设计了一系列(x=0, 0.25, 0.5, 0.75, 1)的固溶体光催化剂,并通过微波溶胶热法和后处理煅烧相结合的方法进行合成,通过各种技术对所制备的固溶体光催化剂的物理化学和光学性能进行了表征。通过模拟阳光照射下双酚A(BPA)的光降解,评估了光催化活性。结果表明,所有的光催化剂都呈花状,Bi2W0.25Mo0.75O6微球显示出最好的光催化活性。在双酚A浓度为15mgL-1时,120分钟和180分钟内双酚A和总有机碳的去除率分别为96.2%和87.1%。在反应过程中,-O2-和h+起着主导作用,而-OH也对双酚A的降解做出了贡献。12种中间产物被鉴定出来,它们逐渐被矿化为CO2和H2O。在此基础上根据对活性物种和中间产物的分析,提出了BPA的主要降解途径。即羟化作用、烷基氧化作用、脱氢作用和芳香族开环作用。在双酚A降解过程中的毒性变化,还通过标准化的生物发光法对发光细菌的抑制率进行了研究。发光细菌Vibrio qinghaiensis sp. Q67,并结合定量结构-活性关系分析。双酚A及其中间体对青海弧菌Q67的抑制作用,随着双酚A及其中间体对青海弧菌Q67的抑制作用逐渐消除。Q67的抑制作用随着BPA的分解而逐渐消除。综上所述,Bi2W0.25Mo0.75O6是一种有效的光催化剂,用于降解和降低BPA的毒性。水溶液中双酚A的降解和毒性降低。
方案详情

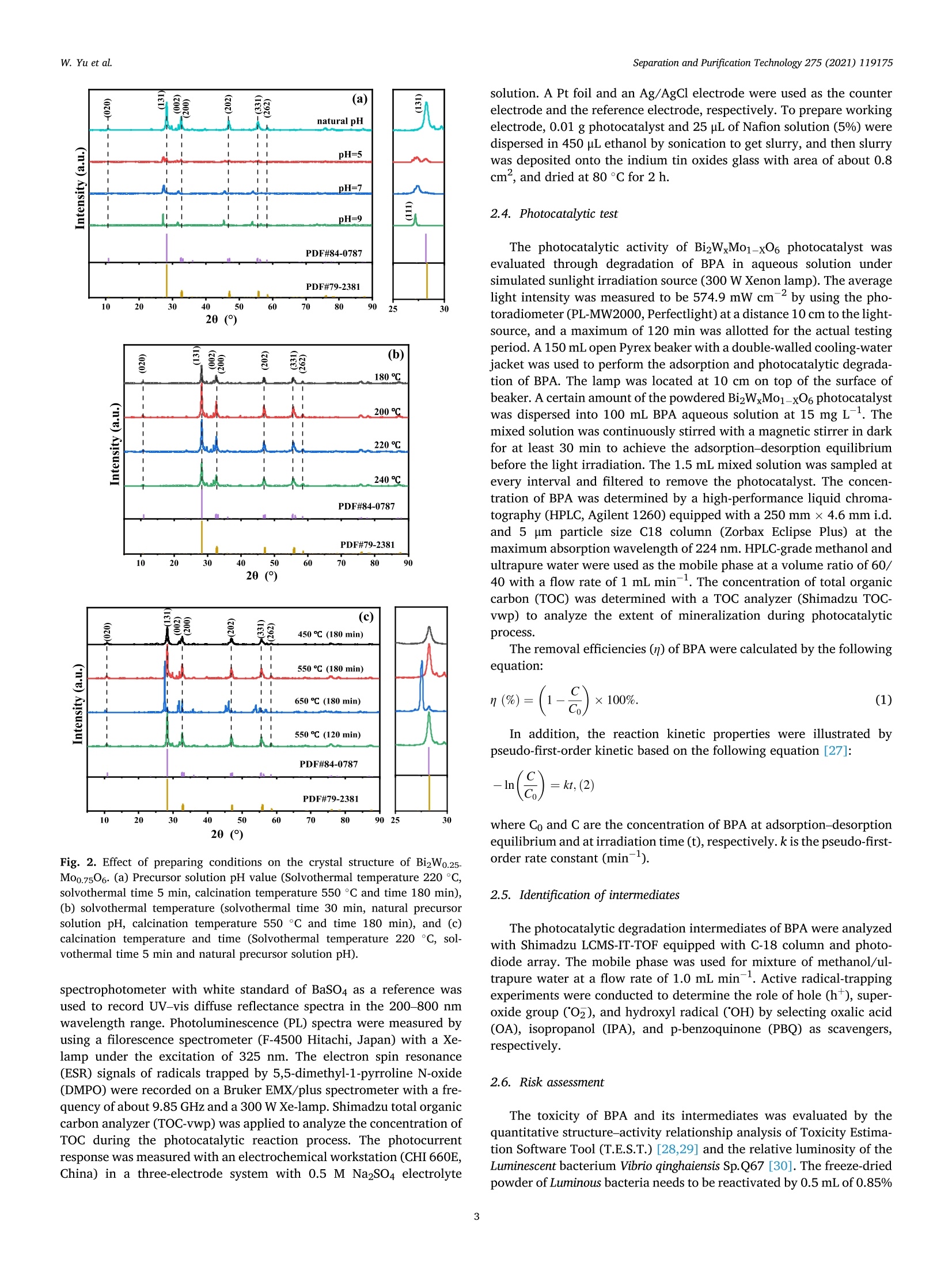
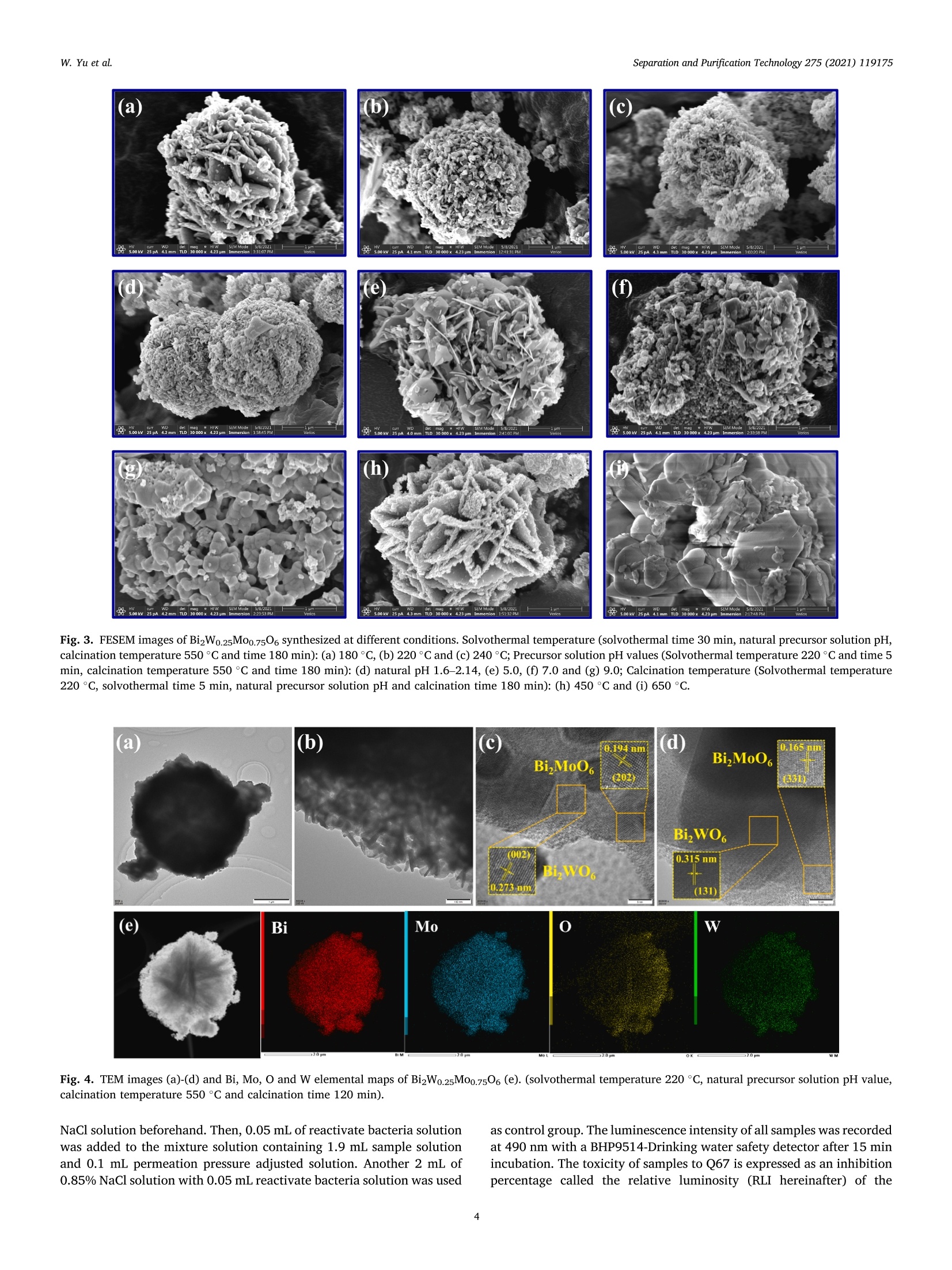
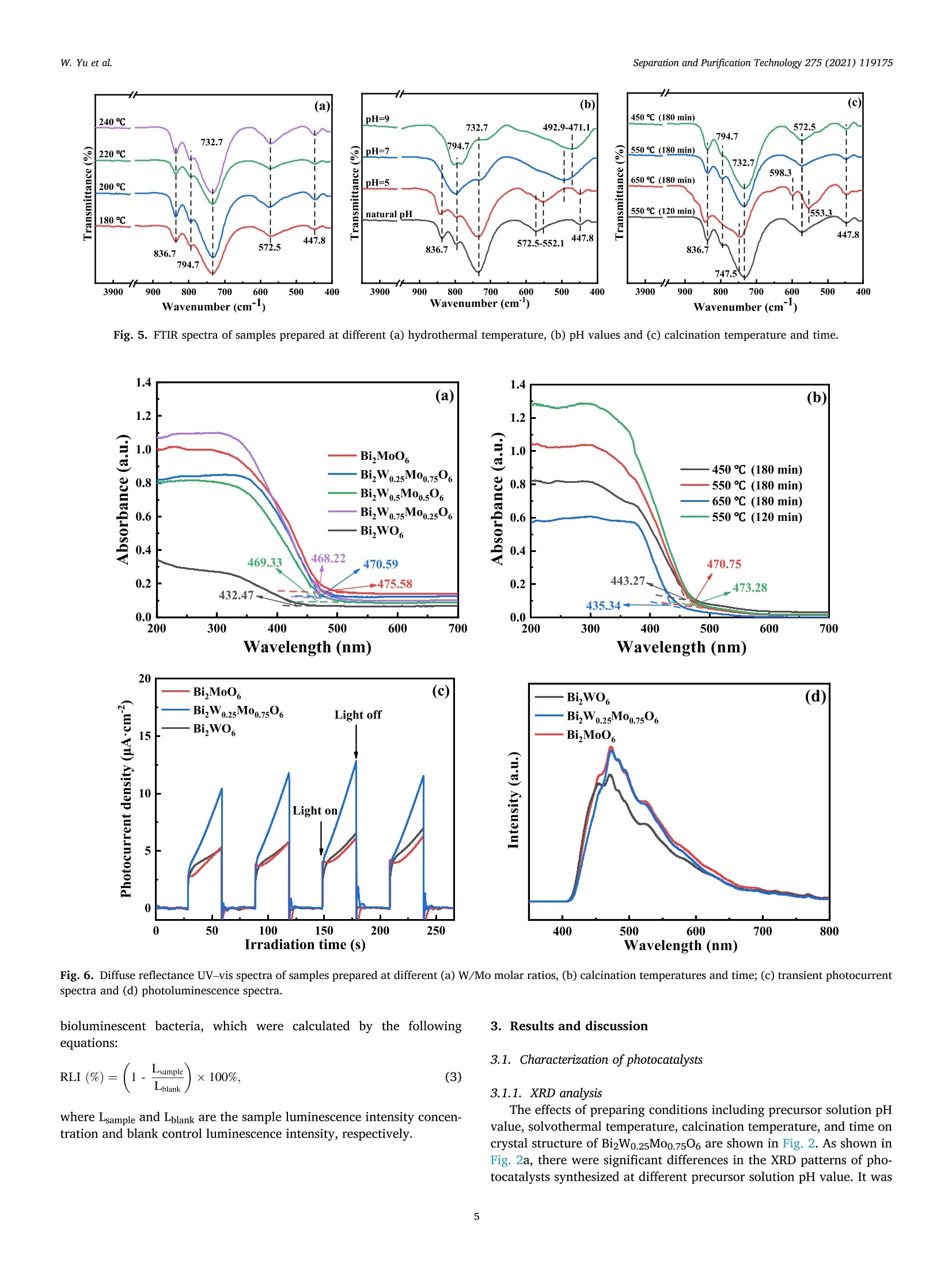
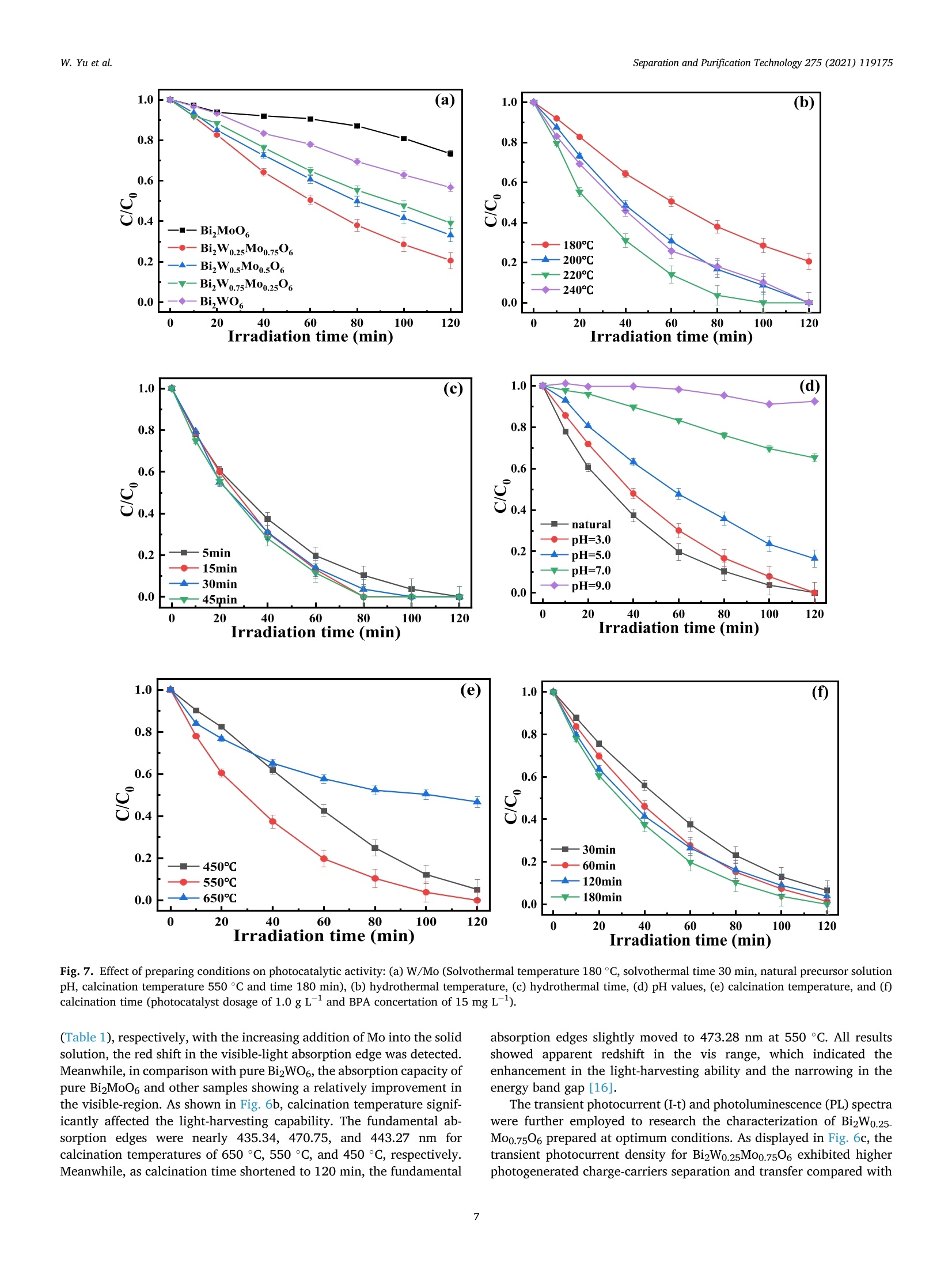
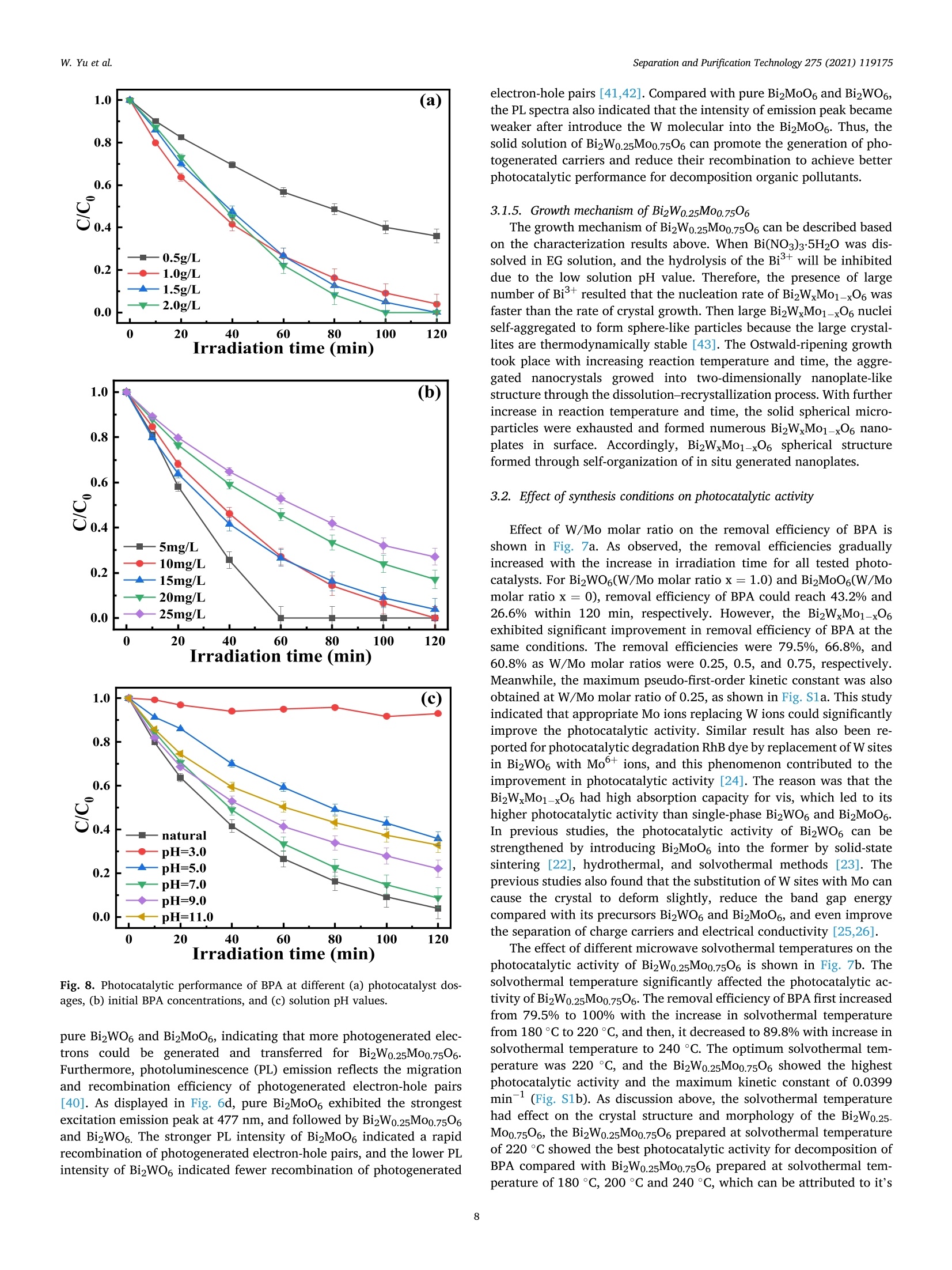
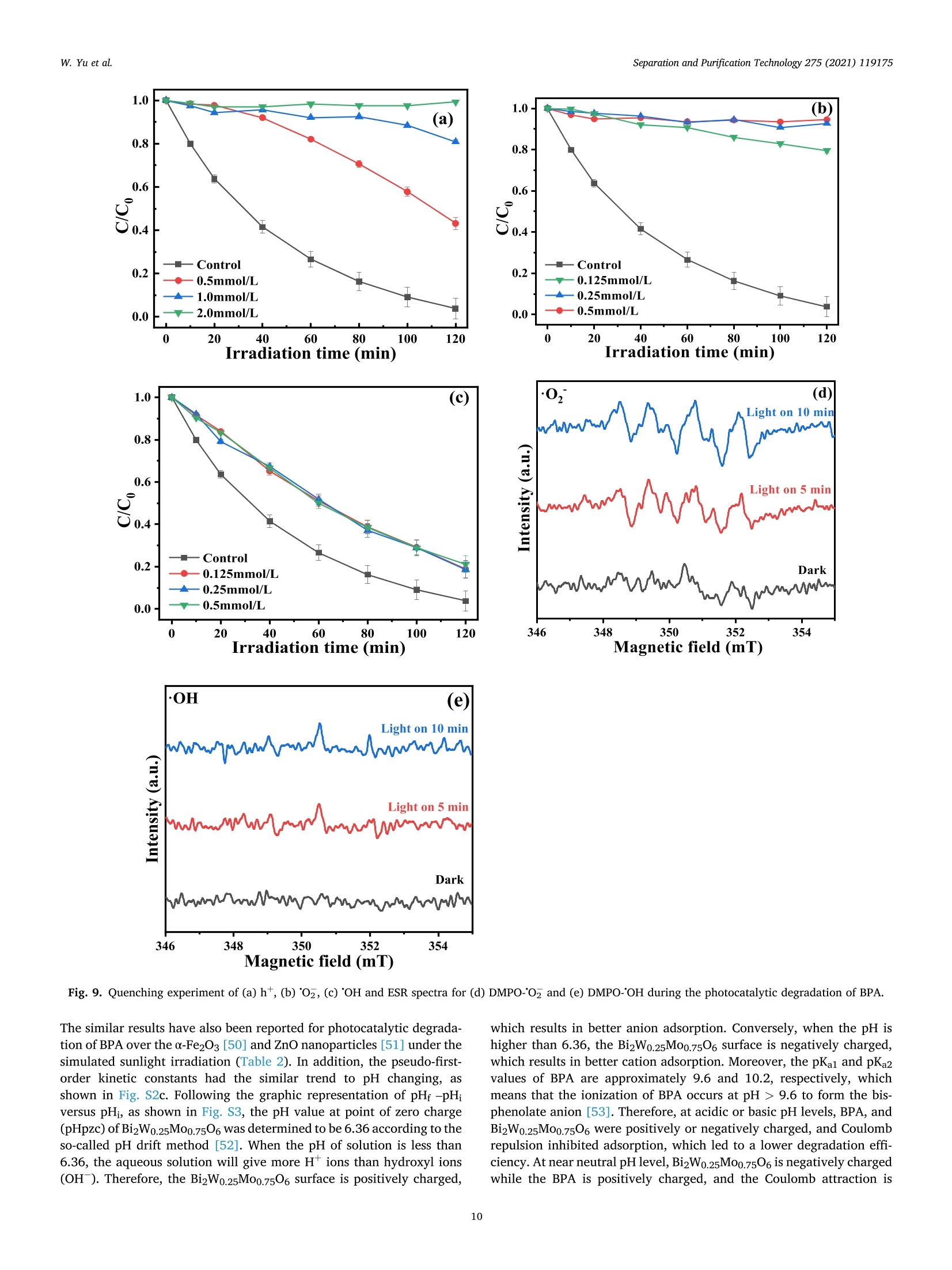
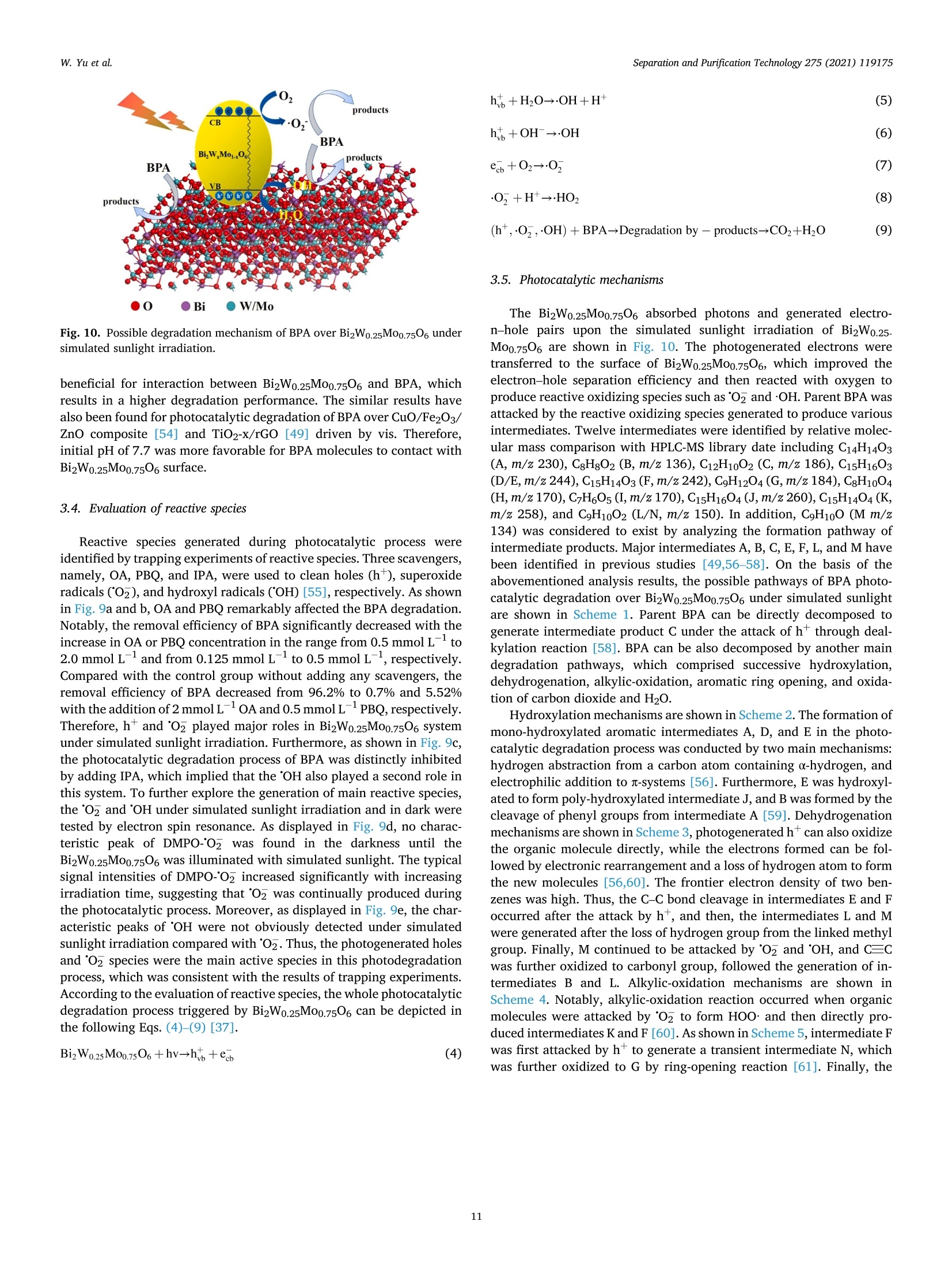
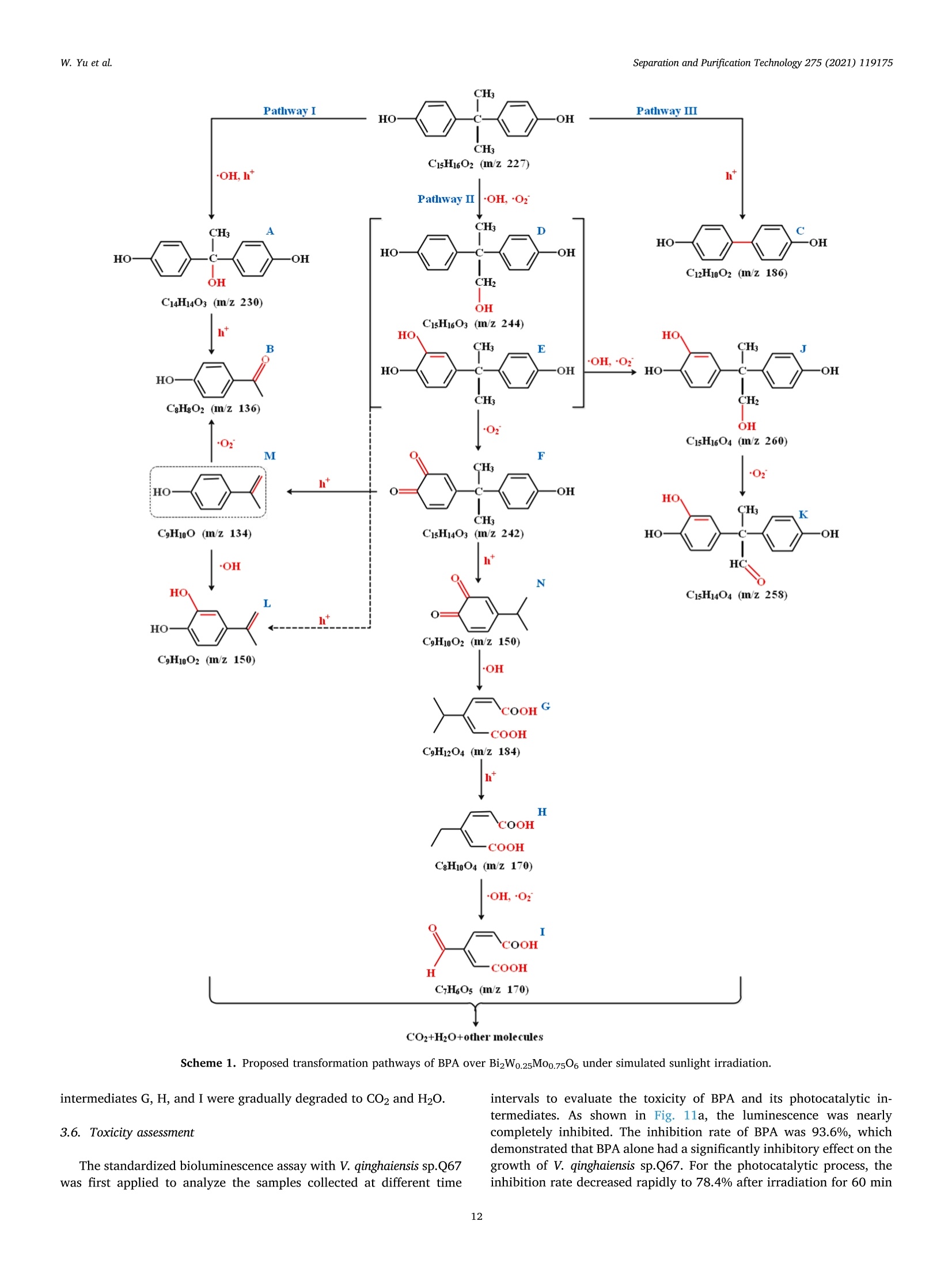
Separation and Purification Technology 275 (2021) 119175 Contents lists available at ScienceDirect Separation and Purification Technology journal homepage: www.elsevier.com/locate/seppur Microwave solvothermal-assisted calcined synthesis of Bi2WxMo1-x06 solidsolution photocatalysts for degradation and detoxification of bisphenol Aunder simulated sunlight irradiation Weili Yual, Shungang Wan b,cd,1, Dan Yuan, Lei Sun b,cd,*, Yan Wang,Mengmeng Wang College of Ecology and Environment, Hainan University, Haikou 570228, China School of Chemical Engineering and Technology, Hainan University, Haikou 570228, China Key Laboratory of Advanced Materials of Tropical Island Resources, Ministry of Education, Haikou 570228, China “Key Laboratory of Solid Waste Resource Utilization and Environmental Protection of Haikou City, Haikou 570228, China ARTICLEINFO ABSTRAC T Keywords: Bismuth-based photocatalyst Endocrine disrupting chemicals Solid solution photocatalyst Degradation mechanism Detoxification A series of (x=0, 0.25, 0.5, 0.75, 1) solid solution photocatalysts was designed and synthesized by the com-bination of microwave solvothermal method and post-treatment calcination. The physicochemical and opticalproperties of the as-prepared solid solution photocatalysts were characterized by various techniques. The pho-tocatalytic activity was also evaluated through the photodegradation of bisphenol A (BPA) under simulatedsunlight irradiation. Results showed that all photocatalysts were flower-like, and the BizWo.25M00.7506 micro-spheres showed the best photocatalytic activity. The removal efficiencies of BPA and total organic carbon were96.2% and 87.1% at BPA concentration of 15 mg Lin 120 and 180 min, respectively. During the reactionprocess, O2 and ht played the dominant role, while OH also contributed to the BPA degradation. Twelve in-termediate products were identified, and they were gradually mineralized to CO2 and H2O. On the basis of theanalysis of active species and intermediates determined, the main degradation pathways for BPA were proposed,namely, hydroxylation, alkylic-oxidation, dehydrogenation, and aromatic ring opening. The toxicity changeduring BPA degradation was also investigated by standardized bioluminescence assay of inhibition rate onluminescent bacterium Vibrio qinghaiensis sp. Q67 in combination with quantitative structure-activity relation-ship analysis. The inhibition effect of BPA and its intermediates to V. qinghaiensis sp. Q67 was gradually elimi-nated with the decomposition of BPA. In conclusion, the BizWo.25Mo0.7506 is an effective photocatalyst fordegradation and toxicity reduction of BPA in aqueous solution. 1. Introduction Endocrine disrupting chemicals (EDCs) including pharmaceuticals,personal care products, plasticizers, and other man-made organic pol-lutants can result in adverse health effect by disrupting hormonal bal-ance in humans and wildlife [1]. Various EDCs have been widelydetected in various surface water [2], effluent of wastewater treatmentplant [3], and even in vegetables and fruits at levels between 5.8 and580 ng kg-[4]. EDC concentrations may increase from a few nano-grams per liter (ng L-) to 15 ug L-, which are sufficiently high togenerate acute and chronic toxicity. The sources of EDCs in environmentcould be due to artificial introduction through human activity includingproduction and use of products containing EDCs. For instance, bisphenol A (BPA) as EDC has been widely used in the plastic industry [5], and BPAhas significant adverse effect on reproductive disorders in humans,poultry, and ruminants [6,7]. Thus, an effective technology to removeEDCs is urgently needed given its toxicity. Several technologies havebeen used to remove EDCs, such as homogeneous and heterogenousphotocatalytic oxidation [8], nonthermal plasma discharge [9,10],fenton oxidation [11], adsorption [12], and biodegradation [13].Among these methods, photocatalysis becomes a promising approachfor degrading EDCs because of its potentials with low energy con-sumption, nonselective mineralization, no secondary pollution, andmild reaction conditions [1]. Bismuth-based photocatalysts have received more and more atten-tions in photocatalysis areas including hydrogen evolution from water ( Co-first a uthors. ) Fig. 1. Schematic for the fabrication process of BizWMo1-x06. splitting, refractory pollutant removal, organic synthesis, and so on[14].Bi2MoO6 and Bi2WO6 are important Aurivillius oxides with specialperovskite-like layer structures of excellent photocatalytic activityunder vis irradiation, and they have been used to remove pollutants[15,16] and for water splitting to produce hydrogen [17]. However,photocatalytic activity of single-phased Bi2WO6 with a relatively wideband gap energy (Eg = 2.75-2.77 eV) needs to be further improvedbecause of the low utilization efficiency of vis [18]. Bi2MoO6 generallyhas a narrow band gap (2.42-2.63eV) with similar Aurivillius-layeredstructure; thus, it can utilize more sunlight than single Bi2WO6, butthe high recombination rate of photogenerated electron-hole pairs isone of limitations in photocatalysis [19]. This method can effectivelyreduce the recombination of electron-hole pairs and further improve thephotocatalysis activity by form solid solution photocatalyst to adjust theband gap structure of semiconductors [20,21]. Solid-state sintering[22], hydrothermal/solvothermal methods[23], and even anion-exchange method [24] have been used to prepare composite photo-catalyst of Bi2MoO6 and Bi2WO6. All the results indicated that the sub-stitution of W sites with Mo can slightly deform the crystal and reducethe band gap energy compared with its precursor Bi2WO6, accelerate theseparation of charge carriers, and improve the photocatalytic activity[25,26]. However, few studies have focused on the synthesis of Bi2WxMo1-x06 solid solution photocatalyst by combination of rapid micro-wave solvothermal method assisted with post-treatment calcination tofurther enhance photocatalytic activity under simulated sunlight irra-diation. Especially, the transformation pathways and toxicity reductionof typical EDCs during photocatalytic degradation with Bi2WxMo1-x06need to be studied. In this study, BPA is selected as a model pollutant because it is apopular EDC that is commonly found in the environment and difficult tobe biodegraded. A series of Bi2WxMo1-xO6 solid solution photocatalystswas synthesized by combination of microwave solvothermal and calci-nation method. The synthetic parameters and degradation conditionswere optimized to enhance photocatalytic activity under simulatedsunlight irradiation. In addition, the transformation pathways andtoxicity evolution of BPA during degradation process were discussed indetail. 2. Materials and methods 2.1. Chemical and materials Nitric acid (HNO3), sodium hydroxide (NaOH), and ethylene glycol(EG) were obtained from Xilong Chemical Co., Ltd. (Shantou, China).BPA was obtained from Maclean Biochemical Technology Co., Ltd. (Shanghai, China). Hexadecyltrimethylammonium bromide(C19H42BrN), bismuth(III) nitrate pentahydrate (Bi(NO3)35H2O), andsodium tungstate dihydrate (Na2WO4·2H2O) were obtained from HushiChemical Co., Ltd. (Shanghai, China). Sodium molybdate dihydrate(Na2MoO4·2H20) was obtained from Tianjin, China. 2.2. Synthesis of photocatalyst Bi2WM01-x06 A series of BizWMo1-x06 was synthesized by a microwave sol-vothermal assisted with calcination, as shown in Fig. 1. In brief, 2 mmolof Bi(NO3)3.5H2O was dissolved in 30 mL EG with sonication for 30 minto form solution A. A total of 1 mmol of Na2WO4·2H2O andNa2MoO4·2H2O with W/Mo molar ratios of 3/1, 1, 1/3 and 0 weredissolved in 20 mL EG with sonication for 30 min to form solution B.Solution B was slowly added to solution A by vigorous stirring. Then,0.82 mmol C19H42BrN solution was added to the above-mentioned ho-mogeneous solution under sonication for another 30 min. Thereafter,0.1 M NaOH or HCl solution was added dropwise into the mixture toadjust pH=3,5, 7,9, and the mixture was then transferred into a 100mL Teflon-lined autoclave. The solvothermal processes were conductedin a microwave oven (XH-800S, Beijing Xianghu Science and Technol-ogy Development Co., Ltd., Beijing, China) at certain temperature fortime and then cooled to room temperature naturally. Next, the obtainedprecursors were alternately washed by deionized water and absoluteethanol, and the precipitates were dried at 80°C. Subsequently, theseprecursors were calcined in a muffle furnace (KSL-1200×) with aheating rate of 5℃ min-1to a final temperature for certain time, and theresulting products were denoted as Bi2Wo.25Mo0.75O6, Bi2Wo.5Moo.5O6,and Bi2W0.75Mo0.2506. The Bi2WO6 and Bi2MoO6 were also prepared atthe same procedure as control. 2.3. Characterization Field emission scanning electron microscope (FESEM, Thermo-scientific Verios G4 UC) and high-resolution transmission electron mi-croscope (HRTEM,JEOL-2100F) were used to observe the morphologyand microstructure of the photocatalyst. Meanwhile, lattice fringe andelemental distribution analyses were also carried out using the high--resolution transmission electron microscope equipped with an energydispersive X-ray spectroscopic analyzer (EDS). X-ray diffractometer(XRD, Bruker D8 Advance, Germany) was utilized to identify the crystalphase by using CuKa radiation at 40 kV and 40 mA in the range of 5-90°(20). Fourier transform infrared spectroscopy (FT-IR, Bruker TENSOR27, Germany) was adopted to analyze the chemical groups of photo-catalysts in the range of 400-4000 cm-. A Shimadzu UV2600 20 () Fig. 2. Effect of preparing conditions on the crystal structure of BizWo.25.Mo0.75O6.(a) Precursor solution pH value (Solvothermal temperature 220°C,solvothermal time 5 min, calcination temperature 550°C and time 180 min),(b) solvothermal temperature (solvothermal time 30 min, natural precursorsolution pH, calcination temperature 550 C and time 180 min), and (c)calcination temperature and time (Solvothermal temperature 220 °C, sol-vothermal time 5 min and natural precursor solution pH). spectrophotometer with white standard of BaSO4 as a reference wasused to record UV-vis diffuse reflectance spectra in the 200-800 nmwavelength range. Photoluminescence (PL) spectra were measured byusing a filorescence spectrometer (F-4500 Hitachi, Japan) with a Xe-lamp under the excitation of 325 nm. The electron spin resonance(ESR) signals of radicals trapped by 5,5-dimethyl-1-pyrroline N-oxide(DMPO) were recorded on a Bruker EMX/plus spectrometer with a fre-quency of about 9.85 GHz and a 300 W Xe-lamp. Shimadzu total organiccarbon analyzer (TOC-vwp) was applied to analyze the concentration ofTOC during the photocatalytic reaction process. The photocurrentresponse was measured with an electrochemical workstation (CHI 660E,China) in a three-electrode system with 0.5 M Na2SO4 electrolyte solution. A Pt foil and an Ag/AgCl electrode were used as the counterelectrode and the reference electrode, respectively. To prepare workingelectrode, 0.01 g photocatalyst and 25 uL of Nafion solution (5%) weredispersed in 450 uL ethanol by sonication to get slurry, and then slurrywas deposited onto the indium tin oxides glass with area of about 0.8cm, and dried at 80°C for 2 h. 2.4. Photocatalytic test The photocatalytic activity of Bi2WM01-x06 photocatalyst wasevaluated through degradation of BPA in aqueous solution undersimulated sunlight irradiation source (300 W Xenon lamp). The averagelight intensity was measured to be 574.9 mW cm-2 by using the pho-toradiometer (PL-MW2000, Perfectlight) at a distance 10 cm to the light-source, and a maximum of 120 min was allotted for the actual testingperiod. A 150 mL open Pyrex beaker with a double-walled cooling-waterjacket was used to perform the adsorption and photocatalytic degrada-tion of BPA. The lamp was located at 10 cm on top of the surface ofbeaker. A certain amount of the powdered BizWMo1-x06 photocatalystwas dispersed into 100 mL BPA aqueous solution at 15 mg L-1. Themixed solution was continuously stirred with a magnetic stirrer in darkfor at least 30 min to achieve the adsorption-desorption equilibriumbefore the light irradiation. The 1.5 mL mixed solution was sampled atevery interval and filtered to remove the photocatalyst. The concen-tration of BPA was determined by a high-performance liquid chroma-tography (HPLC, Agilent 1260) equipped with a 250 mm x4.6 mm i.d.and 5 um particle size C18 column (Zorbax Eclipse Plus) at themaximum absorption wavelength of 224 nm. HPLC-grade methanol andultrapure water were used as the mobile phase at a volume ratio of 60/40 with a flow rate of 1 mL min. The concentration of total organiccarbon (TOC) was determined with a TOC analyzer (Shimadzu TOC-vwp) to analyze the extent of mineralization during photocatalyticprocess. The removal efficiencies (y) of BPA were calculated by the followingequation: In addition, the reaction kinetic properties were illustrated bypseudo-first-order kinetic based on the following equation [27]: where Co and C are the concentration of BPA at adsorption-desorptionequilibrium and at irradiation time (t), respectively. k is the pseudo-first-order rate constant (min). 2.5. Identification of intermediates The photocatalytic degradation intermediates of BPA were analyzedwith Shimadzu LCMS-IT-TOF equipped with C-18 column and photo-diode array. The mobile phase was used for mixture of methanol/ul-trapure water at a flow rate of 1.0 mL min-. Active radical-trappingexperiments were conducted to determine the role of hole (h), super-oxide group (02), and hydroxyl radical (OH) by selecting oxalic acid(OA), isopropanol (IPA), and p-benzoquinone (PBQ) as scavengers,respectively. 2.6. Risk assessment The toxicity of BPA and its intermediates was evaluated by thequantitative structure-activity relationship analysis of Toxicity Estima-tion Software Tool (T.E.S.T.) [28,29] and the relative luminosity of theLuminescent bacterium Vibrio qinghaiensis Sp.Q67 [30]. The freeze-driedpowder of Luminous bacteria needs to be reactivated by 0.5 mL of 0.85% (a) (b) ioocox 4.23pmi 5.00kv 25 pA 4.1mm TLD 30000x 4.23pm Fig. 3. FESEM images of Bi2Wo.25M00.7506 synthesized at different conditions. Solvothermal temperature (solvothermal time 30 min, natural precursor solution pH,calcination temperature 550°C and time 180 min):(a) 180°C, (b) 220°C and (c) 240°C; Precursor solution pH values (Solvothermal temperature 220 °C and time 5min, calcination temperature 550°C and time 180 min): (d) natural pH 1.6-2.14, (e) 5.0, (f) 7.0 and (g) 9.0; Calcination temperature (Solvothermal temperature220°C, solvothermal time 5 min, natural precursor solution pH and calcination time 180 min): (h) 450°C and (i) 650°C. Fig. 4. TEM images (a)-(d) and Bi, Mo, O and W elemental maps of BizWo.25M00.7506(e). (solvothermal temperature 220°C, natural precursor solution pH value,calcination temperature 550°C and calcination time 120 min). NaCl solution beforehand. Then, 0.05 mL of reactivate bacteria solutionwas added to the mixture solution containing 1.9 mL sample solutionand 0.1mL permeation pressure adjusted solution. Another 2 mL of0.85%NaCl solution with 0.05 mL reactivate bacteria solution was used as control group. The luminescence intensity of all samples was recordedat 490 nm with a BHP9514-Drinking water safety detector after 15 minincubation. The toxicity of samples to Q67 is expressed as an inhibitionpercentage called the relative luminosity (RLI hereinafter) of the Fig. 5. FTIR spectra of samples prepared at different (a) hydrothermal temperature, (b) pH values and (c) calcination temperature and time. Fig. 6. Diffuse reflectance UV-vis spectra of samples prepared at different (a) W/Mo molar ratios, (b) calcination temperatures and time; (c) transient photocurrentspectra and (d) photoluminescence spectra. bioluminescent bacteria, which were calculated by the followingequations: 3. Results and discussion 3.1. Characterization of photocatalysts where Lsample and Lblank are the sample luminescence intensity concen-tration and blank control luminescence intensity, respectively. 3.1.1. XRD analysis The effects of preparing conditions including precursor solution pHvalue, solvothermal temperature, calcination temperature, and time oncrystal structure of BizWo.25Mo0.7506 are shown in Fig.2. As shown inFig. 2a, there were significant differences in the XRD patterns of pho-tocatalysts synthesized at different precursor solution pH value. It was Table 1Band gap energy of photocatalysts. Sample Synthesis conditions Eg (eV) Bi2MoO6 180°C 30 min 550°C° 180 min 2.61 Bi2Wo.25Moo.7506 180C 30 min 550°C°180 min" 2.63 Bi2Wo.sMoo.5O6 180 °C 30 min550°C 180 min 2.64 BizWo.75Moo.25O6 180°C 30 min550°C° 180 min 2.65 Bi2WO6 180°C 30 min550°C180 min 2.87 Bi2Wo.25Mo0.7506 220°C 5 min 450°C° 180 min 2.80 Bi2Wo.25Mo0.7506 220 °C 5 min 550°C180 min 2.63 Bi2Wo.25M00.7506 220°C 5 min 650°C° 180 min 2.85 BizWo.25M00.7506 220°C 5 min 550°C° 120 min 2.62 Solvothermal temperature. Solvothermal time. Calcination temperature. dCalcination time. found that the characteristic diffraction peaks of photocatalysts syn-thesized at natural pH (1.6-2.14) and pH5 matched well with Bi2WO6and Bi2MoO6. The distinct characteristic diffraction peaks at 20 around10.73°, 28.23°,32.73°,46.82°, 55.56°, and 58.35° were indexed to(020), (131),(002)/(200),(202), (331), and (262) crystallographicplanes of the orthorhombic Bi2MoO6 (PDF#84-0787) and Bi2WO6(PDF#79-2381)[31,32]. As pH values of precursor solution increasedfrom 5 to 7 and 9, the intensity of characteristic diffraction peak forBi2O3 (PDF#77-0374) gradually increased, and the (131) crystal sur-face was gradually replaced by Bi203 (111) crystal surface. This isbecause high concentration of OHmay cause production of Bi(OH)3,which was beneficial to producing Bi2O3 rather than Bi2WO6 andBi2MoO6 under neutral or alkaline conditions [33,34]. Fig. 2b displayedstrong and sharp diffraction peaks for Bi2Wo.25Mo0.7506 prepared atdifferent solvothermal temperatures and times, and it indicated thatthey had high level of crystallinity. As shown in Fig. 2c, the intensity of(131) peak gradually increased with increasing calcination temperaturefrom 450 °C to 550°C, indicating that the increase of calcination tem-perature was beneficial to the formation of crystallization. However, thediffraction peaks of Bi2Wo.25Mo0.7506 prepared at calcination temper-ature of 650°C and calcination time of 180 min were obviously differentfrom those of other samples, and it might be due to the phase transitionof the sample caused by high temperature. Meanwhile, no new peak wasfound in the XRD pattern of the other samples, which indicated that thecrystal structure of the composite photocatalyst did not change signifi-cantly. Thus, BizWxMo1-x06 might be a solid solution instead of a simplemixture of Bi2WO6 and Bi2MoO6 as reported in a previous study [31]. 3.1.2. Morphology and microstructures As shown in Fig. 3a-c, the morphology of samples prepared at fixednatural pH by varying the solvothermal temperature were flower-likemicrospheres. The size of superficial nanosheets on flower-like micro-spheres became much smaller with increasing solvothermal temperaturefrom 180 °C to 240 °C. Meanwhile, a significant growth of superficialnanosheets was found, and the superficial gaps were filled fully withsmall nanoplates. In addition, the solvothermal time had no significanteffect on the morphological characteristic of Bi2W0.25Mo0.7506(Fig. 3band 3d). As shown in Fig. 3e-g, the flower-like microspheres was formedwith numerous irregular thin nanosheets at pH 5, meanwhile, a newcrystal phase of cubic Bi2O3 generated based on the XRD analysis, andmore Bi203 was produced as the pH value further increased to 9 [33]. Itis clear that nanosheets had obvious aggregation and stacking, whichwas accompanied by crystallization changes as solution pH valuesincreased to 9. Furthermore,it can be clearly seen from Fig. 3, the calcination temperature had significant effect on the morphologicalcharacteristics of BizWo.25Mo0.7506. As shown in Fig. 3d and h, thecrystallization was incomplete for Bi2Wo.25Mo0.75O6 prepared at 450°C,however, the increasing of calcination temperature from 450 °C to550°C was beneficial for the formation of crystallization, and the in-tensity of (131) peak gradually increased based on the XRD spectra. Asshown in Fig. 3i, the smaller size of nanosheets growed to irregular thicknanoblocks when the calcination temperature further increased to 650C, the similar result has been found in previous study [35]. Thus, goodmorphology and crystal structure resulted in the lowest band gap energyof Bi2Wo.25Mo0.75O6 prepared at 550 °C, which contributed to theenhanced photocatalytic activity. The detail morphology and microstructural features of Bi2Wo.25.Moo.7506 were further characterized by TEM (Fig. 4a-d). As shown inFig. 4a-b, the sample has round-shaped morphology with a diameter ofabout 3 um, and it is composed of stacked nanosheets with dimensionranging from 40 to 60 nm. The above results are consistent with theresults of SEM. The lattice fringes of Bi2Wo.25Mo0.7506 are clearly seen inFig. 4c-d. The lattice spacings of 0.315 and 0.273 nm are consistent withthe 131 and 002 crystal planes of Bi2WO6. In addition, the latticespacings of 0.194 and 0.165 nm are in good agreement with the 202 and331 crystal planes of Bi2MoO6 [31]. This result is also in conformity withXRD analysis. In addition, as displayed in Fig. 4e, It can be seen from theelemental mapping images that the Bi, Mo, O and W elements wereuniformly distributed over the microsphere, providing powerful evi-dence for the preparation of BizWo.25Mo0.7506 solid solutions [36]. 3.1.3. FT-IR analysis The FT-IR spectra of samples prepared at different conditions arepresented in Fig. 5a-c. The band around at 552.1 cm-,447.8 cm-and598.3 cm-were ascribed to the stretching and deformation vibration ofthe Bi-O bond of Bi2Wo.25Mo0.7506 [37,38]. The absorption band at572.5 cm- was assigned to the bending vibration of MoO [23]. Themain absorption bands at 747.5-732.7 cm- corresponded to theasymmetric stretching vibrations of equatorial oxygen atoms in MoO6and W-O stretching modes [25,38], the blueshift of the metal-o band,and increased interface and space, which resulted in the movement ofelectric dipoles [39]. In addition, the weak absorption peak at around794.7 cm- was probably related to the asymmetric stretching mode ofthe apical oxygen atoms in MoO6 structure [23,25]. The characteristicadsorption peak at 836.7 cm-was assigned to the bending vibrationmode of the W-o-W [23]. Fig. 5a showed that there is no obviousdifference among Bi2Wo.25M00.7506 photocatalysts prepared at differentsolvothermal temperature. However, the changes of precursor solutionpH value had significant effect on synthesis of BizWo.25Moo.7506 asshown in Fig. 5b. Increasing pH values from 5.0 to 9.0 decreased themain absorption peak at 747.5-732.7 cm- and 572.5 cm-, respec-tively. Combined with the XRD analysis, the transition from BizWo.5.Mo0.7506 to Bi20s happened. In addition, as shown in Fig. 5c, thecalcination temperatures had similar effect on the FTIR spectra of thesamples, and the calcination time had no effect on characteristics ofinfrared spectra. 3.1.4. Light absorption and PL spectra analysis The effect of W/Mo molar ratios on light-harvesting capability of theBi2WMo1-x06 was determined by UV-vis diffuse reflectance spectra. Itcan be distinctly seen from Fig. 6a, the Bi2WxMo1-x06(x=1, 0.75,0.5,0.25 and 0) composites displayed the absorption edge at about 432.47,468.22, 469.33, 470.59 and 475.58 nm, the corresponding band gapenergies were calculated to be 2.87, 2.65,2.64, 2.63 and 2.61 eV 1.0 (a) 1.0 (b) 120 Irradiation time (min) Irradiation time (min) Fig. 7. Effect of preparing conditions on photocatalytic activity: (a) W/Mo (Solvothermal temperature 180°C, solvothermal time 30 min, natural precursor solutionpH, calcination temperature 550 °C and time 180 min), (b) hydrothermal temperature, (c) hydrothermal time, (d) pH values, (e) calcination temperature, and (f)calcination time (photocatalyst dosage of 1.0 g L- and BPA concertation of 15 mgL-1). (Table 1), respectively, with the increasing addition of Mo into the solidsolution, the red shift in the visible-light absorption edge was detected.Meanwhile, in comparison with pure Bi2WO6, the absorption capacity ofpure Bi2MoO6 and other samples showing a relatively improvement inthe visible-region. As shown in Fig. 6b, calcination temperature signif-icantly affected the light-harvesting capability. The fundamental ab-sorption edges were nearly435.34,470.75, and 443.27 nm forcalcination temperatures of 650 °C, 550°C, and 450°C, respectively.Meanwhile, as calcination time shortened to 120 min, the fundamental absorption edges slightly moved to 473.28 nm at 550 °C. All resultsshowed apparent redshift in the vis range, which indicated theenhancement in the light-harvesting ability and the narrowing in theenergy band gap [16]. The transient photocurrent (I-t) and photoluminescence (PL) spectrawere further employed to research the characterization of BizWo.25-Mo0.7506 prepared at optimum conditions. As displayed in Fig. 6c, thetransient photocurrent density for Bi2Wo.25Mo0.7506 exhibited higherphotogenerated charge-carriers separation and transfer compared with Fig. 8. Photocatalytic performance of BPA at different (a) photocatalyst dos-ages, (b) initial BPA concentrations, and (c) solution pH values. pure Bi2WO6 and BiMoO6, indicating that more photogenerated elec-trons could be generated and transferred for BizWo.25Mo0.7506.Furthermore, photoluminescence (PL) emission reflects the migrationand recombination efficiency of photogenerated electron-hole pairs[40]. As displayed in Fig. 6d, pure BizMoO6 exhibited the strongestexcitation emission peak at 477 nm, and followed by BizWo.25Mo0.7506and Bi2WO6. The stronger PL intensity of Bi2MoO6 indicated a rapidrecombination of photogenerated electron-hole pairs, and the lower PLintensity of Bi2WO6 indicated fewer recombination of photogenerated electron-hole pairs [41,42]. Compared with pure BizMoO6 and Bi2WO6,the PL spectra also indicated that the intensity of emission peak becameweaker after introduce the W molecular into the Bi2MoO6. Thus, thesolid solution of Bi2Wo.25Mo0.7506 can promote the generation of pho-togenerated carriers and reduce their recombination to achieve betterphotocatalytic performance for decomposition organic pollutants. 3.1.5. Growth mechanism of BizW0.25M00.7506 The growth mechanism of BizWo.25Mo0.7506 can be described basedon the characterization results above. When Bi(NO3)35H2O was dis-solved in EG solution, and the hydrolysis of the Bi’will be inhibiteddue to the low solution pH value. Therefore, the presence of largenumber of Biresulted that the nucleation rate of Bi2W.M01-x06 wasfaster than the rate of crystal growth. Then large BizWxM01-x06 nucleiself-aggregated to form sphere-like particles because the large crystal-lites are thermodynamically stable [43]. The Ostwald-ripening growthtook place with increasing reaction temperature and time, the aggre-gated nanocrystals growed into two-dimensionally nanoplate-likestructure through the dissolution-recrystallization process. With furtherincrease in reaction temperature and time, the solid spherical micro-particles were exhausted and formed numerous BizWMo1-x06 nano-plates in surface. Accordingly, BizWxMo1-x06 spherical structureformed through self-organization of in situ generated nanoplates. 3.2. Effect of synthesis conditions on photocatalytic activity Effect of W/Mo molar ratio on the removal efficiency of BPA isshown in Fig. 7a. As observed, the removal efficiencies graduallyincreased with the increase in irradiation time for all tested photo-catalysts. For Bi2WO6(W/Mo molar ratiox=1.0) and Bi2MoO6(W/Momolar ratio x=0), removal efficiency of BPA could reach 43.2% and26.6% within 120 min, respectively. However, the Bi2WxMo1-x06exhibited significant improvement in removal efficiency of BPA at thesame conditions. The removal efficiencies were 79.5%, 66.8%, and60.8% as W/Mo molar ratios were 0.25, 0.5, and 0.75, respectively.Meanwhile, the maximum pseudo-first-order kinetic constant was alsoobtained at W/Mo molar ratio of 0.25, as shown in Fig. Sla. This studyindicated that appropriate Mo ions replacing W ions could significantlyimprove the photocatalytic activity. Similar result has also been re-ported for photocatalytic degradation RhB dye by replacement of W sitesin Bi2WO6 with Mo+ions, and this phenomenon contributed to theimprovement in photocatalytic activity [24]. The reason was that theBi2WxMo1-x06 had high absorption capacity for vis, which led to itshigher photocatalytic activity than single-phase BizWO6 and Bi2MoO6.In previous studies, the photocatalytic activity of Bi2WO6 can bestrengthened by introducing Bi2MoO6 into the former by solid-statesintering [22], hydrothermal, and solvothermal methods [23]. Theprevious studies also found that the substitution of W sites with Mo cancause the crystal to deform slightly, reduce the band gap energycompared with its precursors Bi2WO6 and Bi2MoO6, and even improvethe separation of charge carriers and electrical conductivity [25,26]. The effect of different microwave solvothermal temperatures on thephotocatalytic activity of BizWo.25Mo0.75O6 is shown in Fig. 7b. Thesolvothermal temperature significantly affected the photocatalytic ac-tivity of Bi2W0.25Mo0.7506. The removal efficiency of BPA first increasedfrom 79.5% to 100% with the increase in solvothermal temperaturefrom 180°C to 220°C, and then, it decreased to 89.8% with increase insolvothermal temperature to 240 °C. The optimum solvothermal tem-perature was 220°C, and the BizWo.25Mo0.7506 showed the highestphotocatalytic activity and the maximum kinetic constant of 0.0399min(Fig. S1b). As discussion above, the solvothermal temperaturehad effect on the crystal structure and morphology of the Bi2Wo.25-Moo.7506, the Bi2Wo.25Mo0.7506 prepared at solvothermal temperatureof 220 °C showed the best photocatalytic activity for decomposition ofBPA compared with Bi2Wo.25Mo0.7506 prepared at solvothermal tem-perature of 180°C, 200°C and 240°C, which can be attributed to it’s Table 2The comparison of BPA degradation efficiencies for prepared photocatalysts in this study with partial reported literature. Photocatalyst Light source Catalyst dosage (g/ BPA concentration (mg/ Degradation efficiency Mineralization rate References L L) (%) (%) So.2-BiOBr 入>420 nm (300 W Xenon lamp) 0.25 10 92 (180 min) 21.5(180 min) [8] TiO2-x/rGO > 400 nm (300 W Xenon lamp) 1.0 2.5 91.0 (60 min) 18.9 (60 min) [49] CuO/Fe203/ZnO 入>400 nm (35 W Xenon lamp) 0.04 20 97.5(120 min) 83.75 (120 min) [54] D35-TiO2 入>420 nm (500 W Xenon lamp) 一 5 94 (300 min) 30 (30 min) [57] ZnO Simulated Sunlight (300 W Xenon 1.0 30 99 (60 min) 54.7 (60 min) [51] lamp) 0-Fe2O3 Simulated Sunlight (300 W Xenon 1.0 30 91 (360 min) 81 (360 min) [50] Bi2Wo.25Mo0.7506 lamp) Simulated Sunlight (300 W Xenon lamp) 1.0 15 96.2 (120 min) 87.1 (180 min) This study good crystal structure and morphology. In addition, Fig. 7c and S1cshow that the solvothermal time slightly affected the photocatalyticactivity of Bi2Wo.25Mo0.7506. Therefore, the solvothermal time of 5 minwas selected in the following experiments considering the experimentaleconomy. In conclusion, the microwave solvothermal method is aneffective assisting method for preparing photocatalyst compared withtraditional hydrothermal method. 3.3. Effect of photocatalytic conditions on decomposition of BPA The influence of photocatalyst dosage was first investigated byvarying Bi2Wo.25Mo0.7506 dosage from 0.5 g L- to 2.0 g L- at BPAconcentration of 15 mg L, solution initial pH of approximately 2.0,and solution volume of 100 mL under simulated sunlight irradiation.The results showed that removal efficiencies of BPA (Fig. 8a) andpseudo-first-order kinetic constants (Fig. S2a) increased with the in-crease in Bi2Wo.25M00.7506 dosage. The reason could be attributed to themore reactive sites and active radicals provided by the increased pho-tocatalyst dosage [46]. When Bi2Wo.25Moo.7506 dosage furtherincreased to more than 1.0 g L, the increase in removal efficiency wasnot obvious. The reason was that the light transmission may be sup-pressed in the suspension system, which led to the lowered photo-irradiation on the photocatalyst surface and thus to the reduction inutilization rate of sunlight as reported in previous studies [47,48].Therefore, 1.0 g L-1 BizWo.25M00.7506 was optimized as the efficientphotocatalyst dosage for photocatalytic reaction in the present experi-ment conditions. The effect of BPA concentration was assessed by varying BPA con-centration from 5 mg L- to 25 mg L- at 1.0gL-BizWo.25Mo0.7506,and the results are presented in Fig. 8b. The removal efficiencies of BPAwere 100%, 100%, and 96.2% at BPA concentration 5, 10, and 15 mgL- within 120 min, respectively. However, the removal efficienciesdecreased from 96.2% to 82.9% and 72.9% as the initial BPA concen-tration further increased from 15 mg L- to 20 and 25 mg L-1. Mean-while, the pseudo-first-order kinetic constants gradually decreased from0.0348 min- to 0.0267, 0.0258, 0.0137, and 0.011 min-1 with theincrease in BPA concentration from 5 mg L-to 25 mg L-(Fig. S2b).The reason was that the active sites on Bi2Wo.25Mo0.75O6 provided werelimited for fixed photocatalyst dosage, and it no longer had sufficientactive sites to capture BPA molecule when the BPA concentration washigher than 15 mg L-1. Thus, the removal efficiencies and kinetic con-stants gradually decreased with the increase in BPA concentration, andthe similar result has been reported in previous studies [49,50]. The effect of solution pH on BPA removal was investigated in the pHrange of 3-11 at catalyst dosage of 1.0 g L-l and BPA concentration of15 mg L-. As shown in Fig. 8c, the solution pH significantly affected thephotocatalytic performance. The removal efficiencies of BPA firstincreased when the pH values ranged from 3 to natural pH of7.7, andthe maximum removal efficiency of 96.2% was obtained at natural pH of7.7 within 120 min. However, the removal efficiencies decreased from96.2% to 67.2% with the further increase in pH from natural 7.7 to 11. 1.0 1.0· (b) (a) 346 348 ·350 352 354 Magnetic field (mT) Fig. 9. Quenching experiment of (a) ht, (b) Oz, (c)OH and ESR spectra for (d) DMPO-02 and (e) DMPO-OH during the photocatalytic degradation of BPA. The similar results have also been reported for photocatalytic degrada-tion of BPA over the o-Fe203[50] and ZnO nanoparticles [51] under thesimulated sunlight irradiation (Table 2). In addition, the pseudo-first-order kinetic constants had the similar trend to pH changing, asH(shown in Fig. S2c. Following the graphic representation of pHf -pH;versus pHi, as shown in Fig. S3, the pH value at point of zero charge(pHpzc) of Bi2Wo.25M00.7506 was determined to be 6.36 according to theso-called pH drift method [52]. When the pH of solution is less than6.36, the aqueous solution will give more Htions than hydroxyl ions(OH). Therefore, the Bi2Wo.25Mo0.7506 surface is positively charged, which results in better anion adsorption. Conversely, when the pH ishigher than 6.36, the Bi2Wo.25Mo0.7506 surface is negatively charged,which results in better cation adsorption. Moreover,the pKa1 and pKa2values of BPA are approximately 9.6 and 10.2, respectively, whichmeans that the ionization of BPA occurs at pH> 9.6 to form the bis-phenolate anion [53]. Therefore, at acidic or basic pH levels, BPA, andBizWo.25Mo0.7506 were positively or negatively charged, and Coulombrepulsion inhibited adsorption, which led to a lower degradation effi-ciency.At near neutral pH level, BizWo.25Mo0.7506 is negatively chargedwhile the BPA is positively charged, and the Coulomb attraction is Fig.10. Possible degradation mechanism of BPA over BizWo.25M00.7506 undersimulated sunlight irradiation. beneficial for interaction between Bi2Wo.25M00.7506 and BPA, whichresults in a higher degradation performance. The similar results havealso been found for photocatalytic degradation of BPA over CuO/Fe203/ZnO composite [54] and TiO2-x/rGO [49] driven by vis. Therefore,initial pH of 7.7 was more favorable for BPA molecules to contact withBi2Wo.25M00.7506 surface. 3.4. Evaluation of reactive species Reactive species generated during photocatalytic process wereidentified by trapping experiments of reactive species. Three scavengers,namely, OA, PBQ, and IPA, were used to clean holes (h), superoxideradicals (02), and hydroxyl radicals ("OH) [55],respectively. As shownin Fig. 9a and b, OA and PBQ remarkably affected the BPA degradation.Notably, the removal efficiency of BPA significantly decreased with theincrease in OA or PBQ concentration in the range from 0.5 mmol L-l to2.0 mmol L-1 and from 0.125 mmol L-1 to 0.5 mmol L-l, respectively.Compared with the control group without adding any scavengers, theremoval efficiency of BPA decreased from 96.2% to 0.7% and 5.52%with the addition of 2 mmolL-1OA and 0.5 mmol L- PBQ,respectively.Therefore, ht and O2 played major roles in Bi2Wo.25Mo0.7506 systemunder simulated sunlight irradiation. Furthermore, as shown in Fig.9c,the photocatalytic degradation process of BPA was distinctly inhibitedby adding IPA, which implied that the OH also played a second role inthis system. To further explore the generation of main reactive species,the 02 and OH under simulated sunlight irradiation and in dark weretested by electron spin resonance. As displayed in Fig. 9d, no charac-teristic peak of DMPO-O2 was found in the darkness until theBi2Wo.25Mo0.7506 was illuminated with simulated sunlight. The typicalsignal intensities of DMPO-02 increased significantly with increasingirradiation time, suggesting that 02 was continually produced duringthe photocatalytic process. Moreover, as displayed in Fig. 9e, the char-acteristic peaks of OH were not obviously detected under simulatedsunlight irradiation compared with Oz. Thus, the photogenerated holesand O2 species were the main active species in this photodegradationprocess, which was consistent with the results of trapping experiments.According to the evaluation of reactive species, the whole photocatalyticdegradation process triggered by BizWo.25Mo0.7506 can be depicted inthe following Eqs. (4)-(9)[37]. (h*,·0,OH)+BPA→Degradation by -products→CO2+H O (9) 3.5. Photocatalytic mechanisms The BizWo.25Mo0.7506 absorbed photons and generated electro-n-hole pairs upon the simulated sunlight irradiation of BizWo.25.Moo.7506 are shown in Fig. 10. The photogenerated electrons weretransferred to the surface of Bi2Wo.25Mo0.7506, which improved theelectron-hole separation efficiency and then reacted with oxygen toproduce reactive oxidizing species such asO2 and ·OH. Parent BPA wasattacked by the reactive oxidizing species generated to produce variousintermediates. Twelve intermediates were identified by relative molec-ular mass comparison with HPLC-MS library date including C14H1403(A, m/z 230), CgHgO2 (B, m/z 136), C12H1002 (C, m/z 186), C15H1603(D/E, m/z 244),C15H1403(F,m/z 242), C9H12O4 (G, m/z 184),CgH1004(H, m/z170),C7H605 (I, m/z 170),C15H1604 (J, m/z 260),C15H1404 (K,m/z 258), and C9H10O2 (L/N,m/z 150). In addition, C9H10O (M m/z134) was considered to exist by analyzing the formation pathway ofintermediate products. Major intermediates A, B, C, E, F, L, and M havebeen identified in previous studies [49,56-58].On the basis of theabovementioned analysis results, the possible pathways of BPA photo-catalytic degradation over Bi2Wo.25Moo.7506 under simulated sunlightare shown in Scheme 1. Parent BPA can be directly decomposed togenerate intermediate product C under the attack of h+ through deal-kylation reaction [58]. BPA can be also decomposed by another maindegradation pathways, which comprised successive hydroxylation,dehydrogenation, alkylic-oxidation, aromatic ring opening, and oxida-tion of carbon dioxide and H2O. Hydroxylation mechanisms are shown in Scheme 2. The formation ofmono-hydroxylated aromatic intermediates A, D, and E in the photo-catalytic degradation process was conducted by two main mechanisms:hydrogen abstraction from a carbon atom containing a-hydrogen, andelectrophilic addition to n-systems [56]. Furthermore, E was hydroxyl-ated to form poly-hydroxylated intermediate J, and B was formed by thecleavage of phenyl groups from intermediate A [59]. Dehydrogenationmechanisms are shown in Scheme 3, photogenerated h* can also oxidizethe organic molecule directly, while the electrons formed can be fol-lowed by electronic rearrangement and a loss of hydrogen atom to formthe new molecules [56,60]. The frontier electron density of two ben-zenes was high. Thus, the C-C bond cleavage in intermediates E and Foccurred after the attack by ht, and then, the intermediates L and Mwere generated after the loss of hydrogen group from the linked methylgroup. Finally, M continued to be attacked by 0 and OH, and C三Cwas further oxidized to carbonyl group, followed the generation of in-termediates B and L. Alkylic-oxidation mechanisms are shown inScheme 4. Notably, alkylic-oxidation reaction occurred when organicmolecules were attacked byOz to form Hoo. and then directly pro-duced intermediates K and F [60]. As shown in Scheme 5, intermediate Fwas first attacked by h to generate a transient intermediate N, whichwas further oxidized to G by ring-opening reaction [61]. Finally, the HO- CO2+H2O+other mnolecules Scheme 1. Proposed transformation pathways of BPA over Bi2Wo.25Mo0.7506 under simulated sunlight irradiation. intermediates G, H, and I were gradually degraded to CO2 and H2O. 3.6. Toxicity assessment The standardized bioluminescence assay with V. qinghaiensis sp.Q67was first applied to analyze the samples collected at different time intervals to evaluate the toxicity of BPA and its photocatalytic in-termediates. As shown in Fig. 1la, the luminescence was nearlycompletely inhibited. The inhibition rate of BPA was 93.6%, whichdemonstrated that BPA alone had a significantly inhibitory effect on thegrowth of V. qinghaiensis sp.Q67. For the photocatalytic process, theinhibition rate decreased rapidly to 78.4% after irradiation for 60 min BPA-A→B CH CH or D-H ·OH HO· -OHI HO- ·OH· → HO C OH CH 02 -HOO HO HO- BPA→D CH2 CH3 CH OI 2 ·OH HO· ·OH HO -OH +HO→ -OH -HOO CH CH2 CH2 H OH BPA→E E→L F-M→B Scheme 3. Dehydrogenation mechanisms. E→F Scheme 4. Alkylic-oxidation mechanisms. Scheme 5. Ring-opening reaction mechanisms. and then further decreased to 63.3%, 50.5%, and 29.1% as photo-catalytic times prolonged to 120, 150, and 240 min. The resultsdemonstrated that toxicity of BPA and its intermediates was reducedduring photocatalytic process. Theoreticalaanalysis of acute toxicity,mutagenicity,bio-accumulation factor, and developmental toxicity was also performed toevaluate the toxicity evolution of BPA and its intermediates usingToxicity Estimation Software [28,29,41].As displayed in Fig. 11b, theacute toxicity represented by oral rat LD5o for BPA was 3034.02 mgkg--1, which was regarded as "toxicity." The intermediates D, E, J, andK derived from BPA showed higher acute toxicity, especially for inter-mediate product J, which was considered “very toxic." For the bio-accumulation factor, as shown in Fig. 11c, the values of all intermediateswere significantly lower than BPA (101.62) due to that the more hy-drophilic groups of these intermediates have difficulty accumulating inorganisms [28]. Fig. 11d shows that all the intermediates were “muta-genicity negative”(<0.5) except for intermediate L. Compared with BPAalone, Fig. 11e shows that the developmental toxicity values of in-termediates were significantly decreased except for those of in-termediates F, J, N, and K. Especially, the values for the B, E, I, and Meven became“developmental nontoxic."The removal efficiencies ofTOC were 28.2%, 37.9%, and 87.1% at irradiation times of 60, 120, and180 min (Fig. 11f), which indicated that all intermediates were gradu-ally detoxified step by step by further oxidizing to CO2. In summary, BizWo.25Mo0.7506 is an effective photocatalyst for mineralization of BPAand reduction in toxicity. 4. Conclusions In this study, BizWxMo1-x06 photocatalysts were successfully syn-thesized by microwave solvothermal assisted with calcination method.The synthesis conditions including W/Mo molar ratio, precursor solu-tion pH value, solvothermal temperature, calcination temperature, andtime significantly affected the photocatalytic activity of Bi2WyMo1-x06.The BizWo.25Mo0.7506 prepared at optimum conditions showed excel-lent photocatalytic activity for decomposition of BPA under simulatedsunlight. The removal efficiency of BPA was influenced by photo-catalytic parameters such as photocatalyst dosage, initial BPA concen-tration, and solution pH. Meanwhile, the photocatalytic process wasdescribed well by pseudo-first-order kinetic model. The removal effi-ciencies of BPA and TOC were obtained as 96.2% and 87.1% at 120thand 180th min of irradiation, respectively. In addition, O2 and h*played key roles in the degradation process, whereas OH played a sec-ond role. The hydroxylation, dehydrogenation, alkylic-oxidation, aro-matic ring opening, and the oxidation of carbon dioxide were proposedto be the dominant mechanisms of BPA over Bi2Wo.25Mo0.75O6. Detailedstudies showed that the Bi2Wo.25Mo0.7506 can effectively reduce thetoxicity of BPA and its intermediates during the photocatalytic process 7000 100 (a) (b) Oral rat H 0.4 Irradiation time (min) Fig. 11. Analysis of toxicity and change of TOC during degradation process. (a) Luminescent bacterium acute toxicity, (b) oral rat LDso, (c) bioaccumulation factor,(d) mutagenicity, (e) development toxicity, and (f) TOC removal. based on the acute exposure experiment of V. qinghaiensis sp. Q67 andtheoretical analysis of acute toxicity, mutagenicity, bioaccumulationfactor, and developmental toxicity. Data curation. Declaration of Competing Interest CRediT authorship contribution statement The authors declare no known competing financial interests or per-sonal relationships that can influence the work reported in this paper. Weili Yu: Investigation, Data curation, Formal analysis, Visualiza-tion, Writing-original draft. Shungang Wan: Conceptualization,Formal analysis, Visualization, Writing-review & editing. Dan Yuan:Data curation, Formal analysis, Software. Lei Sun: Conceptualization,Writing-review & editing, Funding acquisition, Project administration,Supervision. Yan Wang: Data curation, Software. Mengmeng Wang: Acknowledgments The authors gratefully acknowledge the support provided by theNationalNaturalSScience Foundation of China (grant numbers51768002 and 51368004), High Level Talent Project of Basic and Applied Basic Research Plan (Natural Science Field) of Hainan Provincein 2019 (grant number 2019RC057), and the Excellent Talent Project ofHainan University (grant numbers KYQD(ZR)1948 and KYQD(ZR)1949). Appendix A. Supplementary material ( Supplementary data to this article can be found online at h ttps://doi. org / 1 0.1016/ j .seppur . 2021.119175. ) ( References ) ( [ 1 ] R . Wang, X. Ma , T. Li u , Y. Li, L. Song, S.C. Tjong, L . Cao, W . Wang, Q. Yu, Z. Wan g , D egradation a s pects of e ndocrine disrupting chemicals: a review on photocatalytic p rocesses a nd photocatalysts, A ppl Catal A: G en 5 9 7 (2020) 11 7 547,http s :/ / d oi . o rg / 1 0 . 1 0 16/ j.a p ca ta.2 020.117 5 47. ) ( [2 ] C . Su, Y. C ui, D . Liu, H.Zhang, Y. B a ninla, Endocrine disrupting compounds, p harmaceuticals a n d personal care products in the aquatic environment of China: w hich chemicals are t h e prioritized o nes? S ci. Total Environ. 720 ( 2020) 13 7 652, ht t ps : // d oi . org / 10.1016/ j. s c it o tenv . 2020.1 3 7652. ) ( [3] T.K. Kasonga,M.A.A. C oetzee, I. Kamika, V.M.Ngole-Jeme, M.N. Benteke M omba,Endocrine-disruptive chemicals as contaminants of emerging concern i nwastewater and surface water: a review,J. Environ. Manage 277 (2021 ) 1 11485, ht tp s: / / doi. or g /10.101 6 /j .jenvman.2020 . 111485 . ) ( [4] L . Hejji, A . A zzouz, L . P. C o lon, B. Souhail, E. Ba l lesteros, A m ulti-residue method f or determining twenty-four e n docrine disrupting chemicals in vegetables andfruits using ultrasound-assisted s o lid-l i quid extraction an d continuous soli d -phase e xtraction, Chemosphere 263 ( 2021) 1 28158, h ttps : / / do i . o r g / 10.10 1 6 / j. c he m osphere. 2 0 2 0. 1 28158. ) ( [5] I . Bahelka, R. Stupka, J. Citek, M. Sprysl, The impact of bisphenols on reproductive system a nd on offspring in pigs - a review 2011 - 2020, Chemosphere 2 6 3 (2021) 1 28203, h t tp s: / / doi . o rg / 10.1 0 16 / j . chemosph e re.20 2 0.128203. ) ( [6] R .P. S ingh, C .M. Shafeeque, S . K. Sharma, R . S i ngh, M . Kannan, K. V .H. Sastry, S . Raghunandanan, J. Mohan, P.A . Azeez, Effects of bisphenol-A on male r eproductive success i n adult Kadaknath chicken, Ecotox Environ. Safe 128 (2016) 6 1-66, h t t ps: / /d oi. o rg / 1 0.1 0 16/j . e c o env.2 0 16 . 02.012. ) ( [7] A . T omza-Marciniak, P. S t epkowska, J. Kuba, B. P i larczyk, Effect of bisphenol A on r eproductive processes: a review of in v itro, in vivo and epidemiological studies, J . Appl. Toxicol. 38 (2018) 51-80, h ttps ://d oi . org / 10. 1 002/ j at. 3 480. ) ( [8] C .Y. Wang, Q. Z eng, G . Z hu, Novel S -doped BiOBr n a nosheets for the enhanced p hotocatalytic degradation of bisphenol A u n der visible light irradiation, C hemosphere 268 (2020) 128854, ht t p s : / / do i .org/ 10 .1 0 1 6 /j . c h e m o spher e . 2 020. 1 28854. ) ( [9] Y . C hen, L. S un, Z . Yu, L . Wang, G. Xiang, S. W an, Synergistic degradation p erformance and mechanism of 17p-estradiol by dielectric barrier discharge non- t hermal plasma c o mbined with Pt-TiO 2 , Sep . Purif. Te c hnol. 152 (20 1 5) 46-54, h t tp s: / / doi.org/ 1 0. 1 016/j . s e p p ur. 20 15 . 0 7 .061. ) ( [10] L . G ao, L. S un, S . Wan, Z. Y u, M. Li, D egradation kinetics a nd mechanism of e merging c o ntaminants in wa t er by dielectric barrier d i scharge n o n-thermal p lasma: T he case of 1 7β-Estradiol, Chem. Eng.J. 228 (2013)7 9 0-79 8 , ht t p s:/ /d o i. o rg /1 0. 1 016/ j . ce j .20 1 3 .05.079. ) ( [11] D . Liang, N. Li, J. An, J . Ma, Y. Wu, H . L iu, Fenton-based technologies as efficient a dvanced o x idation processes for m i crocystin-LR degradation, Sc i . T o tal E n vir o n. 753 (2021)141809, ht t ps: // d o i.org/1 0 . 1 0 1 6/ j . s c i t ot env.2 0 20.141 8 0 9 . ) ( [12] W . B u nmahotama, T.F. Lin , X. Yang, Pre d iction of adsorption capacity for p harmaceuticals, personal care products a n d endocrine disrupting chemicals ontovarious adsorbent materials, Chemosphere 238 (2020)124658, h t t p s: //d oi . o rg / 1 0 .101 6 / j .c h em o s phere.2019. 1 2 4658. ) ( [13] X . W u, Y. Wang, R . L i ang, Q. Dai, D . Jin, W. Chao, Biodegradation of an endocrine-disrupting chemical d i-n-butyl phthalate by newly i solated Agrobacterium sp. andthe biochemical pathway, P r ocess B i ochem. 46 (2011) 1090-1094, http s :// doi. or g / 1 0. 1 016/ j .proc b i o .2011 . 01 . 03 1 . ) ( [14 ] ] Q. Han, Advances i n preparation methods ofbismuth-based photocatalysts, Chem. E ng. J. 414 ( 2021) 127877, h t tps:/ /d oi.or g /1 0 . 1 0 16 / j .c e j.2020.1 2 7 877. ) ( [ 1 5] J. B ai, Y . Li, J. Liu , L . Liu, 3D Bi2MoO6 h ollow mesoporous nanostructure s with h igh p hotodegradation for t etracycline, Micropor Mesopor Mater 240 (2017) 9 1-95, h ttps: / / d o i . o rg / 1 0.10 1 6/ j . micromeso. 2 016. 1 1. 0 08. ) ( [16] J . Wang, H . L i ang, C. Zhang, B . Jin, Y. M en, BizWO6-xna n osheets with tun a ble Bi quantum dots and oxygen va c ancies fo r photocatalytic selective oxidation of alcohols, Appl. C atal. B : Environ. 2 56 (2019) 117874, htt p s: / / d oi .or g/1 0 .1 0 1 6 /j. a pcatb.2019.1 1 7874 . ) ( [17] M . A r if, Z. M in, L. Yuting, H. Yin, X. Liu, A Bi2WO6-based hybrid heterostructuresphotocatalyst with enhanced p hotodecomposition and photocatalytic hydrogenevolution t hrough Z-scheme pr o cess, J. Ind. Eng. Chem. 69 (2019) 345-357, h ttps ://d o i . or g / 1 0.101 6 /j . ji e c .2 0 1 8 .09.026. ) ( [18] T . Hu, Y. Yang, K. Dai, J . Zhang, C . L iang, A n ovel Z -scheme Bi2MoO6/BiOBr p hotocatalyst for e n hanced pho t ocatalytic acti v ity unde r visible light irradiation, a A r ? /10 ppl. S urf. Sci. 456 (2018) 473- 4 81, h t tps :/ / d oi .o r g/1 0 . 1 0 16/ j . a ps u s c. 2018.06. 1 86 . ) ( [ 20] M .B. T a hir, Construction o f visible l ight driven CuBi2O4/Bi2WO6 solid solutions a nchored w i th B i 12O17ClyBr2-x nanoparticles for improved ph o tocatalytic a ctivity, C eram. Int. 47 (2021) 320-328, ht t p s :/ / do i. org /1 0. 10 1 6/j. c er a m i nt.2 0 2 0 .08 . 1 37. ) ( [ 21] X .u. Hou, Z. Wang, J. C h en, J. Wang,Q. L u, D. W u, Facile construction of s il v er- b ased solid s olution h eterophase for e f ficient v isible-light-driven photocatalyticdegradation of tetracycline, Chem. Eng. J. 4 14 (2021) 1 2 8915, ht t p s: // d o i . org/ 1 0. 1 016/ j. c e j.202 1 . 1289 1 5 . ) ( [ 22] V .I. Voronkova, E.P. K haritonova, O.G. Rudnitskaya, P o lymorphism and propertiesof Bi2W1- x MoxO6 aurivillius phases, Inorg. M ater. 47 ( 2011) 183-191, h t tps:/ / d oi. o r g/ 1 0 .1 1 3 4/SO02016851 1 01 0 146. ) ( [23] H . Salari, Facile synthesis of new Z-scheme Bi2WO6/Bi2MoO6 p-n ju n c t ion p hotocatalysts w i th high photocatalytic activity: structure, kinetics and mechanismapproach, Mater. Res. Bull. 131 ( 2020) 110979, ht t ps:/ /do i.org/1 0 . 1 01 6 / j . m aterre s bull . 2020.110979. ) ( [ 24] W . L i , X . Ding, H . W u, H . Y a ng, Bi2MoxW1-x06 solid solutions wi t h tunable band s tructure and enhanced visible-light photocatalytic activities, Appl. Surf. Sci . 447 (2018)636-647, h t tps: / /d o i. org/ 1 0.101 6 /j. a p susc . 20 1 8.04 . 039 . ) ( [25] S .M. Ghoreishian, K.S. Ranjith, H. Lee, H.I. J u , N.S. Zeinali, Y .K. H an, Y . S. Huh, H ierarchical N-doped TiO2@Bi2WMo1-x06 core-shell nanofibers for b oostingvisible-light-driven photocatalytic and p hotoelectrochemical activities, J . H a zard. M ater. 391 ( 2020) 1 22249, h t tps :/ / doi . org / 10 . 1016/ j .j h azmat.2 0 20 . 1 2 2249. ) ( [ 26] F .J. Z hang, S.F. Zhu, F .Z. Xie, J. Zhang, Z.D. Meng, Plate-on-plate structured B izMoO6/Bi2WO6 heterojunction with high-efficiently gradient charge transfer fordecolorization of MB, S e p. P u rif. T e chnol. 1 1 3 (2013) 1- 8 , htt ps : // do i .or g / 1 0. 1 016/ j. s e p pur . 2 013 . 04. 0 0 8. ) ( [27] H . Y a ng, T. A n , G. Li, W. Song, W.J. Cooper, H. Luo, X. G uo, Photocatalytic d egradation kinetics and mechanism of environmental pharmaceuticals in aqueoussuspension of TiO2: a case of p-blockers, J. Hazard. Mater. 179 (1-3)(2010) 8 34-8 3 9, h t t ps: //d oi.or g/ 1 0. 1 0 16 / j. j h a z m a t.2 0 10.03 . 07 9 . ) ( [28] C ( . Dang, F. S un, H. J iang, T . Huang, W. L i u, X. C hen, H. Ji, Pre-accumulation and i n-situ destruction of diclofenac by a photo-regenerable activated carbon f iber s upported titanate nanotubes composite m a terial: intermediates, DFT calculation, a nd ecotoxicity, J. Hazard M a ter. 400 (2 0 20) 123 2 25, https: / / do i. o r g / 1 0 . 101 6/ j. jhazmat.2020.123225. ) ( [ 29] S . Xin, G . Liu, X. Ma, J. G ong, B. Ma, Q. Y a n, Q . C h en, D. Ma, G. Zhang, M. Gao , Y .Xin, High e f ficiency heterogeneous Fenton-like catalyst biochar modifiedCuFeO2 for the degradation of tetracycline: economical synthesis, catalytic p erformance and me c hanism, Appl. Catal. B: Environ. 280 (2021) 119386, https : / / P . . doi. org /10 . 1 0 1 6 / j. a p ca t b . 2020 . 1 1 9 3 86 . ) ( [ 30] F . Fu, H. Shen, X . Sun, W. Xue, A. Sh o neye, J. Ma, L. Luo, D. W an g , J. Wang, J . Tang, S ynergistic effect of surface oxygen vacancies and interfacial charge t ransfer o n F e(III)/Bi2MoO6 fo r efficient p h otocatalysis, A p pl. Catal. B : E n viron. 2 47 (2019) 1 50-162,ht t p s: //d oi.org/ 1 0 . 1 01 6 / j . a pcat b .2 0 1 9 .01 . 056 . ) ( [ 31] Y. B i an, W. Zeng, M. He, Y. Ma, Y.i. Liu, Y.i. Kong, J. Pa n , Boosting charge tran s fervia molybdenum doping and electric-field effect in bismuth t u ngstate: density f unction theory calculation and potential applications, J. Coll o id Interface Sci. 5 34 ( 2019) 20-30, h t tp s :/ / d o i.o r g/ 1 0 .1 0 16/j. j ci s .2018.09.012. ) ( [ 32] J . Guo, L. S hi, J. Zhao, Y. Wang, K . Tang, W. Zhang, C. Xie, X. Yuan, E n hancedvisible-light photocatalytic activity of Bi2MoO6 n anoplates w i th heterogeneousBi2MoO6x@Bi2MoO6 core-shell structure, Appl. Catal. B : Environ. 2 2 4 (2018) 6 92-7 0 4, h t t p s: //do i . o r g/1 0 .1 0 1 6/j. a pc a t b. 2 0 17 . 1 1 . 0 3 0. ) ( [ 33] J. Li u , Q. N ie, Z .Tan, Y . Luo, S . Wang, H. Y u , In s ights in t o the impurities of Bi 2 WO6 s ynthesized using the hydrothermal method, RSC Adv. 10 ( 2020) 4 0597-40607, h ttps :/ / doi.or g /10.1039 / D0RA07559K. ) ( [ 34] L . Zhang, H. Wang, Z . Chen, P .K. Wong, J. Liu,Bi2WO6 micro/nano-structures: s ynthesis, m odifications and visible-light-driven p hotocatalytic applications, Appl. C atal. B: Environ. 106 (2011) 1 -13, h ttps: // d o i.org /1 0. 1 016/j . a p ca t b . 20 1 1.05.008. ) ( [ 35] M . Sun, P . Guo, M . Wang, F. Re n , Th e effect of calcination temperature on the p hotocatalytic p erformance of Bi2 M oO6 for the degradation of phenol under visible light, Optik 1 99 (2019) 163319, htt ps:// d oi. or g / 1 0. 1 0 1 6 /j. i jl eo.2 0 1 9.16 3 3 1 9. ) ( [ 36] Y . Xie, D. Liu, B . W ang, D. Li, Z. Y an, Y. Chen, J . Shen, Z. Z h ang, X. Wang,Monolayer Bi2W1-xMox06 solid solutions fo r structural polarity t o b oost p hotocatalytic r eduction o f nitrobenzene under v i sible light, ACS S ustain. Chem. E ng. 9 (6) (2021) 2465-2474, h t t p s: // doi.o rg / 10 . 102 1 /a cssusc h emeng. 0c 073 2 4. ) ( [37] Z. Mengting, T.A. Kurniawan, Y. Y anping, M .H. Dzarfan Othman, R . Avtar, D. Fu, G .H. Hwang, Fabrication, characterization, and a p plication o f ternary magnetic r ecyclable B izWO6/BiOI@Fe3O4 composite for phot o degradation of tetracycline in a queous s o lutions, J . Environ. Manage. 2 7 0 (2020) 11 0 839, htt p s :// d o i .o r g / 1 0 . 1016 /j.j e nvman .2 020. 1 1 0 839. ) ( [38] D. Zhu, Q . Zhou, Novel Bi2WO6 modified by N-doped g r aphitic c arbon nitride p hotocatalyst for efficient photocatalytic d e gradation of phenol und e r visible ligh t , A ppl. Catal. B: Environ . 268 (2020) 118426,h ttps: / / d o i . org /1 0. 1 01 6 / j . a th apca t b.2019.118426 . ) ( [39] L . Zhang, Z . Wang, C . Hu, B. Shi, Enhanced photocatalytic performance by the synergy of Bi vacancies and Bi0 in B i 0-Bi2-8MoO6, Appl. Cat a l. B: Environ. 257 ( 2019) 1 17785, h tt p s :/ / do i .org / 1 0. 1 01 6 / j. a pc a t b . 201 9 .1 1 7 785 . ) ( [ 40] K . Zhao, Z. Zhang, Y. F e ng, S. Lin, H. Li, X. Gao, Surface oxygen vacancy modified B i2MoO6/MI L -88B(Fe) heterostructure with enhanced spatial ch a rge separation at t he b ulk & inter f ace, Appl. Catal. B : En v iron . 268 (2020) 118740, https :// d o i.org/ 10 . 1 0 16/ j .ap c a tb.2020 . 1 1 8740. ) ( [ 41] Y . Wang, L. Rao, P. Wang, Z. S hi, L. Zhang, Photocatalytic activity of N-TiO2/O-doped N vacancy g - C3N4 and the intermediates toxicity evaluation undertetracycline hydrochloride and Cr(VI) coexistence environment, Appl. Catal. B: E nviron. 2 62 (2020) 1 1 8308, ht tp s: // do i.o r g/ 1 0 . 10 1 6/j. a pc a t b .2019.1183 0 8. ) ( [42] J. Lv, X. Li u , P. Li, W . Jin, J. X u, Y. Z hao, AgI loading BiOI com p osites with e nhanced photodegradation efficiency fo r bisphenol A under simulated solar li g ht, S ci. Total Environ. 669 (2019) 194-204, h ttps: / / d oi. o rg/ 1 0.10 1 6/ j . s c i totenv . 201 9 .03.077. ) ( [ 43] L . Zhang, W. W ang, L. Z hou, H . Xu, Bi2WO6 nano- and microstructures: shapecontrol and associated visible-light-driven photocatalytic activities, Small 3 (2 0 07) 1618-1625, h t t ps: / / doi.org / 10 . 1002 / sml l . 200700043. ) ( [44] Y . Tian, G. Hua, W. X u, N . Li, M. Fang, L . Zhang, Bismuth tungstate nano/microstructures: controllable m orphologies, growth mechanism and photocatalyticproperties, J. Alloys Compd. 5 0 9 (2011) 724-730 , http s://do i. org/1 0 .1 0 1 6/j. j a ll c om .20 10.09.0 1 0. ) ( [45] | J. Yu, J . Xiong, B . Cheng, Y. Yu, J . Wang, Hydrothermal p r eparation and visible-light photocatalytic activity of BizWO6 powders, J. Solid S t ate Chem. 17 8 (2005) 1968-1 9 72, h tt p s : //do i.or g / 1 0.1 0 16/j . jssc .2 005.04.003. ) ( [46] J. Li, J. Yan, G . Y ao, Y. Zhang, X. Li, B . Lai, I mproving the de g radation of atrazinein the three-dimensional (3D) electrochemical p r ocess u s ing C u Fe2O4 as bo t h p article e lectrode and c a talyst f o r p e rsulfate activation, Chem. Eng . J. 361 (201 9 ) 1317-1332, h t t p s : / / d o i . org/ 10 .1 0 16/j . c e j .2018.1 2 . 1 4 4. ) ( [47] S .H. Liu, W.T. Tang, Photodecomposition of ibuprofen over g-C3N4/Bi2WO6/rGO h eterostructured composites under visible/solar li g ht, S c i. Total Environ. 7 3 1 ( 2020) 139172,ht t ps:// d oi .o r g/ 1 0 . 10 1 6 / j. s c i to tenv.2 0 20.1 39 1 7 2. ) ( [48] K . Prakash, K . P. S e nthil, S. P andiaraj, K . Saravanakumar,S. Karuthapandian, C ontrollable synthesis of SnO2 photocatalyst with superior photocatalytic activity f or the degradation of methylene blue dye solution, J. Exp. Nanosci. 1 1 (2016) 1138-1155, h tt p s: / / doi . or g/ 1 0 . 1080/1 7 458080. 2 016.11 8 8222. ) ( [49] L . X u, L . Y ang, E .M.J. J ohansson, Y. Wang, P. J i n, Photocatalytic activity andmechanism o f bisphenol a removal over T iO2- x /rGO nanocomposite driven b y visible light , Chem. Eng. J . 350 (2018) 1043-1055, h ttps : / / d o i . or g/1 0. 1 016/j . c e j .2018.06.0 4 6 . ) ( [50] C . Y e, K . H u, Z. Niu, Y. Lu, L . Zhang, K. Yan, Co n trollable synthesis of rhombohedral o-Fe203 ef f icient for photocatalytic degradation of b isphenol A, J . Water Process E ng. 2 7 (2019) 205-2 1 0, ht tps: / / d o i.org / 1 0 .1016/ j . j wpe . 2 01 8 .1 2 . 0 08. ) ( [51] Y . W ang, K. H u , Z. Y a ng, C. Y e , X . L i , K . Y an, F acile synthesis of porous ZnO n anoparticles efficient for photocatalytic degradation of biomass-derived bisphenolA u nder simulated s u nlight irradiation, Front. Bioeng. Biotechnol. 8 (2020) 616780,h t tps:/ /d oi.org / 1 0. 3 389/ f bioe . 2020.616780. ) ( [52] I. L ung, M . -L. Soran, A . Stegarescu, O. Op r is, S. Gutoiu, C. Le o stean, M.D. Laz a r, I . Kacso, T.-D. Silipas, A. S . Pora v , Evaluati o n of CNT-COOH/MnO2/Fe3O4 ) nanocomposite for ibuprofen and paracetamol removal from aqueous solutions,J. Hazard. Mater. 403 (2021) 123528, https://doi.org/10.1016/j.ihazmat.2020.123528. ( [ 53] Y . Yoon, P . Westerhoff, S.A. S nyder, M. Esparza, HPLC-fluorescence detection and a dsorption of bisphenol A , 1 7p-estradiol, and 17o-ethynyl e stradiol on powdered a ctivated carbon, Water Res. 37 ( 2003) 3530-3537, ht t p s :// do i.o r g/1 0 . 1 0 1 6/ S0043- 1 3 54 ( 03)00239-2 . ) [54] S. Shekoohiyan, A. Rahmania, M. Chamack, G. Moussavi, O. Rahmanian,V. Alipour, S. Giannakis, A novel CuO/Fe2O3/ZnO composite for visible-lightassisted photocatalytic oxidation of Bisphenol A: kinetics, degradation pathways,and toxicity elimination, Sep. Purif. Technol. 242 (2020) 116821,https://doi.org/10.1016/j.seppur.2020.116821. [55] Q.W. Cao, Y.F. Zheng, X.C. Song, The enhanced visible light photocatalytic activityof Bi2WxMo1-xO6-BiOCl heterojunctions with adjustable energy band, Ceram. Int.42 (2016) 14533-14542, https://doi.org/10.1016/j.ceramint.2016.06.066. [56] W.H.M. Abdelraheem, M.K. Patil, M.N. Nadagouda,D.D. Dionysiou, Hydrothermalsynthesis of photoactive nitrogen- and boron- codoped TiO2 nanoparticles for thetreatment of bisphenol A in wastewater: Synthesis, photocatalytic activity,degradation byproducts and reaction pathways, Appl. Catal. B: Environ. 241(2019) 598-611, https://doi.org/10.1016/j.apcatb.2018.09.039. [57] X. Bai, L. Yang, A. Hagfeldt, E.M.J. Johansson, P. Jin, D35-TiO2 nano-crystallinefilm as a high performance visible-light photocatalyst towards the degradation ofbis-phenol A, Chem. Eng. J. 355 (2019) 999-1010, https://doi.org/10.1016/j.cej.2018.08.061. [58] X. Ruan, Y. Hu, Effectively enhanced photodegradation of bisphenol A by in-situ g-C3N4-Zn/Bi2WO6 heterojunctions and mechanism study, Chemosphere 246 (2020)125782, https://doi.org/10.1016/j.chemosphere.2019.125782. ( [59] T .B. Nguyen, C.P. Huang, R.-A. Doong, Photocatalytic degradation of bisphenol A o ver a Z nFe204/TiO2 nanocomposite und e r visible light, S c i. T otal E nviron. 6 46 ( 2019)7 4 5-756, ht tps : / / d oi . o rg / 1 0 .1016/j . s ci totenv . 20 1 8. 0 7 . 3 5 2 . ) ( [60 C ] .S. G uo, M. Ge, L. L iu, G.D. Gao, Y.C. Feng, Y.Q. Wang, Directed s ynthesis ofmesoporous TiO2 microspheres: ca t alysts and their photocatalysis f o r bisphenol A d egradation, E nviron. S ci. Technol. 44 (2010) 419- 4 25, h t t p s ://d oi.o r g/ 1 0. 1 0 2 1 / es9019854. ) ( [ 61] W .H.M. Abdelraheem, X. He, Z.R. Komy, N.M. Ismail, D.D. Dionysiou, Revealing t he mechanism, pathways a n d k i netics o f UV254nm/H2O2-based degradation ofmodel active sunscreen i ngredient PBSA, Chem. Eng. J. 288 (2016) 8 24-833, https: //d oi . or g/ 1 0. 1 01 6/j . cej. 2 015.1 2. 0 46. ) 微波溶热辅助煅烧合成的Bi2WxMo1-XO6固溶体光催化剂在模拟阳光照射下降解和解毒双酚A01前言:设计了一系列(x=0, 0.25, 0.5, 0.75, 1)的固溶体光催化剂,并通过微波溶胶热法和后处理煅烧相结合的方法进行合成,通过各种技术对所制备的固溶体光催化剂的物理化学和光学性能进行了表征。通过模拟阳光照射下双酚A(BPA)的光降解,评估了光催化活性。结果表明,所有的光催化剂都呈花状,Bi2W0.25Mo0.75O6微球显示出最好的光催化活性。在双酚A浓度为15mgL-1时,120分钟和180分钟内双酚A和总有机碳的去除率分别为96.2%和87.1%。在反应过程中,-O2-和h+起着主导作用,而-OH也对双酚A的降解做出了贡献。12种中间产物被鉴定出来,它们逐渐被矿化为CO2和H2O。在此基础上根据对活性物种和中间产物的分析,提出了BPA的主要降解途径。即羟化作用、烷基氧化作用、脱氢作用和芳香族开环作用。在双酚A降解过程中的毒性变化,还通过标准化的生物发光法对发光细菌的抑制率进行了研究。发光细菌Vibrio qinghaiensis sp. Q67,并结合定量结构-活性关系分析。双酚A及其中间体对青海弧菌Q67的抑制作用,随着双酚A及其中间体对青海弧菌Q67的抑制作用逐渐消除。Q67的抑制作用随着BPA的分解而逐渐消除。综上所述,Bi2W0.25Mo0.75O6是一种有效的光催化剂,用于降解和降低BPA的毒性。水溶液中双酚A的降解和毒性降低。02简介:扰乱内分泌的化学物质(EDCs)包括药品、个人护理用品、增塑剂和其他人造有机污染物。可通过破坏人类和Y生动物的荷尔蒙平衡而对健康产生不利影响[1]。各种EDCs已被广泛在各种地表水[2]、废水处理厂的废水中[3],甚至在蔬菜和水果中也检测到,含量在5.8至580纳克-1[4]。EDC的浓度可能从每升几纳克(ngL- 1)增加到15μg L- 1,这足以使其产生急性和慢性毒性。环境中的EDCs来源可能是由于人类活动造成的人为引入,包括生产和使用含有EDCs的产品。例如,双酚A(BPA)作为EDC已被广泛用于塑料工业[5],而BPA对人类、家禽和反刍动物的生殖系统疾病有显著的不利影响[6,7]。因此,鉴于其毒性,迫切需要一种有效的技术来去除EDCs。一些技术已经已被用于去除EDCs,如同质和异质的光催化氧化[8],非热等离子体放电[9,10]。芬顿氧化[11]、吸附[12]和生物降解[13]。在这些方法中,光催化成为一种有前途的方法。因为它具有低能耗、非选择性矿化、无二次污染和反应条件温和等特点。铋基光催化剂在光催化领域受到了越来越多的关注,包括水裂解氢、难降解污染物的去除、有机合成等。[14]. Bi2MoO6和Bi2WO6是重要的Aurivillius氧化物,具有特殊的过氧化物层结构,在紫外线照射下具有良好的光催化活性。在视觉辐照下,它们已被用于清除污染物[15,16],并用于水分离产生氢气[17]。然而。单相Bi2WO6的光催化活性具有相对较宽的带隙能(Eg=2.0)。带隙能量(Eg = 2.75-2.77 eV)的单相Bi2WO6的光催化活性需要进一步提高。因为vis的利用效率低[18]。Bi2MoO6一般有一个窄带隙(2.42-2.63 eV),具有类似Aurivillius层的结构;因此,它可以比单一的Bi2WO6利用更多的太阳光,但但光生电子-空穴对的高重组率是光催化的限制之一[19]。这种方法可以有效地减少电子-空穴对的重新组合,并进一步提高形成固溶体光催化剂来调整半导体的带隙结构,从而进一步提高光催化活性。半导体的带隙结构[20,21]。固态烧结[22]、水热/溶热法[23],甚至阴离子交换法[24]都被用来制备Bi2MoO6和Bi2WO6的复合光催化剂。所有的结果都表明,用Mo取代W位点可以使晶体轻微变形并降低带隙能,加速电荷载流子的分离,并提高光催化的活性。[25,26]. 然而,很少有研究专注于通过快速微波溶胶热法与后处理煅烧相结合来合成Bi2WxMo1- xO6固溶体光催化剂,以进一步提高光催化活性。在模拟太阳光照射下进一步提高光催化活性。特别是,典型的EDCs在光催化过程中的转化途径和毒性降低,在使用Bi2WxMo1- xO6进行光催化降解的过程中,典型的EDCs的转化途径和毒性降低需要研究。在这项研究中,双酚A被选为模型污染物,因为它是一种流行的EDC,通常存在于人们的生活环境中,并且难以被生物降解。一系列Bi2WxMo1- XO6固体溶液光催化剂采用微波溶热和煅烧相结合的方法合成了一系列Bi2WxMo1- XO6固溶体光催化剂。合成参数和降解条件在模拟阳光照射下,对合成参数和降解条件进行了优化以提高光催化活性。此外,还研究了双酚A在降解过程中的转化途径和毒性演变。03祥鹄仪器在此文献中的使用过程:光催化剂Bi2WxMo1- XO6的合成一系列的Bi2WxMo1- XO6是通过微波溶热与煅烧辅助合成的,如图1所示。简而言之,2mmol的Bi(NO3)3⋅5H2O溶解在30mL EG中,超声处理30分钟,形成溶液A。共计1mmol的Na2WO4⋅2H2O和将W/Mo摩尔比为3/1、1、1/3和0的Na2WO4⋅2H2O溶解在20mL EG中,超声处理30分钟,形成溶液A。溶解在20毫升EG中,超声处理30分钟,形成溶液B。通过剧烈搅拌将溶液B缓慢加入到溶液A中。然后。0.82mmol C19H42BrN溶液在超声处理下再加入上述均匀溶液中30分钟。此后。0.1M NaOH或HCl溶液滴入混合物中,以调整pH值=3,5,7,9,然后将该混合物转移到一个100mL Teflon内衬高压釜。溶热过程在微波水热平行合成仪中进行(微波水热平行合成仪 XH-800S,北京祥鹄科技发展有限公司,中国,北京),在一定的温度下进行溶胀热处理。,然后自然冷却到室温。接下来,得到的前驱体交替用去离子水和绝对乙醇清洗,然后将沉淀物送入实验室。乙醇洗涤,沉淀物在80◦C下干燥。随后,这些前驱体在一个熔炉(KSL-1200 ×)中进行煅烧,加热速度为5◦C/min。加热速度为5℃/min,并在一定时间内达到最终温度。所得产品被称为Bi2W0.25Mo0.75O6、Bi2W0.5Mo0.5O6制备方法也与对照组相同。04结论:在这项研究中,通过微波溶热辅助煅烧法成功合成了Bi2WxMo1- XO6光催化剂。合成条件包括W/Mo摩尔比、前体溶液pH值、溶热温度、煅烧温度和时间对Bi2WxMo1- XO6的光催化活性有明显的影响。在最佳条件下制备的Bi2W0.25Mo0.75O6在模拟阳光下对双酚A的分解表现出了优异的光催化活性,如光催化剂用量、双酚A初始浓度和溶液pH值。同时,光催化过程被伪一阶动力学模型很好地描述了这一过程。在照射120分钟和180分钟时,BPA和TOC的去除率分别为96.2%和87.1%。此外,-O2-和h+在降解过程中起着关键作用,而-OH起着次要作用。羟基化、脱氢、烷基氧化、芳香族开环和二氧化碳的氧化被认为是Bio的主要机制。详细的研究表明Bi2W0.25Mo0.75O6可以有效地降低双酚A及其中间体的毒性。在光催化过程中,基于V. qinghaiensis sp.的急性毒性、诱变性、生物蓄积性和发育毒性的理论分析。文献原文
确定






还剩15页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《环境水中双酚A在光催化剂下降解检测方案(微波合成仪)》,该方案主要用于环境水(除海水)中有机污染物检测,参考标准--,《环境水中双酚A在光催化剂下降解检测方案(微波合成仪)》用到的仪器有Nanocube 多功能微波水热平行合成仪、微波水热合成仪 Hydrocube XH-800SE型
推荐专场
相关方案
更多

















