方案详情
文
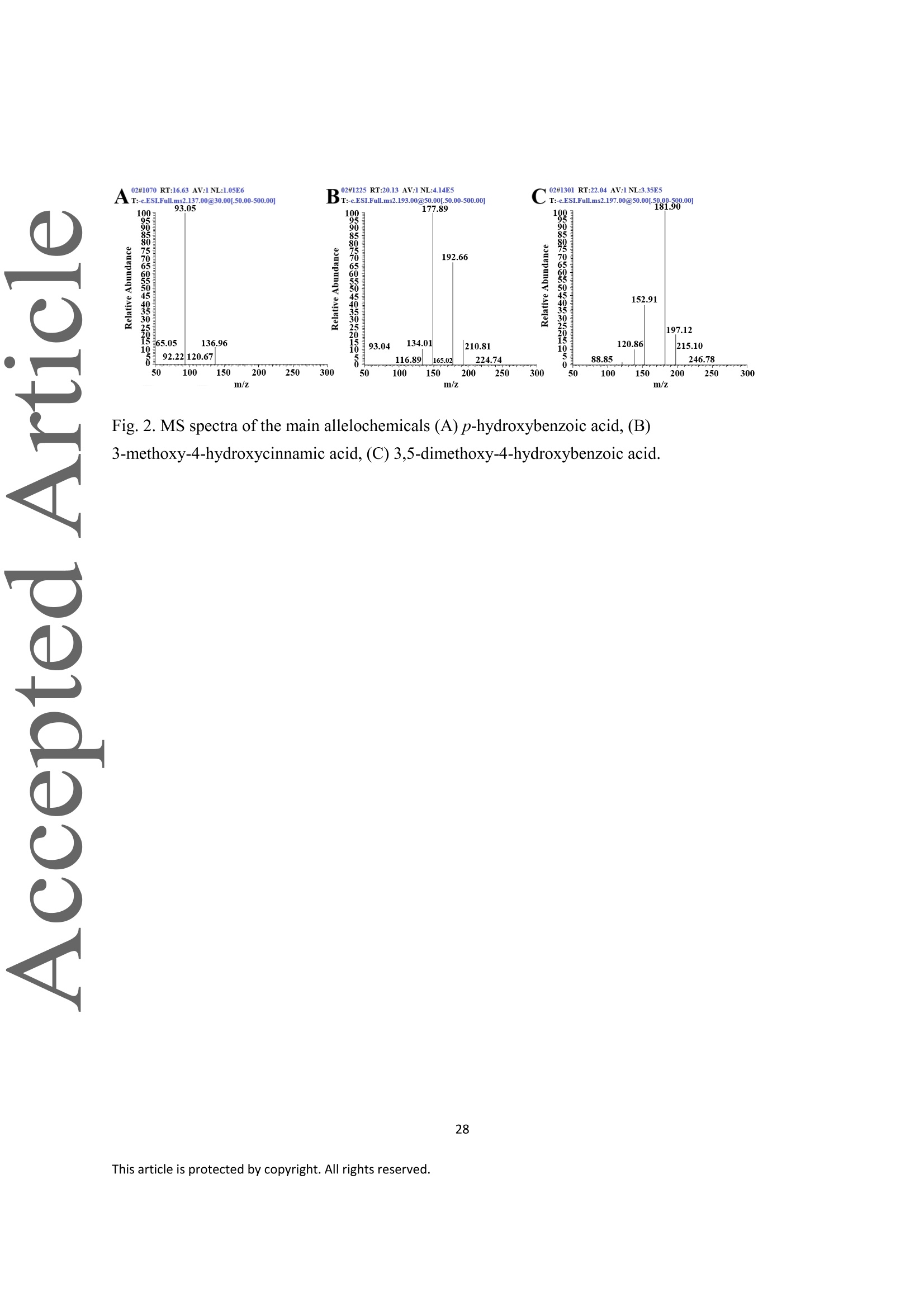
采用响应面方法对发芽的小麦种子中的等离子化合物的微波辅助提取(MAE)进行了优化。在最佳条件下(70℃,功率283W,液固比17mL/g,时间20.0min),对黄瓜种子萌发的抑制率为64.21%±1.99%。三种主要的对羟基苯甲酸、3-甲氧基-4-羟基肉桂酸和3,5-二甲氧基-4-羟基肉桂酸等三种主要的异黄酮类化合物。3,5-dimethoxy-4-hydroxybenzoic acid)。这些等位素化学物质明显抑制了黄瓜种子和根的生长。在使用等效物处理后,黄瓜的丙二醛含量增加,根的活力和淀粉酶活性下降。G胱甘肽含量、G胱甘肽过氧化物酶和G胱甘肽转移酶的活性先上升,然后随着异丙醇化合物浓度的增加而下降。这些结果表明,从发芽的小麦种子中提取的提取物可以通过影响黄瓜的生理和生物化学过程来抑制黄瓜的生长。
方案详情

Extraction of allelochemicals from germinated wheat seeds and their inhibitory effects on cucumber Jingwen Bai , Xuedong Bai, Yu Yang *, Bo Tao *o College of Arts and Sciences, Northeast Agricultural University, Harbin 150030, China' College of Agronomy, Northeast Agricultural University, Harbin 150030, China * Corresponding author. Tel.: +86 451 551911442; fax: +86 451 55190243 E-mail address: yangyu_002@163.com(Y. Yang). * Corresponding author. Tel.: +86 451 551910990; fax: +86 451 55190243 E-mail address: Botaol@163.com (B. Tao). Abstract Microwave-assisted extraction (MAE) ofallelochemicals from germinated wheat seeds was optimized by using response surface methodology. Under the optimal conditions70 °C, power 283W, liquid-to-solid ratio 17 mL/g and time 20.0 min), the inhibitory rate ofallelochemicals on cucumber seeds germination was 64.21%±1.99%. Three mainallelochemicals including p-Hydroxybenzoic acid, 3-methoxy-4-hydroxycinnamic acid and 3,5-dimethoxy-4-hydroxybenzoic acid were identified by LC-MS/MS analysis. These This article has been accepted for publication and undergone full peer review but has not been throughthe copyediting, typesetting, pagination and proofreading process, which may lead to differencesbetween this version and the Version of Record. Please cite this article as doi: 10.1002/agj2.20703. This article is protected by copyright. All rights reserved. allelochemicals significantly inhibited the growth of cucumber seeding and root. oo malondialdehyde content increased and root vitality and amylase activity decreased after thetreatment of allelochemicals. Glutathione content, glutathione peroxidase and glutathionetransferase activities rose first and then fell with the allelochemicals concentration increasedThese results suggested that the extract from germinated wheat seeds could inhibit the growthof cucumber by affecting the physiological and biochemical processes in cucumber. Keywords: Germinated wheat seeds; Allelochemicals; Microwave-assisted extraction;indicators; Physiological activities 1. Introduction Blind and indiscriminate use of chemical herbicides has caused crop loss and weedresistance in most arable lands worldwide. Furthermore, herbicides also lead to soil, waterpollution, and herbicide residues in food, which was a big threat to food safety and humanhealth (Farhoudi and Lee 2013). Allelopathy, as a common biological phenomenon in whichan organism produces biochemicals that influence the growth, survival, development andreproduction of other organisms, may be a possible alternative weed management strategy(Macias et al. 2014). Recently, allelochemicals have drawn much attention because of their.potential applications, such as growth regulators, herbicides, insecticides, and antimicrobialcrop protection products (Cheng and Cheng 2015; Lam et al. 2012; Macias et al. 2014). ao Wheat (Triticum aestivum L.) is the second most produced food among the cereal crops inthe world, and has been extensively studied for its allelopathic potential for the managementweeds (Marta et al. 2006; Tischler et al. 2018). Previous studies revealed that the allelopathic activity of wheat was due to the release of a broad range of allelochemicals, such as phenolicacids, hydroxamic acids and short-chain fatty acids (Albuquerque et al. 2011; Wu et al.Many researchers have confirmed that the diluted extracts of wheat seedlings, straws, roots,shoot tissues, leaves, stems, and residues displayed allelopathic effects on the germinationgrowth of agricultural weeds (Wu et al. 2000; Wu et al. 2001). However, limited reports onallelochemicals from germinated wheat seeds were available. Marta et al. (2006) found thattype of metabolites in the germinated wheat seeds depended strongly on the working upprocedure of the plant material, as well as the plant growth stage. In addition, allelochemicalscan affect the physiological and biochemical processes of plants when they are absorbed intothe plant circulatory system (Weir et al. 2004). Zhang et al.(2011) revealed thatmalondialdehyde (MDA) content and glutathione (GSH) level in M. aeruginosa cellswith the increase of allelochemicals concentration and the prolongation of exposure time. Tannic acid can reduce the synthesis of amylase and acid-phosphatase in the endosperm (Cheng and Cheng 2015). Glutathione peroxidase (GSH-Px) and glutathione transferase increased remarkably under allelochemical stress, suggesting that they might serve as an effective defense tool in avoidance of oxidative damage (Erica Marusa Pergo et al. 2011). Nowadays, the allelopathic effect of allelochemicals from germinated wheat seeds on thegrowth of other plants has not been studied, and the mechanism is unclear. In general, allelochemicals extraction from different plants is the first step for the study ofao allelopathy mechanism. During the past decades, solvent extraction with distilled water,ethanol, and acetone at ambient temperature is the most common method (Tesio et al. 2011;Wu et al. 2001). However, this method is time consuming, solvent wasting, and low yield. Atpresent, microwave-assisted extraction (MAE) is increasingly widespread for different activecompounds extraction (Farhadpour et al. 2016; Moreira et al. 2017; Yang et al. 2020; Zhao etal.2021).MAE performance depends on its operational mode of heating, and allows theextraction of bioactive components without distillation and evaporation, and thereby isfor extracting thermal-labile compounds (Felkai-Haddache et al. 2016; Zhao et al. 2021).Generally, response surface methodology (RSM) model can be established to simultaneouslydesign and optimize the variables, and moreover can predict the operating condition for theresponses (Felkai-Haddache et al. 2016; Yang et al. 2020). To our knowledge, no detailedinvestigation has been conducted to predict and optimize the allelochemicals extraction fromgerminated wheat seeds with MAE using RSM. In the present study, MAE conditions (temperature, liquid-to-solid ratio, power, andfor allelochemicals from germinated wheat seeds were optimized based on the inhibitory rateusing RSM. Then, the inhibitory effect of allelochemicals on the growth of cucumber was analyzed. Finally, root vitality, GSH-Px and GST activities, MDA, GSH and amylasewere determined. 2. Materials and methodsao 2.1.Materials Nineteen wheat seed varieties were obtained from Northeast Agricultural University inHarbin, which were coded as DN-1 to DN-19. The wheat seeds including Longfu series(from Longfu-1 to Longfu-16) and the other five varieties were provided by HeilongjiangInstitute of Agricultural Science (Harbin, China). Seventeen indicating crop seeds werebought from Xinhua seed company (Harbin). All other chemicals were of analytical grade. 2.2. Germination ofwheat seeds Wheat seeds were surface-sterilized for 15 min with 2.5% (v/v) sodium hypochloritesolution, and then rinsed with sterilized distilled water. Two pieces of filter paper (9 cmdiameter) soaked with 5.0 mL sterilized aqueous were placed in each Petri dish, and then 20wheat seeds were placed. The dishes were incubated in darkness in a growth chamber at 22C and 80% humidity. After 36 h of incubation, the seeds with embryo root breaking throughseed coat 2 mm were collected. 2.3. Screening ofindicating crop Seventeen crops,including cucumber, chicory, valerian, spinach, beans, lettuce, carrot,tomato, loofah, big radish, mustard, ramie, xanthium, radish, shallot, Chinese cabbage, and 5 This article is protected by copyright. All rights reserved. ooo rape were treated with the same concentration of extract from germinated wheat seeds. Then,the suitable indicating crop was selected according to response index (RI) of germinationrate, root length, seeding height, and seeding fresh weight. RI was determined according tothe previous reference (Williamson and Richardson 1988): if T> C, then RI=1-(C/T), ifT从发芽的小麦种子中提取等量的化学物质及其对黄瓜的抑制作用01前言:采用响应面方法对发芽的小麦种子中的等离子化合物的微波辅助提取(MAE)进行了优化。在最佳条件下(70℃,功率283W,液固比17mL/g,时间20.0min),对黄瓜种子萌发的抑制率为64.21%±1.99%。三种主要的对羟基苯甲酸、3-甲氧基-4-羟基肉桂酸和3,5-二甲氧基-4-羟基肉桂酸等三种主要的异黄酮类化合物。3,5-dimethoxy-4-hydroxybenzoic acid)。这些等位素化学物质明显抑制了黄瓜种子和根的生长。在使用等效物处理后,黄瓜的丙二醛含量增加,根的活力和淀粉酶活性下降。G胱甘肽含量、G胱甘肽过氧化物酶和G胱甘肽转移酶的活性先上升,然后随着异丙醇化合物浓度的增加而下降。这些结果表明,从发芽的小麦种子中提取的提取物可以通过影响黄瓜的生理和生物化学过程来抑制黄瓜的生长。02简介:盲目和滥用化学除草剂造成了全世界大多数可耕地上的作物损失和杂草的抗药性。此外,除草剂还导致土壤、水污染和食物中的除草剂残留,这是对食品安全和人类健康的一大威胁(Farhoudi和Lee 2013)。同化作用(Allelopathy),作为一种常见的生物现象,一个生物体产生的生化物质会影响其他生物体的生长、生存、发展和繁殖,可能是一种可能的替代杂草管理策略(Macías等人,2014)。最近,等离子化合物由于其潜在的应用,如生长调节剂、除草剂、杀虫剂和抗菌作物保护产品,已经引起了很多关注(Cheng和Cheng2015;Lam等人,2012;Macías等人,2014)。小麦(Tritum aestium L.)是世界上第二大粮食作物,并已被广泛研究其在管理杂草方面的等效效应潜力。杂草(Marta等人,2006;Tischler等人,2018)。以前的研究显示,小麦的等位素活性是由于释放了广泛的等位素化学物质,如酚类 酸、羟肟酸和短链脂肪酸(Albuquerque等人,2011年;Wu等人,2011年)。许多研究人员已经证实,小麦幼苗、秸秆、根部的稀释提取物。小麦苗、秸秆、根、芽组织、叶、茎和残留物的稀释提取物对农业杂草的发芽 (Wu et al. 2000; Wu et al. 2001)。然而,有限的报告是关于 然而,关于发芽的小麦种子中的等效化学物的报告是有限的。Marta等人(2006)发现发芽的小麦种子中的代谢物类型在很大程度上取决于植物材料的加工过程以及植物的生长阶段。此外,当它们被吸收到植物的循环系统中时,等位素化学物质可以影响植物的生理和生化过程(Weir等人,2004)。Zhang等人(2011)发现,铜绿假单胞菌细胞中丙二醛(MDA)含量和G胱甘肽(GSH)水平随着异丙醇浓度的增加和暴露时间的延长而增加。单宁酸可以减少胚乳中淀粉酶和酸性磷酸酶的合成(Cheng and Cheng 2015)。G胱甘肽过氧化物酶(GSH-Px)和G胱甘肽转移酶在等位素化学胁迫下显著增加,表明它们可能成为避免氧化损伤的有效防御工具(Érica Marusa Pergo等人,2011)。现在,发芽的小麦种子的等位素对其他植物生长的影响还没有被研究,其机制也不清楚。一般来说,从不同的植物中提取等价物是研究等价物机制的第一步。在过去的几十年里,用蒸馏水、乙醇和丙酮在环境温度下进行溶剂提取是最常用的方法(Tesio等人,2011。Wu等人,2001)。然而,这种方法费时费力,浪费溶剂,而且产量低。目前,微波辅助提取(MAE)越来越广泛地用于不同活性化合物的提取(Farhadpour等人,2016;Moreira等人,2017;Yang等人,2020;Zhao等人,al. 2021). MAE的性能取决于其加热的操作模式,并允许萃取生物活性成分,而不需要蒸馏和蒸发,因此是用于提取热降解化合物(Felkai-Haddache等人,2016;Zhao等人,2021)。一般来说,可以建立响应面方法(RSM)模型来同时设计和优化变量,而且可以预测反应的操作条件。(Felkai-Haddache et al. 2016; Yang et al. 2020)。就我们所知,我们还没有进行过详细的调查,以预测和优化从发芽的小麦种子中提取等位素的情况。使用RSM对发芽的小麦种子进行预测和优化。在本研究中,MAE的条件(温度、液固比、功率、和 根据抑制率,对发芽的小麦种子中的等离子化合物进行了优化。使用RSM。然后,分析了等离子化合物对黄瓜生长的抑制作用。最后,对根系活力、GSH-Px和GST活性、MDA、GSH和淀粉酶测定。03祥鹄仪器在此文献中的使用过程2.5. 从发芽的小麦种子中提取赤霉烯类化合物MAE实验采用电脑温压双控微波消解仪(XH-800C,北京祥鹄科技发展有限公司,中国,北京,)进行。5.0g的样品,以不同的液固比(10:1-30:1 mL/g)加入一定体积的蒸馏水。混合物在不同的微波辐照下(100- 500W)、不同温度(30-70℃)、不同时间(5-30分钟)下提取混合物。最后 混合物在真空下通过0.45μm的微孔膜过滤,然后将溶液储存在4±1℃。04结论在本研究中,RSM分析证实,萃取温度在MAE法的等离子化合物萃取中起着重要作用。对羟基苯甲酸、3-甲氧基-4-羟基肉桂酸和被确定为发芽小麦种子中的主要等位素。测定了发芽的小麦种子中主要的等位素化学物质。在经过以下处理后 黄瓜的生长、根系活力和α-淀粉酶的产生受到抑制。都受到了抑制。GSH含量、GST和GSH-Px活性呈现出低剂量刺激和高剂量抑制的趋势。进一步研究从发芽的小麦种子中提取的等位素化学物的田间试验。05文献原文
确定




























还剩27页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《发芽小麦种子中赤霉烯类化合物前处理提取检测方案(微波消解仪)》,该方案主要用于种子中前处理检测,参考标准--,《发芽小麦种子中赤霉烯类化合物前处理提取检测方案(微波消解仪)》用到的仪器有微波消解工作站 Acidcube XH-800DE型、微波消解工作站 Honeycomb
推荐专场