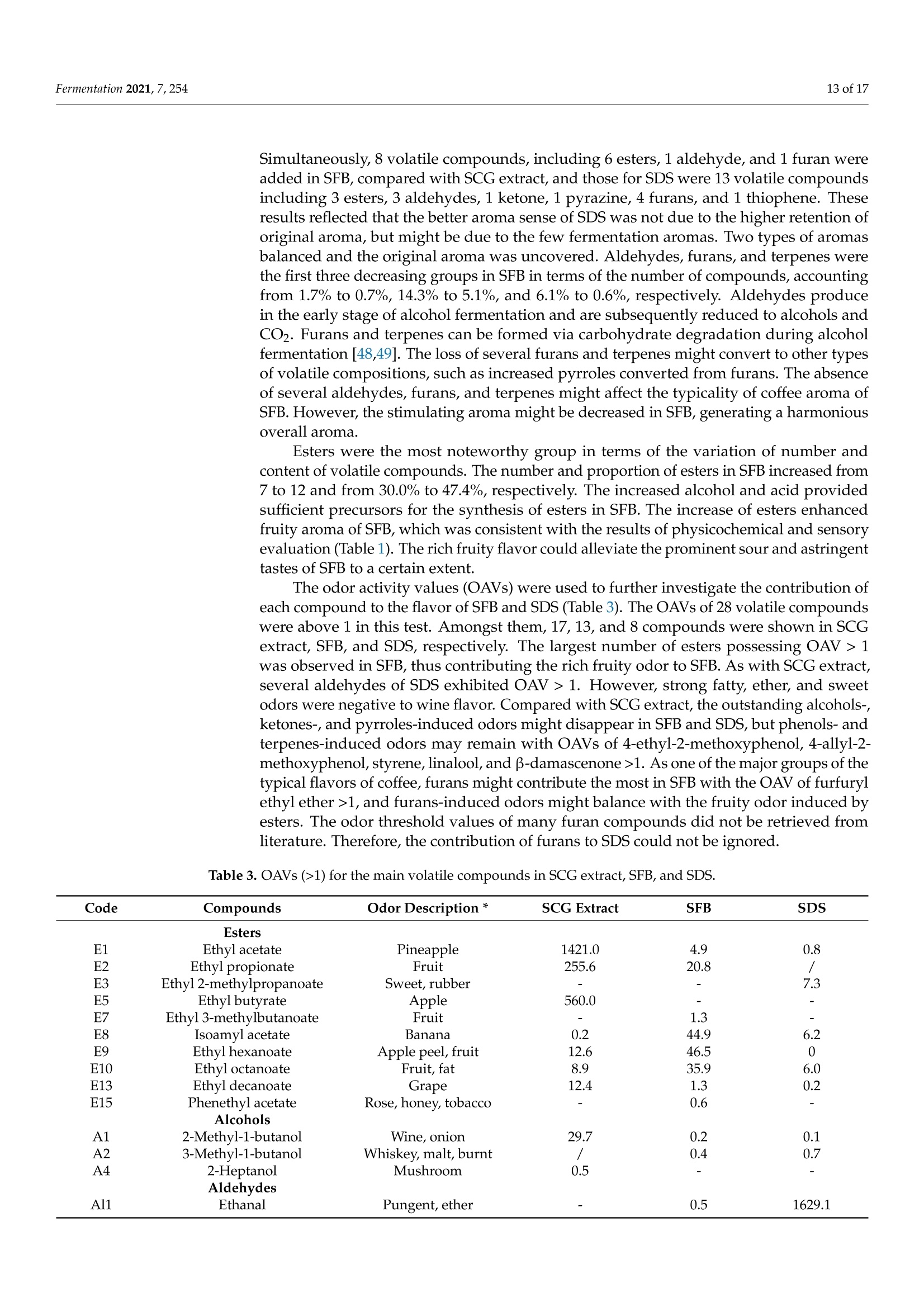
fermentation 2 of 17Fermentation 2021,7,254 Fermentation 2021, 7, 254. https://doi.org/10.3390/fermentation7040254https://www.mdpi.com/journal/fermentation Citation: Wang, L.; Yang, X.; Li, Z.;Lin, X.; Hu, X.; Liu, S.; Li, C. SensoryCharacteristics of Two Kinds ofAlcoholic Beverages Produced withSpent Coffee Grounds Extract Basedon Electronic Senses andHS-SPME-GC-MS Analyses. Fermentation 2021,7,254. https:// doi.org/10.3390/fermentation7040254 Academic Editors: Bin Tian and Jicheng Zhan Received: 10 September 2021 Accepted: 28 October 2021 Published:1 November 2021 Publisher's Note: MDPI stays neutralwith regard to jurisdictional claims inpublished maps and institutional affil-iations. Copyright: O 2021 by the authors.Licensee MDPI, Basel, Switzerland.This article is an open access articledistributed under the tconditions of the Creative CommonsAttribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/). Sensory Characteristics of Two Kinds of Alcoholic BeveragesProduced with Spent Coffee Grounds Extract Based onElectronic Senses and HS-SPME-GC-MS Analyses Lu Wang 12, Xu Yang1, Zhuoting Li l, Xue Lin 1,2*D, Xiaoping Hu 12, Sixin Liu and Congfa Li 1,2,* 1 College of Food Science and Engineering, Hainan University, Haikou 570228, China;lwang@hainanu.edu.cn (L.W.); xu.yang@cansinotech.com (X.Y.); huanyuanjiang@hainanu.edu.cn (Z.L.);huxiaoping03@hainanu.edu.cn (X.H.) 2Key Laboratory of Food Nutrition and Functional Food of Hainan Province, Haikou 570228, China3 College of Sciences, Hainan University, Haikou 570228, China;990495@hainanu.edu.cn Correspondence: 993048@hainanu.edu.cn (X.L.); congfa@hainanu.edu.cn (C.L.); Tel./Fax: +86-898-66278326 (X.L. &C.L.) Abstract: In this work, the hydrothermal extract of spent coffee grounds (SCG) was used to makealcoholic beverages with commercial S. cerevisiae strain D254. The sensory characteristics of the SCGalcoholic beverages were analyzed using sensory description,electronic nose, electronic tongue, andgas chromatography-mass spectrometry (GC-MS). The results suggested that the supplement of 0.20%(NH4)2HPO4 was effective at improving growth and alcohol fermentation of Saccharomyces cerevisiaeD254 in SCG extract. SCG fermented beverages (SFB) and SCG distilled spirits (SDS) produced atthe optimized fermentation conditions had appropriate physicochemical properties and differentsensory characteristics. Fermentation aromas, especially esters, were produced in SFB, increasing thecomplexity of aroma and lowing the irritating aroma. The combination of original and fermentationcomponents might balance the outstanding sourness, astringency, and saltiness tastes of SFB. Thefermentation aroma was partially lost and the sourness, bitterness, astringency, and saltiness tasteswere relieved in distillation, leading to the relatively more prominent aroma typicality of coffee and asoft taste. These findings lay a foundation for producing new high-quality coffee-flavored alcoholicbeverages or flavoring liquors. Keywords: spend coffee grounds; fermentation; sensory property; volatile profile 1.Introduction Coffee is one of the most popular beverages worldwide because of its unique flavorand positive effects on health [1]. It has been consumed for over 1000 years and the demandof coffee production is growing steadily [2]. Nevertheless, spent coffee grounds (SCG)are inevitably produced during the processing of instant coffee [3,4]. Producing 1 kg ofinstant coffee could yield 0.9 kg of by-product SCG simultaneously [4,5]. As the maincoffee industry residue, the vast majority of SCG are unconsumed and disposed of into theenvironment,leading to serious environmental pollution [6]. The reuse of SCG is thus ofhuge importance from environmental and economic viewpoints. Resource utilization of SCG is currently of increasing concern. To improve the addedvalue of SCG, some scholars and enterprises try to use SCG in many ways, such as beingprocessed to feed and fertilizer [7], extraction of oil [8],obtaining polyphenols and dietary fiberfrom SCG [9,10], or using them for fuel production and alcoholic beverages making [11-14]. InSouth America and Vietnam, people have a tradition of drinking alcoholic beverages ofcoffee. Coffee-flavored alcoholic beverages, which can be drunk directly or used to preparecocktails, are generally produced by the mixture of distilled liquor (such as edible alcohols,brandy, rum, and rice wines), coffee, sugar, cream, and other ingredients on the market.This method is relatively convenient for preparing coffee-flavored alcoholic beverages. However, the fusion of aromas is not satisfactory enough in this type of alcoholic beverageand the flavor is not typical or full. SCG are rich in high value molecules and organic compounds and might retainthe typical flavor and taste of coffee [14,15], making it a source of brewing potential fornew coffee-flavored alcoholic beverages or flavoring liquors. Liu et al. [16-18] developsSCG fermented beverages (SFB) using different inoculation strategies and analyzes thechanges of strains growth and chemical compositions in fermentation. Sampaio et al.[11]produces SCG distilled spirits (SDS) using SCG extract and analyzes the sensory profile.Machado et al. [14] produces SFB and SDS using SCG extract and also conducts a sensoryevaluation. Sensory attributes are one of the most important factors affecting alcoholicbeverages' quality and consumer preference. At present, electronic sensing technologiesare widely used in the flavor evaluation of fermented food. The amount of researchreporting on the combination of electronic nose/electronic tongue, gas chromatography-mass spectrometry (GC-MS), and sensory description to evaluate the sensory characteristicsof food is increasing [19-21]. However, the sensory evaluation of SCG alcoholic beveragesby integrating the above-mentioned methods has not been reported. In this study, to further characterize the fermentation potential of SCG extract andenrich the understanding of the sensory quality of the two kinds of SCG extract productsSFB and SDS, the sensory characteristics of SFB and SDS produced at the optimized fer-mentation conditions were evaluated using sensory description, electronic nose, electronictongue, and headspace solid-phase microextraction (HS-SPME) combined with GC-MS. 2. Materials and Methods 2.1. Raw Material and Yeast Strain SCG used in this study were the by-products created during the processing of Arabicagreen coffee beans to instant coffee, in which countercurrent continuous extraction was con-ducted, and were provided by Hainan Lisun investment holding Co., Ltd. (Haikou, China).SCG were dried at 68°C to a moisture content of about 10% and refrigerated for later use. Thecommercial S. cerevisiae strain D254 was purchased from LALVIN (Fredericia, Denmark). 2.2. Fermentation Process The fermentation process of SCG alcoholic beverages is shown in Figure S1. SCGand distilled water were mixed in a proportion of 1:5 (g/mL) and heated at 95°C for45 min. After cooling and filtering with 500 mesh filter cloth, the SCG extract was obtained(2.5°Brix, pH 5.26). According to Sampaio et al. [11] and Machado et al. [14], 200 mg/Lsodium metabisulfite was used to avoid bacterial contamination For the analysis of fermentation blocked in the SCG extract, the following media wereused: (1) SCG extracts were mixed with 20% sucrose and pH was adjusted to 4.0 by HCl,obtaining the SCE medium, (2) the modified RAE medium contained 20% sucrose, 1%yeast extract, 1% peptone, 0.3381%Na2HPO4·12H2O, and 0.151% citric acid and pH wasadjusted to 4.0 by HCl, (3) water in the RAE medium was replaced by SCG extract andother compositions remained unchanged, obtaining the RS medium, (4) Na2HPO4·12H2Owas removed from the RS medium to obtain the RS-P medium, and (5) yeast extract andpeptone were removed from the RS medium to obtain the RS-N medium. To ensure the smooth fermentation of SCG alcoholic beverages, theoptimization ofnutrition was conducted at the following condition: SCG extract was mixed with 20%sucrose and pH was adjusted to 4.0 by citric acid (equivalent to 2.54 ±0.09 g/L citricacid in SCG extract) according to Sharma et al. [22] and Hlaing and Joshy [23]. Natively,2.45 ± 0.03 g/L of citric acid exists in the SCG extract used in this work. Yu et al.[24]reports that citric acid has no obvious effects on the cell count, mortality rate, and alcoholfermentation ability of Saccharomyces cerevisiae at a concentration below 19.0 g/L (equivalentto pH 2.51) in apple wine making. Citric acid has no obvious effects on the volume ofS. cerevisiae cells at a concentration below 5.4 g/L (equivalent to pH 2.97) in the testedconditions. However, citric acid remarkably affects the growth and alcohol fermentation After the main fermentation (in which time alcohol fermentation basically finished andthe difference of CO2 production in two consecutive days was below 0.5 g/L) at the optimalcondition, the post fermentation (during which time a small amount of residual sugar wasused and SCG alcoholic beverages tended to be biologically stable) was conducted at 15 °Cfor 30 days. SCG distilled spirits were obtained using the traditional secondary distillation method.The fresh SCG fermented beverages were distilled in a pre-concentration step to obtain theprimary distillate product at an ethanol content of about 25%, and the head part (1.5% ofthe fresh sample) was removed. Then,the primary distillate product was distilled below85°C in the second distillation. 1.5% of the distillate was removed. The heart distillate wascollected at an ethanol content above 40% and the ethanol content of SCG distilled spiritswas adjusted to 50% using ultrapure water. The fermentations were conducted three times. 2.3. Analysis of Yeast Growth The cell density was measured at 30C using a UV spectrophotometer (T6, Persee,Beijing, China). The yeast cells were centrifuged at 1500× g for 15 min and washed twotimes with 4°C sterile water. Then, yeast cells were dried at 105°C to constant weight. Thefinal biomass yield was determined by the gram of dry cells per liter fermentation broth. 2.4. Analysis of CO2 Production CO2 weight loss was measured using an analytical balance (EL602, Mettler Toledo,Shanghai, China). The CO2 weight loss rate was expressed as the gram of CO2 weight lossproduction per liter of fermentation broth per fermentation time. 2.5. Analysis of Physicochemical Properties Residual sugar was determined by titration with methylene blue indicator. An appro-priate glucose standard solution (2.5 g/L) mixed with 5 mL Fehling solutionI, 5 mL FehlingsolutionII, and 50 mL distilled water. After boiling for 2 min,two drops of methyleneblue indicator were added and the glucose standard solution was used to titrate. The totalvolume of glucose standard solution consumed (V) was recorded when the reaction solu-tion was colorless. The volume of glucose standard solution consumed (V') was recordedwhen the appropriate samples replaced the glucose standard solution in the first step ofabove-mentioned method. The gram of glucose that was equivalentams of aicoS to that in 5 mL FehlingsolutionI and II(F)= gre 118nhydrous glucose used×V/1000. The residual sugar ofsample=F×1000/(volume of sample used/volume of sample used after dilution)×V’.The results were expressed as glucose. Total acidity was determined by direct titration oforganic acids in samples with 0.05 mol/L NaOH solution. A pH of 8.2 was used as theend point of potentiometric titration. The content of total acid of sample=0.05 ×75 x(volume of NaOH solution for titrating the sample-volume of NaOH solution for titrat-ing the control group)/volume of sample used. The results were expressed as tartaricacid. Ethanol content was obtained by distillation combined with a portable densimeter(DA-130N, KEM, Tokyo, Japan). pH was determined by using a pH meter (SevenEasyS20, METTLER TOLEDO, Zurich, Switzerland). Total esters were determined by titrationwith 0.1 mol/L sulfuric acid solution. An appropriate 0.1 mol/L NaOH solution wasused to neutralize the free acid in 50 mL samples. Two drops of phenolphthalein wereused as the reaction indicator. After adding 25 mL of 0.1 mol/L NaOH solution, esters saponification was conducted by heating reflux on a boiling water bath for 30 min. Then,0.1 mol/L sulfuric acid solution was used to titrate the sample. The content of total ester ofsample=0.01× 88 × (volume of sulfuric acid solution for titrating the sample-volume ofsulfuric acid solution for titrating the control group)/volume of sample used. The resultswere expressed as ethyl acetate. 2.6. Analysis of Volatile Profile HS-SPME-GC-MS was used to extract and analyse the volatiles. The SPME fiber(75 um CAR/PDMS, Supelco, PA, USA) was pretreated in the GC injector at 250°C for30 min with 1.0 mL/min flow rate of carrier gas, and 5 g samples were sealed in a 20 mLheadspace vial and stirred at 400 r/min. After equilibrating in a 50 °C water bath for10 min, the aged SPME fiber was suspended 1.6 cm above the sample for 40 min anddesorbed in the GC injector at 250 °C for 5 min. Compound analysis was performed using an Agilent 7890A GC (Agilent, Santa Clara,CA, USA) coupled with an Agilent :d Wi5975C mass spectrometer system equipped with aDB-WAX capillary column (60 m x 0.25 mm x0.25 um, Agilent, Santa Clara,CA, USA).Helium (purity 99.999%) was used as the carrier gas at 2.0 mL/min. The oven was initiallyheld at 40 °C for 6 min, subsequently raised to 100°C at a rate of 3 °C/min, and finallyraised to 230°C at a rate of 5 °C/min for 10 min. The electron ionization energy of themass-selective detector was 70 eV. The chromatogram was recorded by monitoring thetotal ion currents in the m/z range of 30 to 450 atomic mass units. The volatile compounds were identified by comparing retention indices and retentiontimes with those obtained for authentic standards, or those of literature data, or withmass spectra in the NIST/WILEY Database. The relative quantification of volatile com-pounds was performed by peak area method, and 20 uL 2-Methoxy-d3-phenol-3,4,5,6-d4(410 mg/L) was used as an internal standard. 2.7. Electronic Nose Analysis The Germany Airsense PEN 3 portable electronic nose system (Schwerin,Mecklenburg-Vorpommern) was used to estimate the aroma profile of SCG alcoholic beverages. Theperformance of ten metal oxide semiconductor sensors is listed in Table S1. A 10 mL sample (diluted to 10% vol ethanol with ultrapure water) was sealed in asample vial for 60 min for data collection. The headspace sampling method was used forelectronic nose detection. Detection conditions were set as follows: the sensor cleaningtime was 170 s, the auto-zero time was 10 s, the sample preparation time was 5 s, theinjection rate of gas and carrier gas speed were 400 mL/min, and the detection time was120 s. Samples were analyzed using WinMuster statistical analysis software self-containedwith PEN 3 and the sensor response values at 98 s were used to establish the radar chart. 2.8. Electronic Tongue Analysis Electronic tongue analysis was performed on an SA402B system (Insent, Kanagawa,Japan) equipped with seven sensors and reference electrodes, which could analyze bitter-ness, sourness, saltiness, sweetness,astringency, and bitter anion tastes. The compositionof electrode and standard solutions is shown in Table S2. The ethanol concentration ofsamples was diluted to 10% vol with ultrapure water and the supernatants were used foranalysis. 2.9. Sensory Evaluation SFB and SDS were evaluated by a well-trained panel of 11 members (6 men and5 women, ranging from 20 to 55 years old). Then, 20 mL of samples was presented to thepanelists at 21±1°C. The sensory scoring criteria and rules were set based on those of drygrape wine, taking into account the sensory characteristics of coffee. The specific sensoryscoring rules of SCG fermented beverages were shown in Table S3. 2.10. Statistical Data Analysis Results were shown as averages of at least three independent experiments ± SD.Statistically significant differences (p <0.05) were determined using one-way ANOVA andTukey’s honestly significant difference (HSD) tests. 3. Results and Discussion 3.1. Analysis of Fermentation Blocked in SCG Extract In our previous study, the fermentation capacity of four yeast stra)mmins, S. cerevisiae D254,S. bayanus BV818, S. bayanus DV10, and S. bayanus EC1118, commonly used in fruit wineindustry, was evaluated in SCG extract [26]. Surprisingly, the four yeast strains exhibitedinferior capacities of producing CO2 and ethanol in SCE (Table S4, [26]). We speculatedthat this problem may be related to insufficient nitrogen and phosphorus sources andthe existence of a toxic component, such as coffee polyphenols, in the SCG extract. Inprevious studies, Liu et al. [16-18] comprehensively analyzed the chemical composition ofSCG hydrolysates before and after the microbial transformation, and found the insufficientassimilable nitrogen of unfermented SCG hydrolysates. As shown in Table S5, regarding thechemical parameters of unfermented SCG extract used in this work, a similar phenomenonwas observed, revealing the necessity of supplementing with a nitrogen source in SCGextract fermentation. In this study, firstly, the reasons for the weak fermentation of yeast strains in the SCGsubstrate were analyzed using the "medium components addition/subtraction" method.The strain S. cerevisiae D254, which was relatively more suitable for SCG extract fermen-tation in terms of ethanol content and sensory characteristics, was used in this work. Asshown in Figure 1a, compared with that in SCE, the growth of S. cerevisiae D254 improvedobviously in RAE, RS,and RS-P media. The biomass yield of S. cerevisiae D254 achieved oreven exceeded 3.0 g/L in RAE, RS, and RS-P media, whereas only 1.4 g/L biomass yieldwas observed in SCE (Figure 1b). Simultaneously, the residual sugar and ethanol content inRAE, RS, and RS-P were close to the theoretical values (Figure 1c). However, similar slowgrowth and fermentation to those in SCE were displayed in the RS-N medium. These results were corresponding to the analysis of chemical parameters of SCGextract and also indicated that an insufficient nitrogen source was the main factor of weakfermentation in SCG substrate, whereas SCG extract and phosphorus source were not thekey limiting factors for yeast strains in SCG substrate. Generally speaking, the lack of assimilatable nitrogen source is a common cause ofslow and stagnant fermentation in yeast [27]. The level of nitrogen source could influencethe genes expression of yeasts [28]. Yeast cells behave similar as when under carbonstarvation at a low concentration of nitrogen, inhibiting their growth and metabolism [29].In this work, the growth of S. cerevisiae D254 was slow with the lack of nitrogen sources;no rapid growth period and few biomass accumulations were observed under the sameconditions. This result was consistent with that of Hernandez-Orte et al. [30], whichshowed that nitrogen source directly affected the biomass of yeast in the rapid growthperiod at the initial stage of fermentation. Insufficient biomass could lead to the slowfermentation and prolong the fermentation time. a 25 一SCE -RAE -RS ocooc D E 0 0 SCE RAE RS RS-P RS-N Media Figure 1. The growth and fermentation properties of S. cerevisiae D254 in different substrates. (a) Thegrowth of D254 was monitored using OD600 and (b) the biomass yield was calculated using cell dryweight per liter medium. (c) The residual sugar of fermentation end point was tested. SCE medium:Spent coffee ground (SCG) extracts mixed with 20% sucrose and pH was adjusted to 4.0 by HCl. RAEmedium: 20% sucrose, 1% yeast extract, 1% peptone, 0.3381%Na2HPO4·12H2O,and 0.151% citricacid mixed and pH was adjusted to 4.0 by HCl. RS medium: water in the RAE medium was replacedby SCG extract and other compositions remained unchanged. RS-P medium: NazHPO4·12H2O wasremoved from the RS medium. RS-N medium: yeast extract and peptone were removed from theRS medium. Values with different capital letters in the columns of the same color are significantlydifferent (p<0.05). 3.2. Optimization of Nitrogen Source for SFB Considering the high quality, high efficiency, low price, and easy availability of inor-ganic ammonium salts, different concentrations of NH4Cl,(NH4)2SO4,and (NH4)2HPO4were used to try to improve alcohol fermentation [26]. As shown in Figure 2a, the additionof the three nitrogen sources performed different effects on the metabolism of S. cerevisiaeD254.Overall, the growth and fermentation capacities enhanced first and then decreasedwith the increasing concentration of nitrogen sources. The highest biomass yield andethanol content and the lowest residual sugar were observed at the addition of 0.20%of each nitrogen source, and the biomass yield and ethanol content in the addition of(NH4)2HPO4 were higher than those seen with the addition of NH4Cl and (NH4)2SO4 atthe same concentration (Figure S2, [26]). Figure 2. Effects of exogenous nitrogen sources on (a) CO2 weight loss rate of S. cerevisiae D254 and (b) growth of S. cerevisiaeD254 in SCG substrate. The YEPD agar plate was used for the viable count of the yeast cells. Values with different capitalletters in the columns of the same color are significantly different (p<0.05). These results reflected that appropriate addition of nitrogen source was promisingand essential to the growth and alcohol fermentation of S. cerevisiae in SCG extract. Theaddition of 0.20%(NH4)2HPO4 could be used to improve the growth of S. cerevisiae D254(Figure 2b) and solve the slow fermentation in SCG extract. The addition of 0.20%NH4C1l/(NH4)2SO4/(NH4)2HPO4 was beneficial for cell growthand CO2 production. The decreased biomass yields at the nitrogen sources’ levels above0.20% might be caused by the negative effect of excess NH++ on the utilization of aminoacid in an amino acid starvation condition [31]. The low utilization of sugar under the sameconditions might be attributed to the slow growth of yeast cells and the negative effect ofexcess nitrogen sources on the expression of genes related to the transport of ammoniumsalts /amino acids, such as GAP1 and MEP2 [28]. In addition, excessive nitrogen sourcesmay form carcinogens urethane, biogenic amine, and nitrosamine [32,33], which couldnegatively affect the quality and safety of wines. (NH4)2HPO4 could not only complementthe nitrogen source but also provide phosphate ions, further improving the growth andmetabolism of yeast cells. Based on this result, other factors including the ratio of SCG-liquid, inoculationconcentration,sugar degree, pH, and fermentation temperature were optimized by single-factor test (Figure S3). Using the content of residual sugar, total acidity, and ethanol andsensory score as evaluation indexes, the optimal main fermentation condition was set asfollows: SCG-liquid ratio was 1:4;0.2%(NH4)2HPO4 was added; initial sugar level was24% sucrose; pH was adjusted to 4.3 by citric acid (equivalent to 2.51 ±0.02 g/L citric acidin SCG extract, and the content of citric acid kept at 2.51 ±0.10 g/L when the fermentationfinished);6% activated S. cerevisiae strain D254 (equivalent to about 5.3 Log CFU/mL cells)was inoculated; and the main fermentation was conducted at 23°C for 8 days. 3.3. Physicochemical and Sensory Characteristics of SCG Alcoholic Beverages The contents of residual sugar, total acidity, ethanol, and total esters of SFB and SDSwere analyzed at the optimal main fermentation condition. Furthermore, the sensoryattributes including colour and lustre, aroma, and taste of SFB and SDS were described. Asshown in Table 1, SFB and SDS exhibited suitable content of ethanol. The total acidity ofSFB was 9.07 g/L, but that for SDS was only 0.37 g/L. The content of total esters of SDSdecreased from 730.42 mg/L of SFB to 426.93 mg/L. For the sensory attributes, comparedwith SFB, SDS possessed a more typical coffee aroma without fruity aroma, but lacked themulti-level, typical taste of coffee. Table 1. General physicochemical properties of SFB and SDS. Indexes SFB SDS Residual sugar (g/L) 6.27±0.51 / Ethanol (% vol) 12.5十0.1 50.5±0.2 Total acidity (g/L) 9.07 ±0.43 0.37±0.02 Total esters (mg/L) 730.42 ±46.65 426.93±44.28 Colour and lustre Yellowish-brown, clear,and transparent Light amber, clear, and transparent Typical coffee, sweet fruity, and good A typical and rich coffee aroma with a Aroma wine aromas pure wine aroma Taste Full-bodied wine with a harmonious and palatable sour, sweet, and bitter taste Mellow, soft, and elegant wine /: not tested. Spent coffee grounds: namely SCG. SCG fermented beverages: namely SFB. SCG distilled spirits: namely SDS. These results suggested that SFB and SDS had different sensory styles with distin-guished physicochemical characteristics. Acids play a role in presenting flavor in wines [34].The total acidity of SFB was higher than that of most fruit wines, in which the acidity con-tent is commonly below 8g/L. Meanwhile, the total acidity of most SFB samples possessinga high sensory score was higher than 8 g/L (Figure S3). The high content of acidity couldbalance the bitter odor of coffee, contributing to a harmonious and palatable taste of SFB.The acids in SFB include dicaffeoylquinic acid, caffeic acid, quinic acid, citric acid, tartaricacid, and acetic acid [35-37]. As the main acids of coffee, the former three ones hardlyevaporate in distillation, resulting in the low acidity of SDS. Enough acids could providemore precursors of esters. Esters are one of the most important volatile compositions thatcontribute to wine flavor [38]. The high content of total esters of SFB could bring about afruity aroma. Esters were inevitably lost in distillation. The content of total esters of SDSwas lower than that of Baijiu, but this level was similar to that of some distilled wines, suchas brandy [39]. Therefore, SCG alcoholic beverages have development potential in terms ofaroma, flavor, and style. 3.4. Analysis of Electronic Nose and Electronic Tongue 3.4.1. Analysis of Electronic Nose The electronic nose and electronic tongue were used to relatively objectively evaluatethe aroma and taste characteristics of SCG alcoholic beverages, respectively. The responsevalues of SCG extract and coffee liquid were similar in each sensor (Figure 3(a1)). Comparedwith SCG extract and coffee liquor, the response values of SFB and SDS in sensors S2, S6,S7, S8, and S9 were remarkably increased. PCA was used to study the similarities anddifferences of SCG extract,coffee, and SCG alcoholic beverages. The locations of SFB andSDS partially overlapped and separated from SCG extract and coffee, and the two groupsoverlapped in the second quadrant (Figure 3(a2)). Figure 3. The analysis of the (a) electronic nose and (b) electronic tongue. (a1): Radar graph of samples’aroma, (b1): Radargraph of samples'taste. (a2,b2):PCA of samples. C: Coffee. S: SCG extract. SFB: SCG fermented beverages. SDS: SCGdistilled spirits. These results indicated that the SCG extract well retained the aroma of coffee and hadthe value for making coffee-flavored products. Fermentation and distillation affected thearoma profile of SCG extract and coffee. The difference between SCG alcoholic beveragesand SCG extract/coffee on aromas was probably caused by the increase of nitrogen oxides,sulfides, alcohols, and other substances during fermentation. 3.4.2. Analysis of Electronic Tongue Compared with SCG extract,SFB exhibited strong sourness, astringency, umami, andsaltiness tastes and astringency aftertaste with thin sweetness and bitterness tastes andbitter aftertaste. A similar richness taste to SCG extract was observed in SFB. However, theresponse to eight taste attributes, excluding sourness, in SDS was lower than those in SCGextract (Figure 3(b1)). SCG extract, SFB, and SDS were located in three different quadrantsin the PCA result (Figure 3(b2)). Compared with SDS, SFB had a prominent coffee taste with strong sourness and astrin-gency feeling. Nevertheless, typical coffee flavor did not represent high consumer acceptance.Some consumers had low acceptance of pure coffee flavor. The final taste characteristic ofalcoholic beverages depended on the balance and coordination of all taste attributes. Sourness, sweetness, bitterness, and saltiness tastes are four main senses of coffee [40].The sour and astringent tastes of coffee are largely come from a variety of organic acids,such as citric acid, malic acid, tartaric acid, chlorogenic acid, and its degradation prod-uct dicaffeoylquinic acid, in the baking process [35,40,41]. The content of organic acidsincreased during fermentation, and the solubility of dicaffeoylquinic acid in ethanol washigher than that in water [42]. Therefore, sour and astringent substances increased in SFB and the corresponding taste senses enhanced. Sugars of coffee can give a sweet taste,andthe caramel compounds produced in the baking process can give sweet and bitter tastes.WesSith the decomposition of sugars in fermentation, the sweetness and bitterness tastesreduced, which in turn made the sourness and saltiness tastes more prominent. Combinedwith the results of taste evaluation in Table 1, the findings suggested that such changes oftaste of coffee in fermentation were favor to the mutual inhibition and balance of sourness,sweetness, bitterness,and saltiness senses in this study. Distillation was a process that selec-tively extracted the volatile flavor components of fermentation wines. Many compositions withstrong water solubility and weak fat solubility could not be brought out with the distillationof ethanol. The prominent sour and astringent tastes in SFB reduced in distillation with lesssweetness and saltiness senses, which were suitable for consumer groups with low acceptanceof pure coffee. This finding was corresponded to the results of Machado et al. [14], in whichSDS exhibited a high global value in quantitative descriptive analysis. 3.5. Volatile Profile of SCG Alcoholic Beverages A total of 77 volatile compounds were identified in this study, including 15 esters,8 alcohols, 1 acid, 8 aldehydes, 3 ketones, 4 phenols, 9 pyrazines, 16 furans, 7 terpenes,3 pyrroles, 1 lactone, and 2 other compounds (Table 2). The most abundant variety butthe lowest content of total volatile compositions was observed in SCG extract (Figure 4).The lowest variety of volatile compositions was observed in SDS with the moderate totalcontent (Figure 4). SFB displayed the highest content of total volatiles with the moderatevariety (Figure 4). These results suggested that the types and content of volatiles of SCGextract changed in fermentation and distillation. Partial original aromas of SCG extractwere lost and converted in alcohol fermentation with the formation of a large amountof fermentation aromas. Both original and fermentation aromas were partially lost indistillation. The types and contents of volatiles differed from those reported in Machadoet al. [14] and Liu et al. [16]. This discrepancy could be caused by the difference of extractionand test methods. More than 800 aromatic components of coffee have been detected in previous studies,of which pyrazines, furans, aldehydes, and sulphur compounds are the key ones andcontribute the most to coffee aroma [37,40]. Pyrazines have high contents and low odorthresholds, mainly giving nutty and baking odors [43]. Furans commonly contributeto balsamic, wheat, and baking odors [44]. Aldehydes giving fatty, spicy, sweety, andbread odors could confer a richer, more elegant, and unique aroma to wines [45]. Mostsulphur compounds contribute to a negative sulphur aroma. The types and contentsof typical aroma of coffee could be changed by different treatment conditions, such asbaking temperature, extraction temperature, and extraction method, such as that of theextraction of phenolic/flavonoid compounds using different methods [46,47].Only onesulphur compound was detected in this work. This result was not consistent to the strongresponse to sulfides in the electronic nose analysis. The detection method needs to befurther optimized in future study. The above-mentioned four kinds of aromatic componentsaccounted for 34.0%, 11.9%, and 32.2% in SCG extract, SFB, and SDS, respectively. A similarproportion of four key aromatic groups to SCG extract in SDS might bring the outstandingtypicality of coffee aroma in Table 1 and Figure 4a1, though the fewest types of volatileswere observed in SDS. The proportion of original aroma in SFB decreased with increasedfermentation aroma, producing a complex and rich aroma and more flavor levels of SFB. Table 2. The concentration of volatile compounds identified in SCG extract, SFB, and SDS. Code AC1 Table 2. Cont. Code Compounds Odor Description * Concentration (ug/L) SCG Extract SFB SDS F9 Benzofuran 18±1 F10 2-Acetylfuran Balsamic 550±6 B 2350 ±21 A 152±3C F11 5-Methyl-2-propenyl furan 105±2A 4±1C 27±1B F12 Methyl Furfuryl Disulphide Smoke 66±3 F13 2-(2-Furfuryl)furan 768±14A 70±3C 309±6 B F14 5-Methyl-2-propiony furan 252±5 - F15 Difurfuryl ether 1015±10A 608±9B - F16 2-Furfuryl-5-methyl furan 532 ±11A 64±1C 82±1B Terpenes T1 B-Myrcene Balsamic, must, spice 13±1B 99±3A T2 DL-Limonene 80±2B 48±3B 396±9A T3 Styrene Balsamic, gasoline 538±11B - 1030±10A T4 cis-Linaloloxide (2-methyl-2-vinyl-5-(alpha hydroxyisopropyl) tetrahydrofuran) Flower, wood 308 ±3A 213±6B T5 Camphor Camphor 199±4 - T6 Linalool 273±3A 254±6B - T7 B-Damascenone Apple. rose, honey 173±2A 19±1B Pyrroles Pyr1 3-Methylpyrrole 53±4B 368±7A Pyr2 N-methyl-2-acetyl pyrrole 571±14B 808±9A - Pyr3 1-Furfurylpyrrole Lactones y-Butyrolactone Others 893 ± 15 L1 Caramel, sweet 83±2B 167±5A - 3,4-Diethylthiophene - 19±2 O2 4-Methylthiazole Roasted meat 63±4 - - Values shown represent the averages of triplicate samples (data are mean ± SD). Values with different superscript letters in the same roware significantly different (p<0.05).-: not detected. Spent coffee grounds: namely SCG. SCG fermented beverages: namely SFB. SCGdistilled spirits: namely SDS.*http://www.flavornet.org/flavornet.html (accessed on 22 October 2021). Figure 4. Variation in (a) concentration and (b) number of volatile compounds in samples. SFB: SCG fermented beverages.SDS: SCG distilled spirits. Values with different capital letters upon the columns of the same item are significantly different(p<0.05). Compared with SCG extract, 20 volatile compounds including 1 ester, 2 alcohols,5 aldehydes,1 ketone, 1 phenol, 5 furans, 3 terpenes, 1 pyrrole, and 1 thiazole lost in SFB,but those for SDS were 36 volatile compounds including 1 ester, 5 alcohols, 4 aldehydes,2 ketones, 3 phenols, 6 pyrazines,6 furans, 4 terpenes, 3 pyrroles,1 lactone, and 1 thiazole. Esters were the most noteworthy group in terms of the variation of number andcontent of volatile compounds. The number and proportion of esters in SFB increased from7 to 12 and from 30.0% to 47.4%, respectively. The increased alcohol and acid providedsufficient precursors for the synthesis of esters in SFB. The increase of esters enhancedfruity aroma of SFB, which was consistent with the results of physicochemical and sensoryevaluation (Table 1). The rich fruity flavor could alleviate the prominent sour and astringenttastes of SFB to a certain extent. The odor activity values (OAVs) were used to further investigate the contribution ofeach compound to the flavor of SFB and SDS (Table 3). The OAVs of 28 volatile compoundswere above 1 in this test. Amongst them, 17, 13, and 8 compounds were shown in SCGextract, SFB, and SDS, respectively. The largest number of esters possessing OAV>1was observed in SFB, thus contributing the rich fruity odor to SFB. As with SCG extract,several aldehydes of SDS exhibited OAV>1. However, strong fatty, ether, and sweetodors were negative to wine flavor. Compared with SCG extract, the outstanding alcohols-,ketones-, and pyrroles-induced odors might disappear in SFB and SDS, but phenols- andterpenes-induced odors may remain with OAVs of 4-ethyl-2-methoxyphenol, 4-allyl-2-methoxyphenol, styrene,linalool, and B-damascenone>1. As one of the major groups of thetypical flavors of coffee, furans might contribute the most in SFB with the OAV of furfurylethyl ether >1, and furans-induced odors might balance with the fruity odor induced byesters. The odor threshold values of many furan compounds did not be retrieved fromliterature. Therefore, the contribution of furans to SDS could not be ignored. Table 3. OAVs (>1) for the main volatile compounds in SCG extract, SFB, and SDS. Code Compounds Odor Description * SCG Extract SFB SDS Esters E1 Ethyl acetate Pineapple 1421.0 4.9 0.8 E2 Ethyl propionate Fruit 255.6 20.8 / E3 Ethyl2-methylpropanoate Sweet, rubber - - 7.3 E5 Ethyl butyrate Apple 560.0 - - E7 Ethyl3-methylbutanoate Fruit - 1.3 - E8 Isoamyl acetate Banana 0.2 44.9 6.2 E9 Ethyl hexanoate Apple peel, fruit 12.6 46.5 0 E10 Ethyl octanoate Fruit, fat 8.9 35.9 6.0 E13 Ethyl decanoate Grape 12.4 1.3 0.2 E15 Phenethyl acetate Alcohols Rose, honey, tobacco - 0.6 - A1 2-Methyl-1-butanol Wine, onion 29.7 0.2 0.1 A2 3-Methyl-1-butanol Whiskey,malt, burnt / 0.4 0.7 A4 2-Heptanol Mushroom 0.5 - - Aldehydes Al1 Ethanal Pungent, ether 0.5 1629.1 Table 3. Cont. Code Compounds Odor Description* SCG Extract SFB SDS Al3 Acetaldehyde diethyl acetal - - 6.4 Al4 (Z)-4-heptenal Biscuit, cream 12.4 - - A15 Octanal Fat, soap, lemon, green 1.5 - Al6 Nonanal Fat, citrus, green 15.9 一 - A18 Phenylethanal Hawthorne, honey, sweet 55.0 - 1.0 K1 Ketones 2-Methylpentan-3-one Phenols Mint 5.8 / - P2 4-Ethyl-2-methoxyphenol Spice, clove 26.4 37.3 1.8 P4 4-Allyl-2-methoxyphenol Clove, honey 1464.7 39.8 - F2 Furans Furfuryl ethyl ether - 138.0 0.9 Terpenes T3 Styrene Balsamic, gasoline / - 4.1 T6 Linalool 2.7 10.2 - T7 B-Damascenone Apple. rose, honey / 380.0 - Pyrroles Pyr3 1-Furfurylpyrrole Lactones y-Butyrolactone 8.9 - L1 Caramel, sweet 2.4 4.8 - -: not detected./not calculated due to unreferenced odor threshold values.*http://www.flavornet.org/flavornet.html (accessed on22 October 2021). OAVs were calculated by the ratio of the concentration of a compound to its odor threshold value. To calculate the OAVsof compounds in SCG extract, SFB, and SDS, odor threshold values in water, low content of alcohol (<15%), and high content of alcohol(>40%) were referred from literature, respectively. 4. Conclusions The supplement of 0.20%(NH4)2HPO4 was effective for improving growth and alcoholfermentation of S. cerevisiae D254 in SCG extract. SFB and SDS produced at the optimizedfermentation conditions (SCG-liquid ratio 1:4;24% sucrose; pH 4.3; 6% activated S. cerevisiaestrain D254;23 °C for 8 days) had appropriate physicochemical properties (12.5% and 50.5%ethanol, respectively; 9.07 g/L and 0.37 g/L total acidity, respectively) and different sensorycharacteristics, including aroma and taste senses. Fermentation aromas, especially esters,produced in SFB, increased the complexity of aroma and lowered the irritating aromas, suchas nitrogen oxides-induced ones. The combination of original and fermentation aromaticcomponents could balance the outstanding sourness, astringency, and saltiness of SFB.Fermentation aroma was partially lost (e.g., the total contents of esters decreased by 84%,compared with SFB) and the sourness, bitterness, astringency, and saltiness tastes wererelieved in distillation according to the electronic tongue system, leading to the relativelymore prominent aroma typicality of coffee and a soft taste. Supplementary Materials: The following are available online at https://www.mdpi.com/article/10.3390/fermentation7040254/s1, Figure S1: The brewing process of SCG beverages, Figure S2: Effectsof types and concentrations of nitrogen source on a biomass yield of D254, b residual sugar, and cethanol content in SCG substrate, Figure S3: Effects of a the ratio of SCG b the inoculation amount of S.cerevisiae D254c the initial content of sucrose dpH e fermentation temperature on the physicochemicaland sensory properties of SFB, Table S1: Chemical sensors used in electronic nose correspondingto different types of volatile substances, Table S2: Standard solution for electronic tongue analysis,Table S3: Sensory scoring rules for the SCG fermented beverages, Table S4: Performance of differentyeast strains in SCG extract. Table S5: Chemical parameters of unfermented SCG extract. Author Contributions: Conceptualization, C.L. and S.L.; Methodology, Z.L.; Investigation, Z.L.;Resources, C.L. and S.L.; Data Curation, X.Y.; Writing-Original Draft Preparation, L.W., X.Y. andX.L.; Writing-Review & Editing, X.L. and X.H.; Supervision, C.L. All authors have read and agreedto the published version of the manuscript. Funding: This research was funded by [the Hainan Provincial Natural Science Foundation of China]grant number [2019RC083], [the National Natural Science Foundation of China] grant number[31760456], [the Education Department of Hainan Province] grant number [Hnky2019-7], and [theScientific Research Foundation of Hainan University] grant number [KYQD1660]. The APC wasfunded by [the National Natural Science Foundation of China]. Institutional Review Board Statement: Not applicable. Informed Consent Statement: Not applicable. Data Availability Statement: The original contributions presented in the study are included in thearticle/Supplementary Material, further inquiries can be directed to the corresponding author. Acknowledgments: This research was funded by the Hainan Provincial Natural Science Foundationof China (grant number 2019RC083), the National Natural Science Foundation of China, grantnumber 31760456, the Education Department of Hainan Province, grant number Hnky2019-7, andthe Scientific Research Foundation of Hainan University, grant number KYQD1660. Conflicts of Interest: The authors declare no conflict of interest. References 1Acikalin, B.;Sanlier, N. Coffee and its effects on the immune system. Trends Food Sci. Technol.2021,114,625-632.[CrossRef]23 Ruta, L.L.; Farcasanu, I.C. Coffee and yeasts: From flavor to biotechnology. Fermentation 2021, 7, 9. [CrossRef] Campos-Vega, R.;Loarca-Pina,G.; Vergara-Castaneda,H.A.;Oomah, B.D. Spent coffee grounds: A review on current researchand future prospects. Trends Food Sci. Technol. 2015,45,24-36. [CrossRef] 4. Mussatto, S.J.; Machado, E.M.S.; Martins, S.; Teixeira, J.A. Production, composition and application of coffee and its industrialresidues. Food Bioprocess Technol. 2011,4,661-672.[CrossRef] 5. Silva,M.;Nebra, S.A.; Machado Silva, M.J.;Sanchez, C.G. The use of biomass residues in the Brazilian soluble coffee industry.Biomass Bioenerg. 1998,14,457-467.[CrossRef] 6. da Silveira,J.S.; Durand, N.;Lacour,S.; Belleville, M.P.;Perez, A.; Loiseau, G.;Dornier, M. Solid-state fermentation as a sustainablemethod for coffee pulp treatment and production of an extract rich in chlorogenic acids. Food Bioprod. Process. 2019,115,175-184.CrossRefl ( 7. Simoes, G.; Demetrio,G.B.; de Paula, G.F.;Ladeira, D.C.; Matsumoto,L.S. Influence of spent coffee grounds on soil microbiological attributes and maize c rop. Res. Soc. Dev. 2020,9, e818986400. [ Cr ossRef] ) ( 8. Araujo, M.N.; Azevedo, A.Q.P.L.; Hamerski, F; Voll, F.A.P.; Corazza, M.L. Enhanced extraction of spent coffee grounds oil using high-pressure CO2 plus ethanol solvents. Ind. Crop Prod . 2019,141,111723. [CrossR e f ] ) 9. Martinez-Saez,N.; Garcia, A.T.; Perez, I.D.;Rebollo-Hernanz, M.; Mesias, M.; Morales, F.J.;Martín-Cabrejas, M.A.; del Castillo,M.D. Use of spent coffee grounds as food ingredient in bakery products. Food Chem. 2017,216,114-122. [CrossRef] ( 10. Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of natural antioxidants from spent coffee grounds. J.Agric. Food Chem. 2013,61,4162-4168. [ CrossRef ] ) ( 11. Sampaio, A.; Dragone,G.; Vi l anova, M.; Oliveira, J.M.; Teixeira, J.A.; Mussatto, S.I. Production, chemical characterization, andsensory profile of a novel spirit elaborated from spent coffee ground. LWT-Food. Sci. Technol. 201 3 ,54,557-563.[CrossRe f] 12. ) ( Gouvea, B.M.; Torres, C.; Fr a nca, A.S.;Oliveira, L.S. ; Oliveira, E.S. Feasibility of ethanol prod u ction from c o ffee husks. Biotechn o l. Lett. 2009, 31,1315-1319 . [ C r oss R ef ] ) ( 13. Hughes, S . R.; Lopez-Nunez, J.C.;Jones, M.A.; Moser, B.R.; Cox,E.J.; Lindquist, M. ; Galindo-Leva, L.A . ; Riano-Herrera, N.M.; Rodriguez-Valencia, N.; Gast, F.; et al. Sustainable conversion of coffee and other crop wastes to biofuels and bioproducts using coupled biochemical and thermochemical processes in a multi-stage biorefinery concept. Appl. Microbiol. B i otechnol. 2014,98, 8413-8431. [CrossRe f ) ( 14. Machado,E.; Mussatto, S.; Teixeira, J.; Vilanova, M.; Oliveira, J. Increasing the sustainability of t h e coffee agro-industry: Spentcoffee grounds as a source of new beverages. Beverages 2018, 4,105. [Cro s s Ref] ) ( 15. Garcia, C .V.;K i m, Y.T. Spent coffee grounds and coffee silverskin as potential materials for packaging: A review . J . Polym. Environ. 2021,29, 2372-2384. [C rossRef] ) ( 16. Liu, Y.J. ; Yuan, W.Q.; Lu, Y.Y.; Liu , S.Q . Biotransformation of spent coffee grounds b y fermentation w i th monocultures ofSaccharomyces cerevisiae and Lachancea thermotolerans aided by yeast extracts. LWT-Food Sc i . Technol. 2021,138,110751. [CrossRef ] ) ( 17. Liu, Y.J.; Seah, R.H.; Abdul Rahaman, M.S.; L u, Y.Y.; Liu, S.Q. Concurrent inoculations of Oenococcus oeni and Lachanceathermotolerans: Impacts on non-volatile and volatile components of spent coffee grounds hydrolysates. LWT-Food Sci. T echnol. 2021,148,111795.[ C r ossRe f ) ( 18. Liu, Y.J; Lu, Y.Y.; Liu, S.Q. The potential of spent coffee grounds hyd r olysates fermented with Torulaspora delbrueckii and Pichiakluyveri for developing an alcoholic beverage: The yeasts growth and chemical compounds modulation by yeast extracts. Curr. Res. Food Sci. 2021,4,489-498. [CrossRe f ) ( 19. Buratti, S.;Ballabio, D.; Benedetti, S.; Cosio, M.S. Prediction of I talian red wine sensorial descriptors from electronic nose, electronic tongue and spectrophotometric measurements by means of Genetic Algorithm regre s sion models. Food C hem. 2007, 100,211-218. [ C r o ssRef] ) ( 20. Jo, Y.; Gu, S.Y.; Chung, N.; Gao, Y.; Kim, H.J;Jeong, M.H.; Jeong, Y.J.; Kwon, J.H. Comparative analysis of sensory profiles ofcommercial cider vinegars from Korea, China,Japan, and US by SPME/GC-MS,E-nose, and E-tongue October Korean. J. Food S c i.Technol. 2016,48,430-436. ) ( 21. Zhao, G.Z.; Feng, Y.X.; Hadiatullah, H.; Zheng, F.P.; Yao, Y.P. Chemical characteristics of three kinds of Japanese soy sauce basedon electronic senses and GC-MS analyses. Front. Microbiol. 2021, 11,3222.[ Cross R ef] ) ( 22. Sharma, S.; M ahant, K.; Sharma, S.; Thakur, A.D. Effect of nitrogen source and citric acid a d dition on wine preparation from Japanese persimmon. J. I . Brewing2017,123,144-150.[C r o s s Ref ] ) ( 23. Hlaing, M.T.; Joshy, C.G. Optimization of fermentation process for the preparation of coffee wine by response surface methodology.Dagon U n iv. C o mmem. 25th Anniv. Silver Jubil. Res . J. 2019,9,2. ) ( 24. Yu,L . ; Du, J.H.; Wang, X.J.; Yin, Q. Study on r e sis t ance of Saccharomyces cerevisi a e sp. to c i tri c acid. S c i. Te c hnol . Food Ind. 200 8 ,29, 148-151. ) ( 25. Wang, B.S.; L i , L.B.; W u, Z.W.; Zhang, L.; Chen, F.; Chen, L.; Zhang, M.X. Inhibiting effects of high concentration of citric ac i d onthe growth of Saccharomyces cerevisiae. China Brew. 2 018,37,56-60. ) ( 26. Li, Z.T.; Li, C.F.; J i a, Y.Y.; Wang, Q.K.; Lu, Y.S.; Wang, M.Y.; Huang, Q.; Liu, S.X. Screening of yeast for coffee-grounds winefermentation and research on nitrogen source for nutritional condition opt i mization. China Brew. 2016, 35, 61-64. ) ( 27. Gutierrez, A.; Chiva, R.; Sancho, M.; Beltran, G.; Arroyo-Lopez, F.N.; Guillamon, J.M. Nitrogen requirements of commercial wineyeast strains during fermentation of a synthetic grape must. Food Microbiol. 20 1 2,31,25-32.[Cross Ref] ) ( 28. Beltran, G.;Novo, M.; Rozes, N.; Mas, A.; Guillamon,J.M. Nitr o gen catabolite repression in Saccharomyces cerevisiae during winefermentations. FEMS Yeast Res. 2004,4,625-632.[C rossRef] ) ( 29. Lucero, P .; M oreno, E.; Lagunas,R. Catabolite inactivation of the sugar transporters in Saccharomyces cerevisiae is inhibited by t h epresence of a nitrogen source. FEMS Yeast Res. 2002,1,307-314. ) ( 30. Hernandez-Orte, P.; Ibarz, M . J.; Cacho,J.; F e rreira, V. Effect o f the addition of ammonium and amino acids to musts of Aire n variety on aromatic composition and sensory properties of the obtained wine. Food Chem. 2005,89,163-174.[ C r ossRe f ] ) 31. Santos,J.;Leitao-Correia, F.; Sousa, M.J.; Leao,C. Ammonium is a key determinant on the dietary restriction of yeast chronologicalaging in culture medium. Oncotarget 2015, 6, 6511-6523.[CrossRef] [PubMed] ( 32. Forkert, P.G. Mechanisms of lung tumorigenesis by ethyl carbamate and vinyl carbamate. Drug Metab.Rev. 2010, 42, 355-378.[ CrossRef ][ PubMe d ] ) ( 33. Silla, S.M.H. Biogenic amines: Th e ir importance in foods. Int. J . Food Microbiol. 1996 , 29, 213-231. ) ( 35. Bressani, A.P.P.; Martinez, S.J.; Sarmento, A.B.I.; Borem, F.M.; Schwan,R.F. Organic acids produced during fermentation andsensory perception i n specialty coffee using yeast starter culture. Food Res. I nt. 2020, 1 28,108773. [Cr ossRe f ] ) 36. De Bruyn, F;Zhang, S.J.; Pothakos, V.; Torres,J.; Lambot,C.;Moroni, A.V.; Callanan, M.; Sybesma, W.; Weckx, S.; De Vuyst, L. ( Exploring the impacts of postharvest processing on the microbiota and metabolite profiles during green coffee bean production.Appl. E nviron. Microbiol. 2016, 8 3 , e02398-16. [C r ossRef] ) ( 37. Evangelista, S.R.; da Cruz Pedrozo Miguel, M.G.; de Souza Cordeiro, C.; Silva, C.F.; Pinheiro,A.C.M.; Schwan,R.F. Inoculation ofstarter cultures in a semi-dry coffee (Coffea arabica) fermentatio n process. Food Microbiol.2014,44,87-95. [ CrossRef] ) ( 38. Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, ecology and industrial applications of aromaformation in yeast. FEMS Microbiol. R ev. 2 017,41,S95-S128. [C r os s Ref] ) ( 39. Ma, P.X.; Zhang, B.C.; Zhang, Y. Research on th e di f ference between Chi n ese quality brandy and French qualit y brandy . Liquor-Mak. Sci. T echnol. 2011, 1,47-51. ) ( 41. Lee, S.J.; Kim, M.K.; Lee, K.G. Effect of reversed coffee grinding and roasting process on physicochemical properties includingvolatile compound profiles. I nnov. Food Sci. Emerg. Technol. 2017,44,97-102. [ CrossRef] ) ( 42. Ribeiro, L.S. ; Miguel, M.G.; Evangelista, S.R.; Machado Martins, P.M.; van Mullem, J.; Belizario, M.H.; Schwan, R.F. Behavior ofyeast inoculated during semi-dry coffee fermentation and the effect on chemical and sens o rial properties of the final beverage.Food Res. Int. 2017,92,26-32. [ C r ossRef] ) ( 43. Czerny, M.; Christlbauer, M.; Christlbauer, M.; Fischer, A.; Granvogl, M.; Hammer, M.; Hartl, C.; Hernandez, N.M.; Schieberle, P.Re-investigation on odour thresholds of key food aroma compounds and development of an aroma language based on odourqualities of defined aqueous odorant solutions. Eur. Food Res. Technol. 2008 , 228,265-273. [ CrossRef] ) ( 44. Lin, X.; Wang, Q.K.; Wu, W.Y.; Zhang, Y.X.; Liu, S.X.; Li, C.F. Evaluation of different Saccharomyces cerevisiae strains on the profileof volatile compounds in pineapple wine. J. Food Sci. Technol . 2018, 55,4119-4130. [CrossRef ] ) ( 45. Nyanga, L . K.; Nout, M . J.; Smid, E.J.; B oekhout, T . ; Z wietering, M . H. Fe r mentation characteristics of yeasts isolated f r omtraditionally f ermented masau (Ziziphus mauritiana) frui t s. Int. J. Food Microbiol. 20 1 3,166,426-432. [Cros s R ef] ) ( 46. Lin, X.; Wu, L.F.; Wang, X.; Yao, L.L.; Wang, L. Ultrasonic-assist e d extraction for flavonoid compounds content and antioxidant activities of India Moringa oleifera L. leaves: Simultaneous optimization, HPLC characterization and comparison with othermethods. J. Appl. Res. Med. Aromat. Plants 2021,20,100284. ) 47. Wu, L.F.; Li, L.;Chen, S.J.; Wang, L.; Lin, X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compoundsfrom Moringa oleifera L. leaves: Optimization, comparison and antioxidant activity. Sep. Purif. Technol. 2020, 274, 117014.[CrossRef 48. Lin, X.; Jia, Y.; Li, K.; Hu, X.P.; Li, C.F.; Liu, S.X. Effect of the inoculation strategies of selected Metschnikowia agaves andSaccharomyces cerevisiae on the volatile profile of pineapple wine in mixed fermentation. J. Food Sci. Technol. 2021. [CrossRef] ( 49. Sun, S.Y.;Jiang, W.G.; Zhao, Y.P. E valuation o f different Saccharomyces cerevisiae strains on the profile of volatile compounds andpolyphenols in cherry wines. Food Chem. 2011,2,547-555. [C rossRef] ) 以废咖啡渣为原料,采用水热提取液,以商业酿酒酵母菌株D254为原料制备酒精饮料。采用感官描述、德国AIRSENSE电子鼻、日本INSENT电子舌、气相色谱-质谱(GC-MS)对SCG酒精饮料的感官特性进行分析。结果提示,添加0.20% (NH4)2HPO4可有效促进酿酒酵母D254在SCG提取液中的生长和酒精发酵。在优化的发酵条件下生产的SCG发酵饮料(SFB)和SCG蒸馏酒(SDS)具有合适的理化性质和不同的感官特性。SFB中产生了发酵香味,尤其是酯类香味,增加了香气的复杂性,降低了刺激性香气。原始成分和发酵成分的组合可能会平衡SFB突出的酸味、涩味和咸味。发酵香气部分丧失,蒸馏过程中酸、苦、涩、咸的味道得到缓解,咖啡的香气典型性相对突出,口感柔和。这些发现为生产新的高品质咖啡味酒精饮料或调味酒奠定了基础。
确定









还剩15页未读,是否继续阅读?
北京盈盛恒泰科技有限责任公司为您提供《酒精饮料中感官特性检测方案(感官智能分析)》,该方案主要用于其他饮料中理化分析检测,参考标准--,《酒精饮料中感官特性检测方案(感官智能分析)》用到的仪器有德国AIRSENSE品牌PEN3电子鼻
推荐专场
感官智能分析系统(电子鼻/电子舌)
相关方案
更多
该厂商其他方案
更多