本文研究了解淀粉芽孢杆菌发酵米豆(Vigna umellata)的理化性质和生物活性,根据纤维蛋白溶解活性对发酵条件进行了优化,解淀粉芽孢杆菌发酵的米豆可作为功能性食品对预防血栓性疾病有潜在的好处。
方案详情

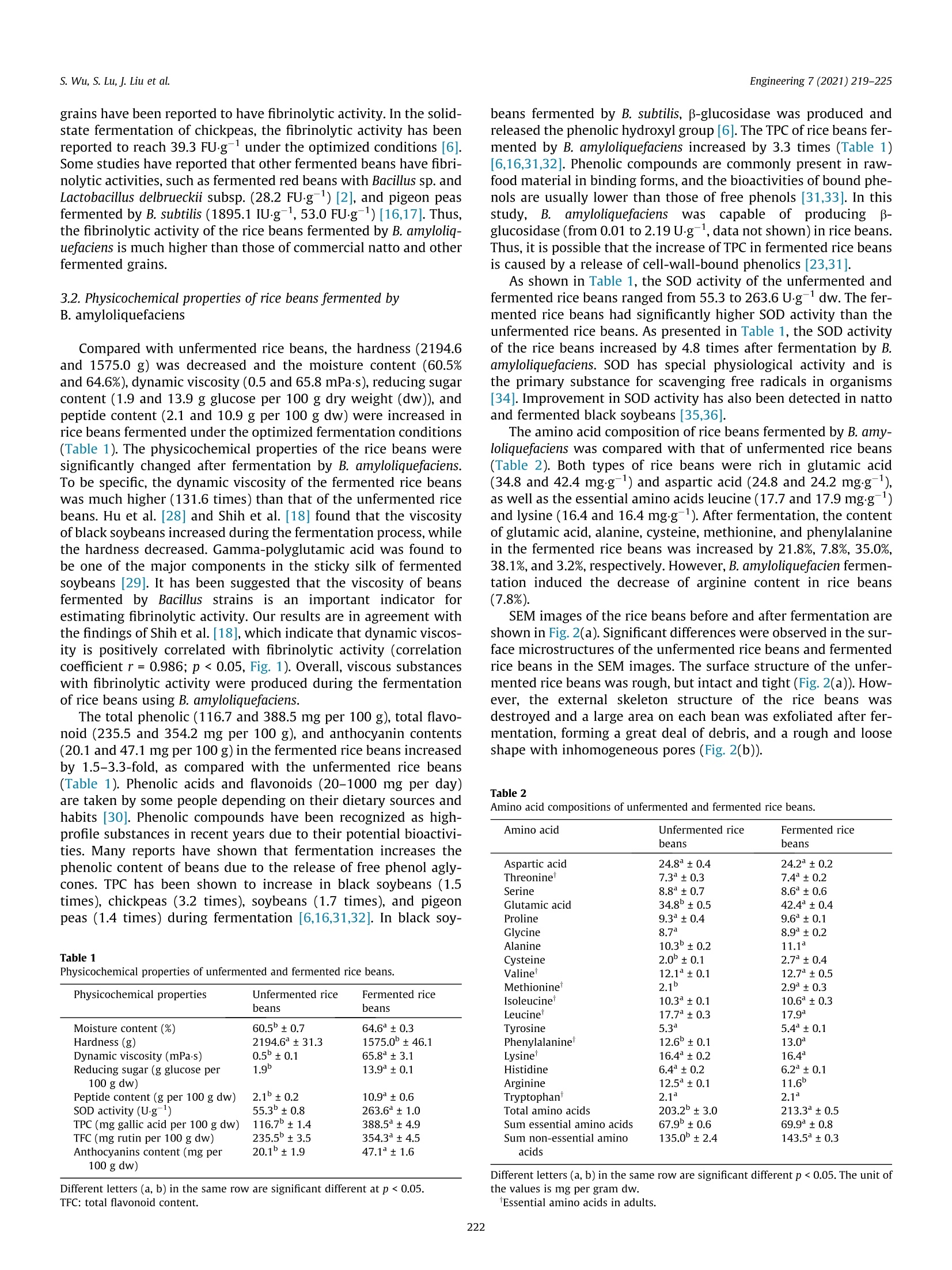
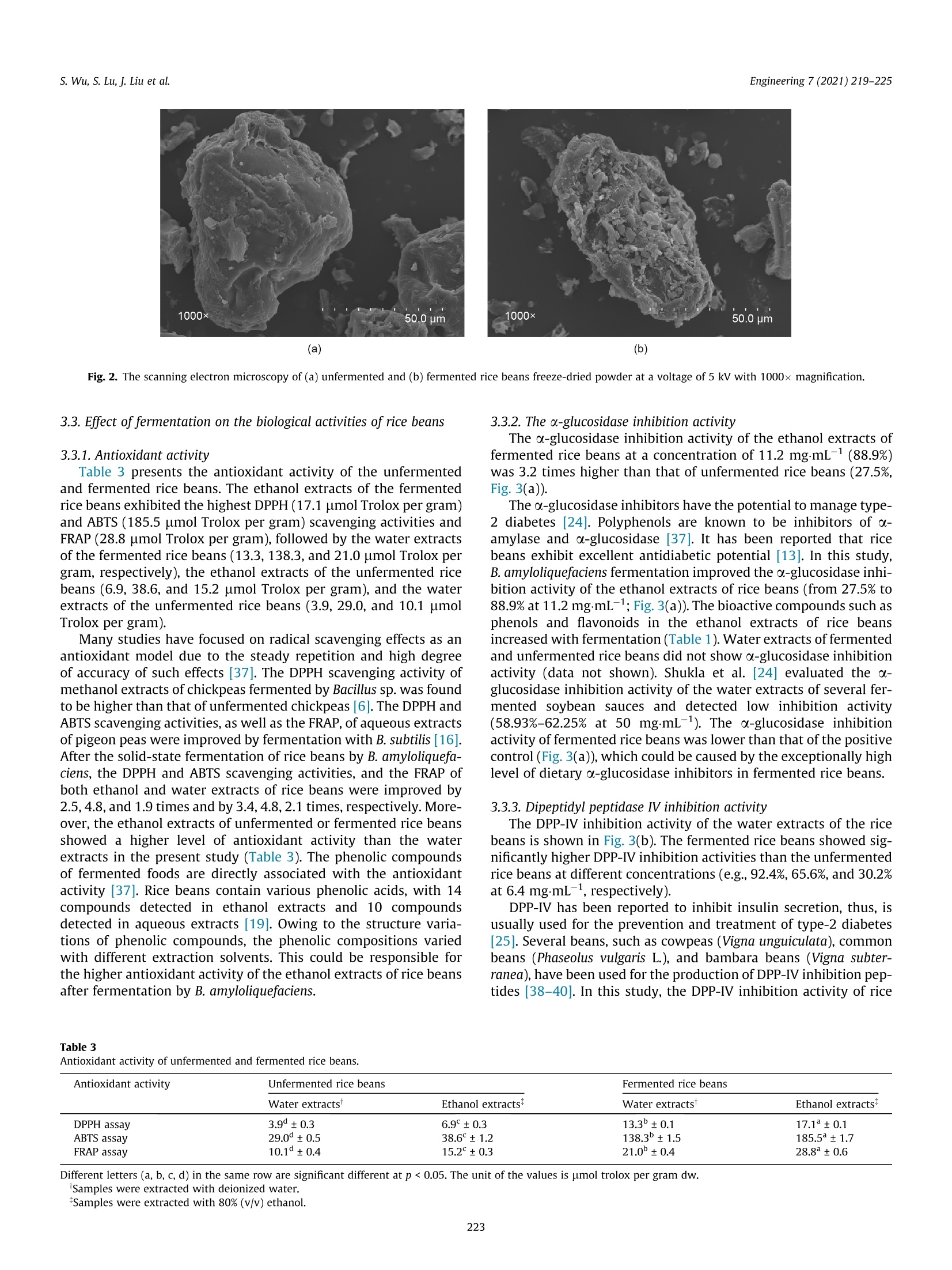
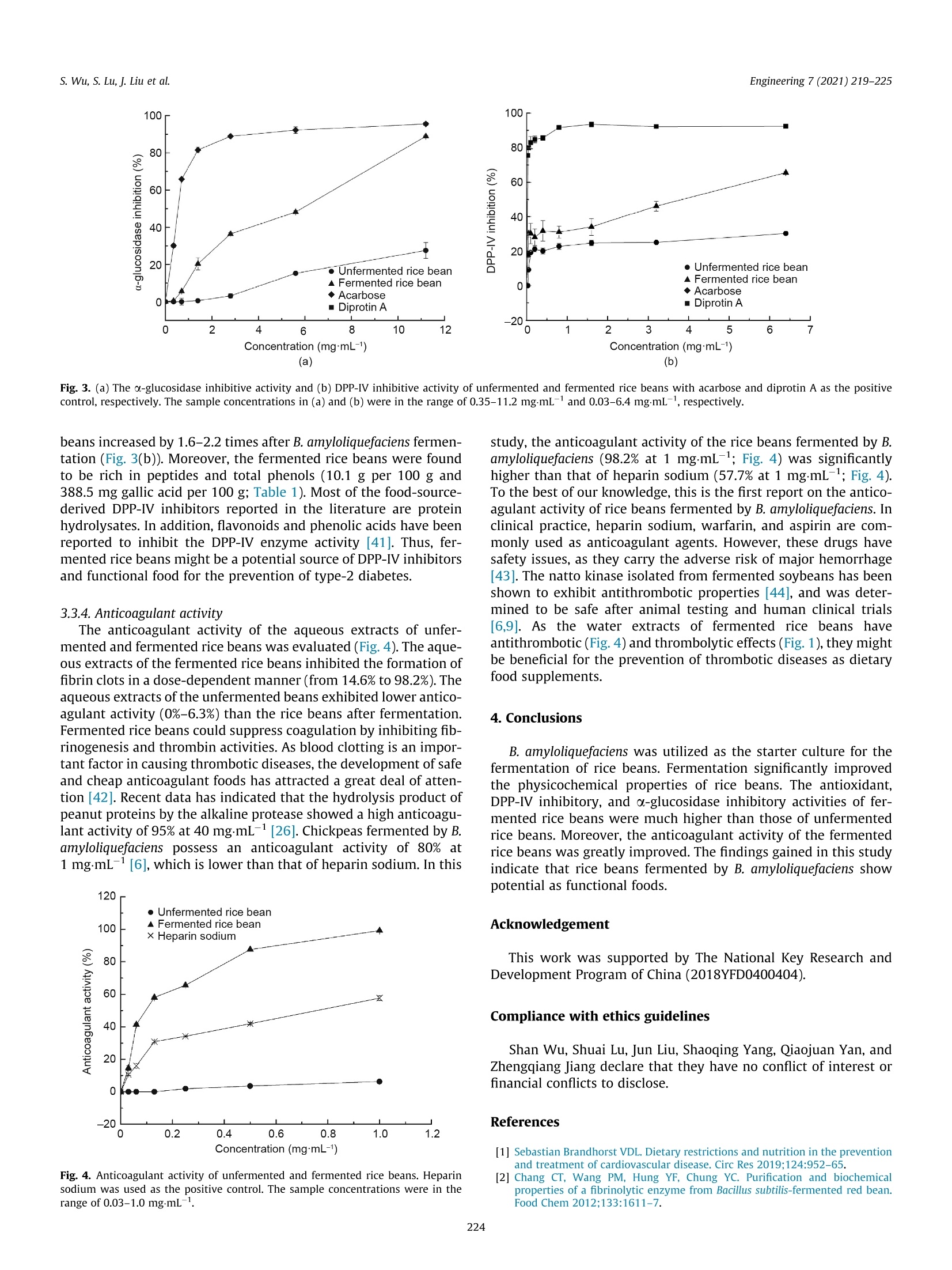
Engineering 7 (2021) 219-225 s. Wu, S. Lu, J. Liu et al.Engineering 7 (2021) 219-225 Contents lists available at ScienceDirect Engineering Engineering journal homepage: www.elsevier.com/locate/eng Research Food Safety and Health-Article Physicochemical Properties and Bioactivities of Rice Beans Fermented byBacillus amyloliquefaciens Shan Wu, Shuai Lu ", Jun Liu, Shaoqing Yang, Qiaojuan Yana.#,*, Zhengqiang Jiangb,#,* Key Laboratory ofFood Bioengineering (China National Light Industry), College of Engineering, China Agricultural University, Beijing 100083, China"Beijing Advanced Innovation Center for Food Nutrition and Human Health, College of Food Science and Nutritional Engineering, China Agricultural University, Beijing 100083, China ARTICLE INFO ABSTRAC T Article history: Received 2 November 2018Revised 12 December 2019Accepted 18 December 2019Available online 29 October 2020 Keywords: Solid-state fermentationRice beansBacillus amyloliquefaciensAntithrombotic activityAntioxidant activityAntidiabetic activity The purpose of this study was to investigate the physicochemical properties and bioactivities of ricebeans (Vigna umbellata) fermented by Bacillus amyloliquefaciens. The fermentation conditions were opti-mized on the basis of the fibrinolytic activity. Under the optimal fermentation conditions, the fibrinolyticactivity reached a maximum of 78.0 FUg-(4890IU.g-, fibrin plate method, FU: fibrin degradation unit).The contents of peptides (which increased from 2.1 to 10.9 g per 100 g), total phenolics (from 116.7 to388.5 mg gallic acid per 100 g), total flavonoids (from 235.5 to 354.3 mg rutin per 100 g), and antho-cyanin (from 20.1 to 47.1 mg per 100 g), as well as the superoxide dismutase activity (from 55.3 to263.6 Ug) in rice beans were significantly increased after fermentation. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt(ABTS) free radical scavenging activities and ferric reducing antioxidant power (FRAP) of fermented ricebeans were 1.9-4.8 times higher than those of unfermented rice beans. Moreover, fermentation inducedan increase in the dipeptidyl peptidase IV (DPP-ⅣV) inhibition,a-glucosidase inhibition, and anticoagulantactivities of rice beans. Rice beans fermented by Bacillus amyloliquefaciens may serve as a functional foodwith potential benefits for the prevention of thrombotic diseases. ◎ 2020 THE AUTHORS. Published by Elsevier LTD on behalf of Chinese Academy of Engineering and 1. Introduction Cardiovascular disease (CVD) is an important factor affectinghuman health, and accounts for approximately one-third of deathsworldwide. Strokes and coronary heart disease are primarilycaused by obstructions in the blood vessels that preclude bloodfrom flowing into the brain or heart. Therefore, thrombolytic ther-apy is an effective way to prevent CVD [1]. During the past decades,many researchers have focused on safe and cheap antithromboticfoods. Studies have reported that fermentation enhances the fibri-nolytic activity of soy foods, including Chinese dochi, Japanesenatto, and Korean soybean pastes [2]. Fermented soy foods havesignificant modifications in flavor and texture due to the actionof microorganisms [3]. Bacteria, especially Bacillus sp., have beenrecognized as the most important genus producing fibrinolyticenzymes. Some fibrinolytic enzymes have been discovered fromfood-grade bacteria such as Bacillus subtilis (B. subtilis) [4], Bacillus ( * Corresponding authors. ) ( E-mail addresses: yanqj@cau.edu.cn (Q .Yan), zh q j i ang@ c au.edu.cn (Z. Jiang). T hese authors contributed e q ually to this work. ) amyloliquefaciens (B. amyloliquefaciens) [5-7], Bacillus vallismortisAce02 [8], and B. megaterium KSK-07 [9].Soybeans fermented byB. subtilis have antithrombotic effects both in vitro and in vivo[4,10]. B. amyloliquefaciens is close to B. subtilis, and can bescreened from Chinese dochi and Korean soy sauce [5,10]. Subtili-sins, a fibrinolytic enzyme, was found to be produced by B. amy-loliquefaciens [7]. Solid-state fermentation is considered to be agood process for the promotion of nutrients and active substances,as well as antioxidant activity, in many legumes and cereals [11].However, there are very few reports on the thrombolytic and anti-coagulant activities of solid-state fermented foods by B. amyloliq-uefaciens [6]. The rice bean is a legume with strong viability that possessesdrought resistance, a short growth period, and simple cultivationtechniques. This crop originates from tropical Asia and has beencultivated in India, Korea, Japan, and South China. In particular,the levels of nutrients and active substances are higher in ricebeans than in many other beans belonging to the same genus[12]. The rice bean is rich in phenolic compounds and shows bioac-tivities that include antioxidant activity and antidiabetic potential[13]. Furthermore, due to its low recognition and high nutritional ( 2095-8099/O 2020 THE AU T HORS. Published by Elsevier LTD on beh a lf of Chinese Academy of Engineering and Hig h er Edu c ation Pre s s Limited Company. This i s an open access article under the CC BY license (htt p://creativecommons.org/licenses/by/4.0/). ) quality, the rice bean has great development potential in the foodmarket [14]. Solid-state fermentation has the advantages of highproductivity,low energy consumption, easy control of the fermen-tation process, and low sterility requirements [15]. In the presentstudy, rice beans were subjected to solid-state fermentation usingB. amyloliquefaciens. The effects of fermentation on the physico-chemical properties and bioactivities of the antioxidant, antidia-betic, and antithrombotic activities of the rice beans wereevaluated. 2. Materials and methods 2.1. Materials, chemicals, and microorganisms Rice beans were purchased from a supermarket in Beijing,China, and were stored at 4C until used. Folin-Ciocalteu phenolreagent, gallic acid, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,2-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammoniumsalt (ABTS), ferric tripyridyltriazine (TPTZ), urokinase from humankidney cells (10 KU), thrombin from bovine plasma,fibrinogen, anddipeptidyl peptidase IV (DPP-Ⅳ) were bought from Sigma-Aldrich(Canada). Heparin sodium, rutin, and acarbose were supplied byBioDee Biotechnology Co., Ltd. (China). B. amyloliquefaciens CAUNDJ118 was screened from mushroomsauce and assigned a preservation number (CGMCC NO. 16050) inthe China General Microbiological Culture Collection Center(CGMCC). 2.2. Fermentation and extraction of rice beans Fifty grams of rice beans were soaked in deionized water for18 h. The swollen beans were then placed on a plate and cookedat 121 C. The cooked beans were cooled, inoculated with variousconcentrations of bacteria, and incubated at 40 ℃ to optimizethe fermentation conditions on the basis of fibrinolytic activity.Un-inoculated rice beans were used as the blank control (unfer-mented rice beans). Both the fermented and unfermented sampleswere freeze-dried and then extracted separately with deionizedwater (1/5 (v/v)), 80% (v/v), ethanol, and 0.5% HCl in methanol,respectively. The extracts were collected and stored at -20 ℃untilsubsequent analyses. 2.3. Measurement of fibrinolytic activity Fibrinolytic activity was evaluated by two methods. One wasthe fibrin degradation method, which was applied based on theprotocols previously described by Lee et al.[16]. In brief, 1.4 mLof 50 mmol·L-1KCl-HgBO3 buffer (pH 8.0) and 0.4 mL of 0.72% fib-rinogen were incubated at 37C for 5 min. Next, 0.1 mL of throm-bin (20 U-mL-1) was added to react at 37 C for 10 min.Subsequently, 0.1 mL of sample water extract was added and incu-bated at 37 C for 60 min. The reaction was stopped with 2 mL of0.2 molL-trichloroacetic acid (TCA), and the absorption valuewas measured at 275 nm after centrifugation for 10 min(10 000r-min-). One unit (fibrin degradation unit, FU) of enzymeactivity is defined as the amount of enzyme producing a 0.01 incre-ment of absorbance at 275 nm per minute. The fibrinolytic activitywas expressed as FU.g-fresh weight. The other method was the fibrin plate method, which wasemployed to measure fibrinolytic activity as previously describedby Feng et al. [17], with slight modifications. The fibrin plate wasprepared by decanting a mixture that incorporated 5 mL of 0.4%fibrinogen in boric acid-borate buffer with 5 mL of 0.8% agaroseand 1 mL of thrombin (200 U-mL-1). To quantify the fibrinolytic activity of the samples, urokinase solution with different activityunits was used as the standard curve. The results were expressedas IU urokinase per gram fresh weight. 2.4. Physical properties of rice beans The hardness of unfermented and fermented rice beans by B.amyloliquefaciens was analyzed according to the methodprevi-ously described by Shih et al. [18], with slight modifications. Unfer-mented and fermented rice beans were equilibrated to 25°℃ beforemeasurement, and the hardness was determined by means of atexture analyzer (TMS-PRO; FTC, USA). A 25 000gload cellwasapplied and the moving speed was set at 1 mm.s-1.In addition, aplunger probe (o38.1 mm) was applied to press the beans to 60%of the original height. Each sample had nine replicates. The maxi-mum force was considered as the hardness (g). The dynamic vis-cosity of the rice beans was determined with a viscometer (DV-1Viscometer;Yueping, China). Ten grams of rice beans were shakenwith 100 mL deionized water for 30 min and the slimy substanceswere extracted. Next, 20 mL of the extracts was put into the beakerand the viscosity was measured at 30 C. The microstructure wasexamined with a scanning electron microscope (SEM, S-3400N;HITACHI, Japan). The sample was covered with gold and observedat a voltage of 5 kV with 1000x magnification. 2.5. Chemical properties of rice beans Reducingssugar content was determined bytthe3,5-dinitrosalicylic acid (DNS) method [19] and expressed inmilligrams of glucose per gram of dry rice beans. Peptide contentwas neasured .u susing the o-phthaldialdehyde method withGly-Leu as the standard [20]. Total phenolic content (TPC) and totalflavonoid content in the ethanol extracts were analyzed using theFolin-Ciocalteu method and the colorimetric method, respectively[13].Anthocyanin content in the 0.5% HCl methanol extracts wasdetermined using the pH differential method as described by Chiouet al. [21]. Superoxide dismutase (SOD) activity in the waterextracts was measured using a commercial assay kit (Nanjing Jian-cheng Bioengineering Institute, China). Powdered samples weretreated by acidic hydrolysis. Amino acid composition analysiswas performed using the Hitachi L-8900 amino acid analyzer withion-exchange chromatography and the post-column ninhydrinderivation methodd[22]. Amino acid contents were quantifiedagainst 0.2 mmolL-working standard solutions for 17 kinds ofamino acids. The results were recorded in mgg-dry weight. 2.6. Measurement of antioxidant, a-glucosidase inhibition, DPP-IVinhibition, and anticoagulant activities The antioxidant activity of both the water extracts and the etha-nol extracts of the samples was analyzed. DPPH and ABTS freeradical scavenging activities and ferric reducing antioxidant power(FRAP) were determined as reported by Dudonne et al. [23]. All theresults were expressed as umol Trolox per gram dry weight The o-glucosidase inhibition activity of the ethanol extracts wasdetermined according to the method previously described byShukla et al. [24], with slight modifications. In brief, the reactionsolution, including phosphate buffer (0.1 mol-L-, pH 6.8, 50 pL),sample (30 uL), and a-glucosidase (1.5 UmL-,30 pL), was incu-bated at 37 ℃ for 10 min. Subsequently, p-nitrophenyl-o-D-glucopyranoside (0.5 mmolL-,40 uL) was added to the solution,and the resulting mixture was maintained at 37 ℃ for 40 min.NazCOs reagent (0.2 molL-, 80 uL) was used to terminate theenzymatic reaction. The solution without extracts was used asthe control and the solution without a-glucosidase was used as the blank control. Absorbance at 405 nm was measured and theinhibitory percentage was calculated as follows: where Acontrol is the absorbance of the solution without a sample,Asample is the absorbance of the samples, and Acontrolblank is theabsorbance of the blank solution without a-glucosidase. The DPP-IV inhibitory activity of the water extracts was mea-sured by the method reported by Wang et al. [25]. In brief, theextracts were diluted with 0.1 molL- Tris-HCl buffer (pH 8.0).The sample solution (25 pL) was combined with 25 pL1.6 mmolL-1Gly-Pro-p-nitroanilide. The reaction solution wasincubated at 37 ℃ for 10 min. Then, 50 uL DPP-IV (8 UL-1) wasadded. The reaction solution was placed at 37 ℃ for 60 min and100 uL sodium acetate buffer (1 molL-1, pH 4.0) was used to stopthe reaction. Absorbance at 405 nm was measured and the DPP-IVinhibitory activity was determined as follows: where Ag is the absorbance of the mixture without extracts (blankgroup); ABc and Asc is the absorbance of the blank mixture andthe extracts mixture without DPP-IV, respectively; and As is theabsorbance of the reaction mixture (the sample group or the posi-tive group). Measurement of anticoagulant activity was performed in 96-well microplates [26]. The inhibition abilities were calculatedaccording to the following equation: where Ac is the absorbance of the mixture without sample (bufferreplaces the sample); and AcB and AsB is the absorbance of the con-trol mixture and the sample mixture without thrombin (bufferreplaces the thrombin), respectively. 2.7. Statistical analysis All experiments were performed at least in triplicate. Theresults were reported as the mean ± standard deviations. Statisticaldifferences were analyzed using one-way analysis of variance(ANOVA) and Duncan’s multiple range tests with SPSS 20.0 soft-ware (SPSS Chicago, IL, USA). A p value below 0.05 was consideredto be significant. 3. Results and discussion 3.1.Optimization of fermentation conditions Rice beans were steamed for 10-50 min and inoculated with B.amyloliquefaciens at a viable count of 1× 10 CFU (CFU: colonyforming unit) per 100 g. After solid-state fermentation for 24 h,the physicochemical properties and fibrinolytic activity of the fer-mented rice beans were analyzed. As shown in Fig. 1(a), the hard-ness decreased (from 2798 to 1058 g) and the fibrinolytic activityand dynamic viscosity increased with an increase in steaming time.The maximum fibrinolytic activity (73.7 FU.g-) and dynamic vis-cosity (68.0 mPas) of the fermented rice beans were obtained after40 min of steaming. Furthermore, the effects of the inoculationamount and fermentation time on the fibrinolytic activity, hard-ness, and dynamic viscosity for 40 min of steaming time wereinvestigated. As shown in Figs. 1(b) and (c), when the viable countwas 10 CFU per 100 g and the fermentation time was 24 h, the Fig. 1. (a) Effect of steaming time,(b)viable count, and (c) fermentation time on thefibrinolytic activity, dynamic viscosity, and hardness of rice beans fermented byBacillus amyloliquefaciens. maximum fibrinolytic activity and dynamic viscosity were78.0 FUg(4890 IUg, fibrin plate method) and 76.0 mPas,respectively. The sensory evaluation, stringiness, and flavor of thefermented rice beans were very similar to those of commerciallyavailable natto, which is a Japanese traditional fermented soybeanproduct [10]. Moreover, the“ammonia"smell of the fermented ricebeans was much less than that of natto. Under the optimized fermentation conditions, the maximumfibrinolytic activity of the fermented rice beans was found to be78.0 FUg-(4890IU.g, fibrin plate method) in the present study.Based on our knowledge, there is no standard method for thedetermination of fibrinolytic activity. Both the fibrin degradationmethod and the fibrin plate method are commonly used for fibri-nolytic activity analysis. In comparison with the fibrin degradationmethod, the fibrin plate method is easier to operate but has lowersensitivity. In comparison with reported data, the fibrinolytic activ-ity of the rice beans fermented by B. amyloliquefaciens was mea-sured by both the fibrin degradation method and the fibrin platemethod. In general, commercially available natto exhibits a fibri-nolytic activity of 20-40 FU·g-[[i27]. In addition, many other grains have been reported to have fibrinolytic activity. In the solid-state fermentation of chickpeas, the fibrinolytic activity has beenreported to reach 39.3 FU.g-under the optimized conditions [6].Some studies have reported that other fermented beans have fibri-nolytic activities, such as fermented red beans with Bacillus sp. andLactobacillus delbrueckii subsp. (28.2 FU.g)[2], and pigeon peasfermented by B. subtilis (1895.1 IU.g-, 53.0 FU.g) [16,17]. Thus,the fibrinolytic activity of the rice beans fermented by B. amyloliq-uefaciens is much higher than those of commercial natto and otherfermented grains. 3.2. Physicochemical properties of rice beans fermented byB. amyloliquefaciens Compared with unfermented rice beans, the hardness (2194.6and 1575.0 g) was decreased and the moisture content (60.5%and 64.6%),dynamic viscosity (0.5 and 65.8 mPas), reducing sugarcontent (1.9 and 13.9 g glucose per 100 g dry weight (dw)), andpeptide content (2.1 and 10.9 g per 100 g dw) were increased inrice beans fermented under the optimized fermentation conditions(Table 1). The physicochemical properties of the rice beans weresignificantly changed after fermentation by B. amyloliquefaciens.To be specific, the dynamic viscosity of the fermented rice beanswas much higher (131.6 times) than that of the unfermented ricebeans. Hu et al. [28] and Shih et al.[18] found that the viscosityof black soybeans increased during the fermentation process, whilethe hardness decreased. Gamma-polyglutamic acid was found tobe one of the major components in the sticky silk of fermentedsoybeans [29]. It has been suggested that the viscosity of beansfermented by Bacillus strains isisan important indicator forestimating fibrinolytic activity. Our results are in agreement withthe findings of Shih et al. [18],which indicate that dynamic viscos-ity is positively correlated with fibrinolytic activity (correlationcoefficient r= 0.986; p<0.05, Fig. 1). Overall, viscous substanceswith fibrinolytic activity were produced during the fermentationof rice beans using B. amyloliquefaciens. The total phenolic (116.7 and 388.5 mg per 100 g), total flavo-noid (235.5 and 354.2 mg per 100 g), and anthocyanin contents(20.1 and 47.1 mg per 100 g) in the fermented rice beans increasedby 1.5-3.3-fold, as compared with the unfermented rice beans(Table 1). Phenolic acids and flavonoids (20-1000 mg per day)are taken by some people depending on their dietary sources andhabits [30]. Phenolic compounds have been recognized as high-profile substances in recent years due to their potential bioactivi-ties. Many reports have shown that fermentation increases thephenolic content of beans due to the release of free phenol agly-cones. TPC has been shown to increase in black soybeans (1.5times), chickpeas (3.2 times), soybeans (1.7 times), and pigeonpeas (1.4 times) during fermentation [6,16,31,32]. In black soy- Table 1Physicochemical properties of unfermented and fermented rice beans. Physicochemical properties Unfermented rice Fermented rice beans beans Moisture content (%) 60.5±0.7 64.6±0.3 Hardness (g) 2194.6±31.3 1575.0±46.1 Dynamic viscosity (mPas) 0.5±0.1 65.8±3.1 Reducing sugar (g glucose per 1.9 13.9±0.1 100 g dw) Peptide content (g per 100 g dw) 2.1±0.2 10.9±0.6 1、 SOD activity (U.g) 55.3±0.8 263.6±1.0 TPC (mg gallic acid per 100 g dw) 116.7b±1.4 388.5°±4.9 TFC (mg rutin per 100 g dw) 235.5±3.5 354.3±4.5 Anthocyanins content (mg per 20.1±1.9 47.1±1.6 100 g dw) ( Different letters (a, b) in the same row are significant different at p<0.05. TFC: total flavonoid c ontent. ) beans fermented by B. subtilis, p-glucosidase was produced andreleased the phenolic hydroxyl group [6]. The TPC of rice beans fer-mented by B. amyloliquefaciens increased by 3.3 times (Table 1)[6,16,31,32]. Phenolic compounds are commonly present in raw-food material in binding forms, and the bioactivities of bound phe-nols are usually lower than those of free phenols [31,33].In thisstudy,B.amyloliquefacienswWascapablee of producingglucosidase (from 0.01 to 2.19 U.g, data not shown) in rice beans.Thus, it is possible that the increase of TPC in fermented rice beansis caused by a release of cell-wall-bound phenolics [23,31]. As shown in Table 1, the SOD activity of the unfermented andfermented rice beans ranged from 55.3 to 263.6 U.gdw. The fer-mented rice beans had significantly higher SOD activity than theunfermented rice beans. As presented in Table 1, the SOD activityof the rice beans increased by 4.8 times after fermentation by B.amyloliquefaciens. SOD has special physiological activity and isthe primary substance for scavenging free radicals in organisms[34]. Improvement in SOD activity has also been detected in nattoand fermented black soybeans [35,36]. The amino acid composition of rice beans fermented by B. amy-loliquefaciens was compared with that of unfermented rice beans(Table 2). Both types of rice beans were rich in glutamic acid(34.8 and 42.4 mg.g-) and aspartic acid (24.8 and 24.2 mg.g--1),as well as the essential amino acids leucine (17.7 and 17.9 mgg)and lysine (16.4 and 16.4 mgg). After fermentation, the contentof glutamic acid, alanine, cysteine, methionine, and phenylalaninein the fermented rice beans was increased by 21.8%,7.8%, 35.0%,38.1%, and 3.2%, respectively. However, B. amyloliquefacien fermen-tation induced the decrease of arginine content in rice beans(7.8%). SEM images of the rice beans before and after fermentation areshown in Fig.2(a). Significant differences were observed in the sur-face microstructures of the unfermented rice beans and fermentedrice beans in the SEM images. The surface structure of the unfer-mented rice beans was rough, but intact and tight (Fig.2(a)).How-ever, the external skeleton structure of the rice beans wasdestroyed and a large area on each bean was exfoliated after fer-mentation, forming a great deal of debris, and a rough and looseshape with inhomogeneous pores (Fig. 2(b)). Table 2Amino acid compositions of unfermented and fermented rice beans. Amino acid Unfermented rice Fermented rice beans beans Aspartic acid 24.8°±0.4 24.2±0.2 ThreonineT 7.3±0.3 7.4±0.2 Serine 8.8±0.7 8.6±0.6 Glutamic acid 34.8±0.5 42.4±0.4 Proline 9.3±0.4 9.6±0.1 Glycine 8.7 8.9±0.2 Alanine 10.3±0.2 11.1 Cysteine 2.0±0.1 2.7±0.4 ValineT 12.1±0.1 12.7°±0.5 MethionineT 2.1 2.9±0.3 Isoleucine 10.3 ±0.1 10.6±0.3 Leucine 17.7±0.3 17.9 Tyrosine 5.3 5.4±0.1 Phenylalanine 12.6±0.1 13.0 Lysine 16.4±0.2 16.4 Histidine 6.4±0.2 6.2 ±0.1 Arginine 12.5±0.1 11.6 Tryptophan 2.1 2.1 Total amino acids 203.2±3.0 213.3±0.5 Sum essential amino acids 67.9±0.6 69.9±0.8 Sum non-essential amino 135.0±2.4 143.5±0.3 acids Different letters (a, b) in the same row are significant different p<0.05. The unit ofthe values is mg per gram dw. (a) (b) Fig. 2. The scanning electron microscopy of (a) unfermented and (b) fermented rice beans freeze-dried powder at a voltage of 5 kV with 1000x magnification. 3.3. Effect offermentation on the biological activities of rice beans 3.3.1. Antioxidant activity Table 3 presents the antioxidant activity of the unfermentedand fermented rice beans. The ethanol extracts of the fermentedrice beans exhibited the highest DPPH (17.1 umol Trolox per gram)and ABTS (185.5 umol Trolox per gram) scavenging activities andFRAP (28.8 umol Trolox per gram), followed by the water extractsof the fermented rice beans (13.3, 138.3, and 21.0 umol Trolox pergram, respectively), the ethanol extracts of the unfermented ricebeans (6.9, 38.6, and 15.2 umol Trolox per gram), and the waterextracts of the unfermented rice beans (3.9, 29.0, and 10.1 umolTrolox per gram). Many studies have focused on radical scavenging effects as anantioxidant model due to the steady repetition and high degreeof accuracy of such effects [37]. The DPPH scavenging activity ofmethanol extracts of chickpeas fermented by Bacillus sp. was foundto be higher than that of unfermented chickpeas [6]. The DPPH andABTS scavenging activities, as well as the FRAP, of aqueous extractsof pigeon peas were improved by fermentation with B. subtilis [16].After the solid-state fermentation of rice beans by B. amyloliquefa-ciens, the DPPH and ABTS scavenging activities, and the FRAP ofboth ethanol and water extracts of rice beans were improved by2.5, 4.8, and 1.9 times and by 3.4, 4.8,2.1 times, respectively. More-over, the ethanol extracts of unfermented or fermented rice beansshowed a higher level of antioxidant activity than the waterextracts in the present study (Table 3). The phenolic compoundsof fermented foods are directly associated with the antioxidantactivity [37]. Rice beans contain various phenolic acids, with 14compounds detected in ethanol extracts and 10 compoundsdetected in aqueous extracts [19]. Owing to the structure varia-tions of phenolic compounds, the phenolic compositions variedwith different extraction solvents. This could be responsible forthe higher antioxidant activity of the ethanol extracts of rice beansafter fermentation by B. amyloliquefaciens. 3.3.2. The a-glucosidase inhibition activity The a-glucosidase inhibition activity of the ethanol extracts offermented rice beans at a concentration of 11.2 mg·mL-(88.9%)was 3.2 times higher than that of unfermented rice beans (27.5%,Fig. 3(a)). The o-glucosidase inhibitors have the potential to manage type-2 diabetes [24]. Polyphenols are known to be inhibitors of o-amylase and a-glucosidase [37]. It has been reported that ricebeans exhibit excellent antidiabetic potential [13]. In this study,B. amyloliquefaciens fermentation improved the o-glucosidase inhi-bition activity of the ethanol extracts of rice beans (from 27.5% to88.9% at 11.2 mg.mL-1;Fig.3(a)). The bioactive compounds such asphenols and flavonoids in the ethanol extracts of rice beansincreased with fermentation (Table 1). Water extracts of fermentedand unfermented rice beans did not show a-glucosidase inhibitionactivity (data not shown). Shukla et al. [24] evaluated the a-glucosidase inhibition activity of the water extracts of several fer-mented soybean sauces and detected low inhibition activity(58.93%-62.25% at 50 mg·mL-1). The a-glucosidase inhibitionactivity of fermented rice beans was lower than that of the positivecontrol (Fig.3(a)), which could be caused by the exceptionally highlevel of dietary a-glucosidase inhibitors in fermented rice beans. 3.3.3. Dipeptidyl peptidase IV inhibition activity The DPP-IV inhibition activity of the water extracts of the ricebeans is shown in Fig. 3(b). The fermented rice beans showed sig-nificantly higher DPP-IV inhibition activities than the unfermentedrice beans at different concentrations (e.g., 92.4%, 65.6%, and 30.2%at 6.4 mg.mL-1,respectively). DPP-IV has been reported to inhibit insulin secretion, thus, isusually used for the prevention and treatment of type-2 diabetes[25]. Several beans, such as cowpeas (Vigna unguiculata), commonbeans (Phaseolus vulgaris L.), and bambara beans (Vigna subter-ranea), have been used for the production of DPP-IV inhibition pep-tides [38-40]. In this study, the DPP-IV inhibition activity of rice Antioxidant activity of unfermented and fermented rice beans. Antioxidant activity Unfermented rice beans Fermented rice beans Water extractsT Ethanol extracts+ Water extracts Ethanol extracts* DPPH assay 3.9±0.3 6.9°±0.3 13.3±0.1 17.1±0.1 ABTS assay 29.0±0.5 38.6±1.2 138.3±1.5 185.5±1.7 FRAP assay 10.1±0.4 15.2±0.3 21.0±0.4 28.8±0.6 7 (b) Fig. 3. (a) The a-glucosidase inhibitive activity and (b) DPP-IV inhibitive activity of unfermented and fermented rice beans with acarbose and diprotin A as the positivecontrol, respectively. The sample concentrations in (a) and (b) were in the range of 0.35-11.2 mg.mL-1and 0.03-6.4 mg.mL-1, respectively. beans increased by 1.6-2.2 times after B. amyloliquefaciens fermen-tation (Fig. 3(b)). Moreover, the fermented rice beans were foundto be rich in peptides and total phenols (10.1 g per 100 g and388.5 mg gallic acid per 100 g; Table 1). Most of the food-source-derived DPP-IV inhibitors reported in the literature are proteinhydrolysates. In addition, flavonoids and phenolic acids have beenreported to inhibit the DPP-IV enzyme activity [41]. Thus, fer-mented rice beans might be a potential source of DPP-IV inhibitorsand functional food for the prevention of type-2 diabetes. 3.3.4. Anticoagulant activity The anticoagulant activity of the aqueous extracts of unfer-mented and fermented rice beans was evaluated (Fig. 4). The aque-ous extracts of the fermented rice beans inhibited the formation offibrin clots in a dose-dependent manner (from 14.6% to 98.2%). Theaqueous extracts of the unfermented beans exhibited lower antico-agulant activity (0%-6.3%) than the rice beans after fermentation.Fermented rice beans could suppress coagulation by inhibiting fib-rinogenesis and thrombin activities. As blood clotting is an impor-tant factor in causing thrombotic diseases, the development of safeand cheap anticoagulant foods has attracted a great deal of atten-tion [42]. Recent data has indicated that the hydrolysis product ofpeanut proteins by the alkaline protease showed a high anticoagu-lant activity of 95% at 40 mg.mL-1[26]. Chickpeas fermented by B.amyloliquefaciens possess an anticoagulant activity of 80% at1 mg·mL-1[6], which is lower than that of heparin sodium. In this 1.2 Fig.4. Anticoagulant activity of unfermented and fermented rice beans. Heparinsodium was used as the positive control. The sample concentrations were in therange of 0.03-1.0 mgmL-. study, the anticoagulant activity of the rice beans fermented by B.amyloliquefaciens (98.2% at 1 mg.mL-; Fig. 4) was significantlyhigher than that of heparin sodium (57.7% at 1 mg.mL-1; Fig. 4).To the best of our knowledge, this is the first report on the antico-agulant activity of rice beans fermented by B. amyloliquefaciens. Inclinical practice, heparin sodium, warfarin, and aspirin are com-monly used as anticoagulant agents. However, these drugs havesafety issues, as they carry the adverse risk of major hemorrhage[43]. The natto kinase isolated from fermented soybeans has beenshown to exhibit antithrombotic properties [44], and was deter-mined to be safe after animal testing and human clinical trials[6,9]. As the water extracts of fermented rice beans haveantithrombotic (Fig. 4) and thrombolytic effects (Fig. 1), they mightbe beneficial for the prevention of thrombotic diseases as dietaryfood supplements. 4. Conclusions B. amyloliquefaciens was utilized as the starter culture for thefermentation of rice beans. Fermentation significantly improvedthe physicochemical properties of rice beans. The antioxidant,DPP-IV inhibitory,and o-glucosidase inhibitory activities of fer-mented rice beans were much higher than those of unfermentedrice beans. Moreover, the anticoagulant activity of the fermentedrice beans was greatly improved. The findings gained in this studyindicate that rice beans fermented by B. amyloliquefaciens showpotential as functional foods. Acknowledgement This work was supported by The National Key Research andDevelopment Program of China (2018YFD0400404). Compliance with ethics guidelines Shan Wu, Shuai Lu, Jun Liu, Shaoqing Yang, Qiaojuan Yan, andZhengqiang Jiang declare that they have no conflict of interest orfinancial conflicts to disclose. References [3] Jhan JK, Chang WF, Wang PM, Chou ST, Chung YC. Production of fermented redbeans with multiple bioactivities using co-cultures of Bacillus subtilis, andLactobacillus delbrueckii. subsp. bulgaricus. LWT-Food. Sci Technol2015;63:1281-7. [4] Sumi H, Hamada H, Tsushima H, Mihara H, Muraki H. A novel fibrinolyticenzyme (nattokinase) in the vegetable cheese natto: a typical and popularsoybean food in the Japanese diet. Experientia 1987;43:1110-1. [5] Peng Y, Huang Q. Zhang RH, Zhang YZ. Purification and characterization of afibrinolytic enzyme produced by Bacillus amyloliquefaciens DC-4 screened fromdouchi, a traditionalChinese soybean food. CompBiochemPhys B2003;134:45-52. [6]| Wei X. Luo M. Xu L. Production of fibrinolytic enzyme from Bacillusamyloliquefaciens by fermentation of chickpeas, with the evaluation of theanticoagulant and antioxidant properties of chickpeas. J Agric Food Chem2011;59:3957-63. [7]Slem B, GezginY, Eltem R. Screening and characterization of thermostablefibrinolytic enzyme from Bacillus amyloliquefaciens EGE-B-2d.1. Turk BiyokimDerg 2016;41:167-76. ( [8] K im JB , Jung W H , R y u J M , Le e YJ, Jung J K , J ang HW, e t a l . Ide n t i fication of a fibr i nolytic enzyme by Bacillus vallismor t is a n d its p o tenti a l a s a bacteriolytic enzyme again s t streptococcus mutans. Biotechn o l Lett 2007;29:605- 1 0. ) ( [9] | K ot b E . Purific a t i on an d p artial c h a racterization o f serine f i br ino lytic enzyme f r om B acillus megateriu m , KSK-0 7 isolated f r om ki s hk, a traditional E g yptian f e r mented f ood. Appl Biochem Microbiol 2015;51:34-43. ) ( [10] Taniguc h i-F u katsu A. Yama n aka-Okumura H , Naniwa-Kuroki Y , N i shida Y , Yamamo t o H, Ta k e tani Y. N a tto a n d viscous vegetables i n a Jap a nese-style b reakfast i mproved insulin s e nsiti v ity, l i p id m e tabolism a n d o x i dative st r ess i n ove r weight s u b ject s with i mp a ired g lu c ose t oleranc e -corrigendum. B r J N ut r 2012:107:1184-91. ) [11]] Dey TB, Chakraborty S, Jain KK, Sharma A, Kuhad RC. Antioxidant phenolicsand their microbial production by submerged and solid state fermentationprocess: a review. Trends Food Sci Technol 2016;53:60-74. [12] |I Bisht IS, Singh M. Asian Vigna. In: Singh M, Upadhyaya H, Bisht IS. Genetic &Genomic Resources of Grain Legume Improvement. 10. Amsterdam: Elsevier;2013;p.237-67. ( [13] Yao Y, Cheng XZ, Wang L X, Wang SH, Ren G. Major p henolic compoun d s, a n t ioxidant c a p a c it y and antid i abet i c po t ential o f ri c e bean (Vigna umbel l ate L.) i n China. I n t J Mol Sci 2012;13:2707- 1 6. ) [14]KatochR. Nutritional potential of rice bean (Vigna umbellata): an underutilizedlegume.J Food Sci 2013;78:8-16. ( [15] M arti n s S , M ussatt o S I , M a rti n ez- A v ila G , M o ntane z - S aenz J, A g u ilar CN , T eixeira J A . Bioactive phenolic compounds: p roduction and e x traction b y solid - state fermentation, a review . Biotechno l A d v 2 0 11;29:365- 7 3. ) ( [16] L ee B H, L ai Y S, W u SC . An t ioxidation, a n giotensin c o nverti ng en zyme i n hibition a ctivi ty , n attokinase, a nd a n tihype rt ension of B ac i llus sub t ilis, ( natt o )-f e rme n t ed pigeon pe a .J Food Drug Anal 2 015;23:750-7 . ) ( [17]| F eng C, Jin S , Luo M, Wang W , Xia XX, Zu YG, et al. Optimization of pr o d u ction p arameters f or p r e paration of natto-pigeon pea w ith im m o bi l ized B a cillus n atto and sensory evaluations of t he p r oduct. I nn o vative F ood S c i E m ergingTec h nol 2015;31 : 160-9 . ) ( [18] S h i h M C, Yang K T , Shu- Y a SU, T s ai ML. Optimization p r ocess o f r o asted brok e n black soybean natto usin g response s u rface m e thodology. J Food Pr o cess Pr eserv 201 3 ;37:474-82. ) ( [19] | Sritongta e B , Sangsuk i am T, Morgan M R, D u ang m al K. Ef f ect of a cid p retreatment t a and the g ermination period on the c omposition andanti o xid a nt t a ctiv i ty of ric e bean ( Vigna u mbellata). Food Chem 2017;227:280-8. ) ( [20] Zhang B , S u n Q. Li u HJ , Li S Z , Ji a n g ZQ . Ch a r a cteri z ation of a ct i nidin fro m Chinese k i w i f ruit c u ltivars a nd i t s a pp l ic a ti o ns i n m e a t te n d e rizat i o n and prod u ct i on o f a ngiotensin I -converting enzyme ( A CE) i n hibitory p eptide s . LWT-Food Sci T echnol 201 7 :78:1 - 7. ) ( [21] Chiou A , Panagop o ul o u E A , Gatzali F , De M S, Kar a thanos VT. Antho c yanins c on t ent a n d antio xi dant cap a city of cor i nthian cu rr a n t s (Viti s vini f era L. , var . Apyrena). Food Chem 2014;146:157-65. ) ( [22] | Wu Z G, J i ang W . Nitin M , B a o X Q . ( Chen S L, T ao ZM. C h aracterizing diversit y b a sed 1 on n ut r itio n a l andd b ioa c tive comp o sitions o f yam ger m plasm ( D ioscorea s pp.) commonly c u ltiv a ted i n C hina. J F ood D rug Anal 201 6 ;24:367- 7 5. ) [23] Dudonne S, Vitrac X, Coutiere P, Woillez M, Merillon JM. Comparative study ofantioxidant properties and total phenolic content of 30 plant extracts ofindustrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays.J Agric FoodChem 2009;57:1768-74. [24]| Shukla S, Park J, Kim DH, Hong SY, Lee js, Kim M. Total phenolic content,antioxidant, tyrosinase and a-glucosidase inhibitory activities of water solubleextracts of noble starter culture doenjang, a Korean fermented soybean saucevariety. Food Control 2016;59:854-61. [25]Wang TY,Hsieh CH, Hung CC, Jao CL, Lin PY, Hsieh YL, et al. A study to evaluate..the potential of an in silico approach for predicting dipeptidyl peptidase-IVinhibitoryaactivity in vitro ofprotein hydrolysates.FoodChem2017;234:431-8. ( [26] ] Zhang S B. I n v itro antithrombotic a c tivities o f p eanut p r otein h y dro l ysates. F ood Chem 2016;202:1 - 8. ) ( [27] W ei X , Z hou Y, Ch e n J, C a i D, Wa n g D, Q i G, e t a l . Eff i c ien t e x p ression of n attokina s e i n B a cillus l i cheniformis: h ost s t ra i n c o n stru c tion an d sig n al p eptide o p timization. J Ind M icrobiol B iotechnol 2 015 ; 42:287-95. ) ( [28] H u Y, G e C . Wei Y, Z h u R , Z hang W, D u L, et a l. Character i z ation of fermented b lack s oybean n a tto i n ocula t ed w i th Bacil l us natto dur in g fermentation. J Sci Food Agr i c 2010;90: 11 94- 2 02. ) ( [29] W ei Q . W ol f -Ha ll C , C h ang KC . N a t to ch a racter i stic s as a f fected b y s t ea m ing t ime, B acillus strain, and fer m entat i on ti m e. J F ood S ci 2001;66:167-73. ) ( [30] Chung IM, Seo SH, A hn J K , Kim S H . E f f ect o f processing, fermentation, and aging t reatment t o c ontent a nd p r ofile o f p henolic c ompou n ds in so y bea n seed, soy curd and soy paste. F oo d Chem 2011;1 2 7:960- 7 . ) ( [31] J u a n M Y, C hou CC. Enh a ncement o f antioxidant activity, to t al phenolic and f lavonoid c ontent of black soybeans by solid state f e r m entatio n wi t h Bacillus subtili s B C RC 1 4 715 . Food Microbiol 2010;27( 5 ) : 586-91 . ) [32]] Ademiluyi AO, Oboh G, Boligon AA, Athayde ML. Effect of fermented soybeancondiment supplemented diet on o-amylase and o-glucosidase activities instreptozotocin-induced diabetic rats. j Funct Foods 2014;9:1-9. ( [33] F R obbins R J . P henolic a cids i n f ood s : an overview of a nalytical m ethodology.J Agr i c Food Chem 20 0 3;51:2866 - 87. ) ( [34] ( I L i mm o ngkon A , S o mb o on T, W o ngshay a P , Pi l ai s angsure e V . L C- M S/M S p rofiles a n d i n terrelationships between t h e a n ti-inf l ammat o ry activity, total p henolic content and antioxidant p o tential o f ka l asin 2 c u l ti v ar p e anut sprout crude ex tr act . Food Chem 20 1 8;239:569- 7 8. ) ( [35] S u mi H, Yatagai C , Sumi A . Superoxide r a dical s cavenging e n zymes de t ected in the fermented soybean n atto. J Brew Soc Jpn 1999;9 4 :10 1 6-8. ) ( [36] Wu C H , C h o u CC. E n ha n cement of ag l ycone, vita m in k 2 a n d su p eroxide d ism u tase act i v i t y o f b l ac k s o ybean th r ough fe r m ent a t io n w i t h B acillus s ubtilis BCRC 14715 at di f ferent temperat u res.J Agric Food Chem 2009;57:10695-70 0 . ) ( [37] I L ee jH , K i m B , Hwan g CE, H a q ue M A , K i m SC , L e e C S , e t a l . C hanges i n conj ug ated l inoleic acid and i soflavo n e contents from f e rment e d s o ymi l ks u si n g L actobacillus plantarum P1201 a nd screening f o r t heir digestive e n zyme i n h ibiti o n and a nt i oxidant p roper t ie s . J Fu n ct F oo ds 2018;43:17 - 2 8 . ) ( [38] R ocha TDS, Hernandez LMR, Chang Y K , M e jia EGD. Imp a ct of ge r minat i on an d enzymati c h yd rolysis o f cowpea be a n (Vigna unguiculata) on t h e generation of p eptid e s cap a ble of inhibiti n g d ipeptidyl p eptid a se I V. Food R es Int 2 014;64 (3):799-809. ) ( [39] Osegueratoledo ME, Gonzalez dME, A mayallano S L. H ard-to-cook bean(Phaseolus vulgari s L. ) protein s hy d rolyze d by a l calas e and bromel ai n p roduce d b i o a ctive p e p ti de fr a ctions t h at i n hi b it t a r gets of t ype-2 d i abet es and oxida t ive str e ss. Food Res In t 2015; 7 6(3):839-5 1 . ) ( [40] M une Mu n e MA, Minka SR , Henl e T. Investigation on a ntioxidant , angiotensin converting e nzyme an d d i pept i dy l pept i da s e IV inhibitory a c tivity o f bambara bean p rotein hydrolysates . Food Chem 2018;250:162-9. ) [41]Lacroix IME, Li-Chan ECY.Food-derived dipeptidyl-peptidase lV inhibitors as apotential approach for glycemic regulation-current knowledge and futureresearch considerations. Trends Food Sci Tech 2016;54:1-16. 42]1R1en Y, Wu H, Lai F, Yang M, Li X, Tang Y. Isolation and identification of a novelanticoagulant peptide from enzymatic hydrolysates of scorpion (Buthusmartensii Karsch) protein. Food Res Int 2014;64:931-8. [43] Alberts MJ, Dawson DV, Massey EW. A follow-up survey of clinical practices forthe use of heparin, warfarin, and aspirin. Neurology 1994;44:618-21. [44]Omura K, Kaketani K, Maeda H, Hitosugi M. Fibrinolytic and antithromboticeffect of the protein from Bacillus subtilis (natto) by the oral administration.BioFactors 2010;22:185-7. https://doi.org/j.eng. 本文研究了解淀粉芽孢杆菌发酵米豆(Vigna umellata)的理化性质和生物活性,根据纤维蛋白溶解活性对发酵条件进行了优化,解淀粉芽孢杆菌发酵的米豆可作为功能性食品对预防血栓性疾病有潜在的好处。 未发酵和发酵米豆的硬度测定:TMS-PRO质构仪(美国FTC)文献来源:Physicochemical Properties and Bioactivities of Rice Beans Fermented by Bacillus amyloliquefaciens
确定




还剩5页未读,是否继续阅读?
北京盈盛恒泰科技有限责任公司为您提供《解淀粉芽孢杆菌发酵黄豆中理化性质及生物活性检测方案(质构分析仪)》,该方案主要用于豆制品中理化分析检测,参考标准--,《解淀粉芽孢杆菌发酵黄豆中理化性质及生物活性检测方案(质构分析仪)》用到的仪器有美国FTC-质构仪、FTC-质构仪TMS-PRO
推荐专场
相关方案
更多
该厂商其他方案
更多

















