方案详情
文
哲斯泰GERSTEL的自动化解决方案可以高效准确的自动提取食用油中的霉菌毒素结合LC-MS/MS分析;可重复性高,平均RSD为 4.32%;四种黄曲霉毒素的平均回收率为101%;线性高,R2 大于 0.999
方案详情

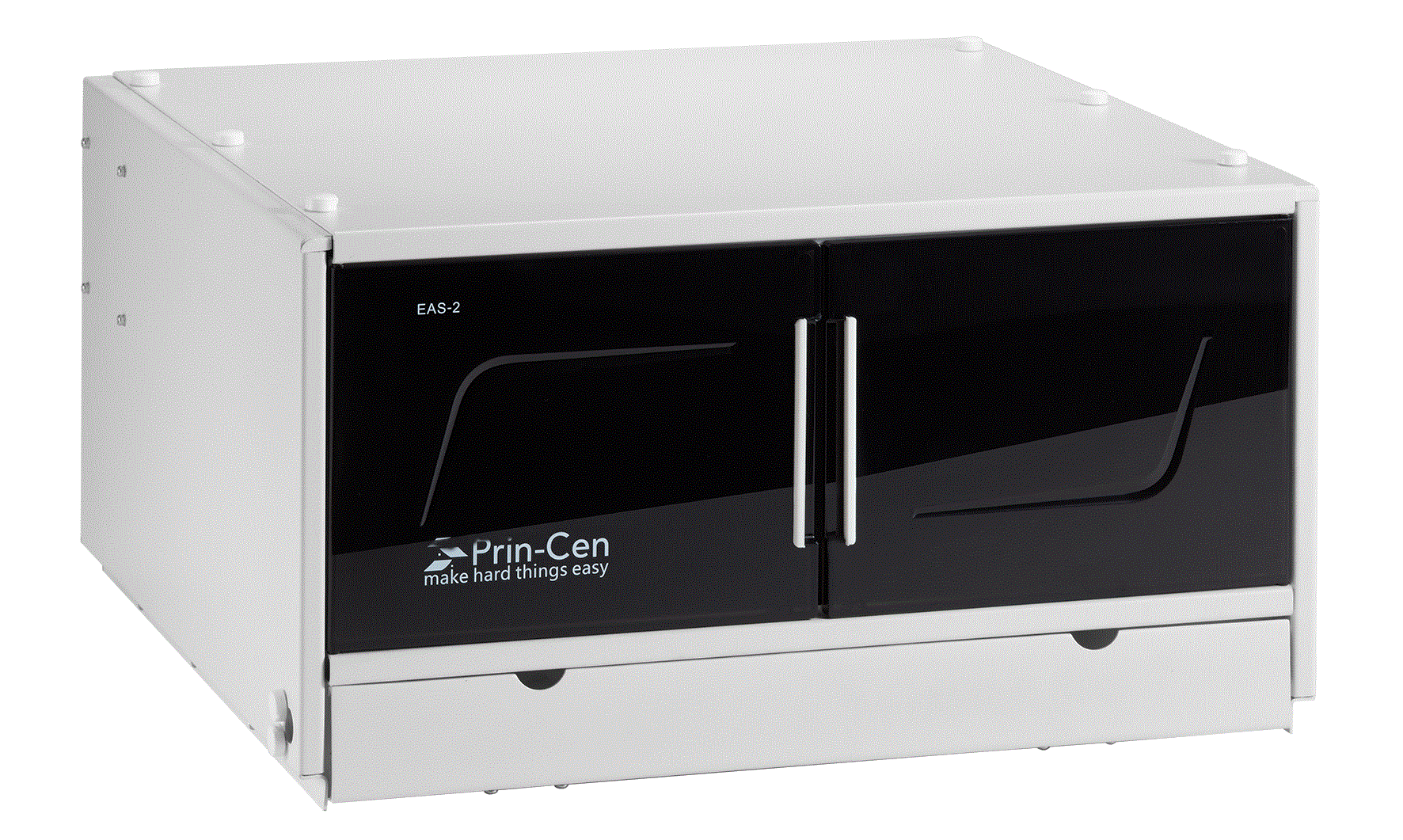
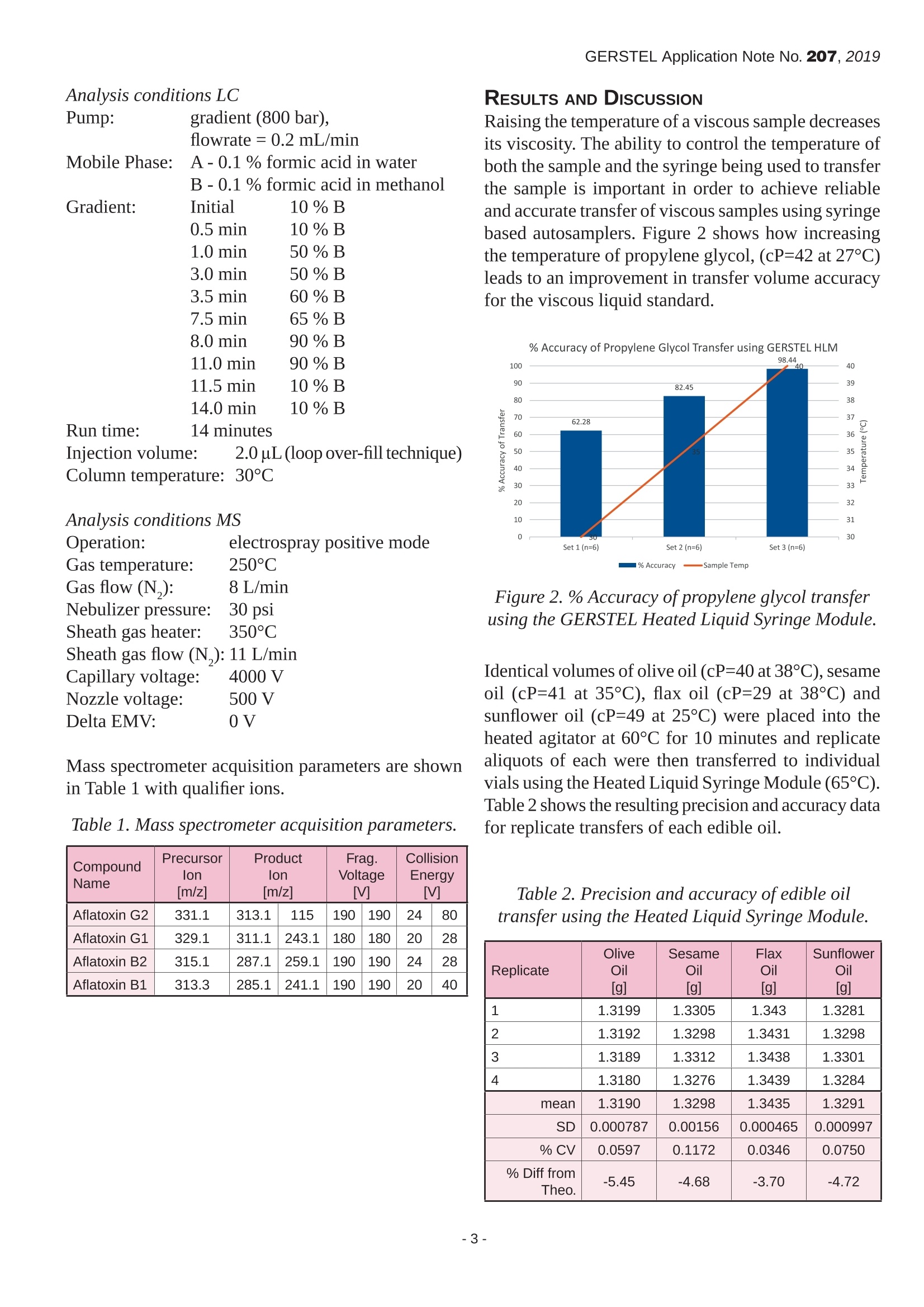
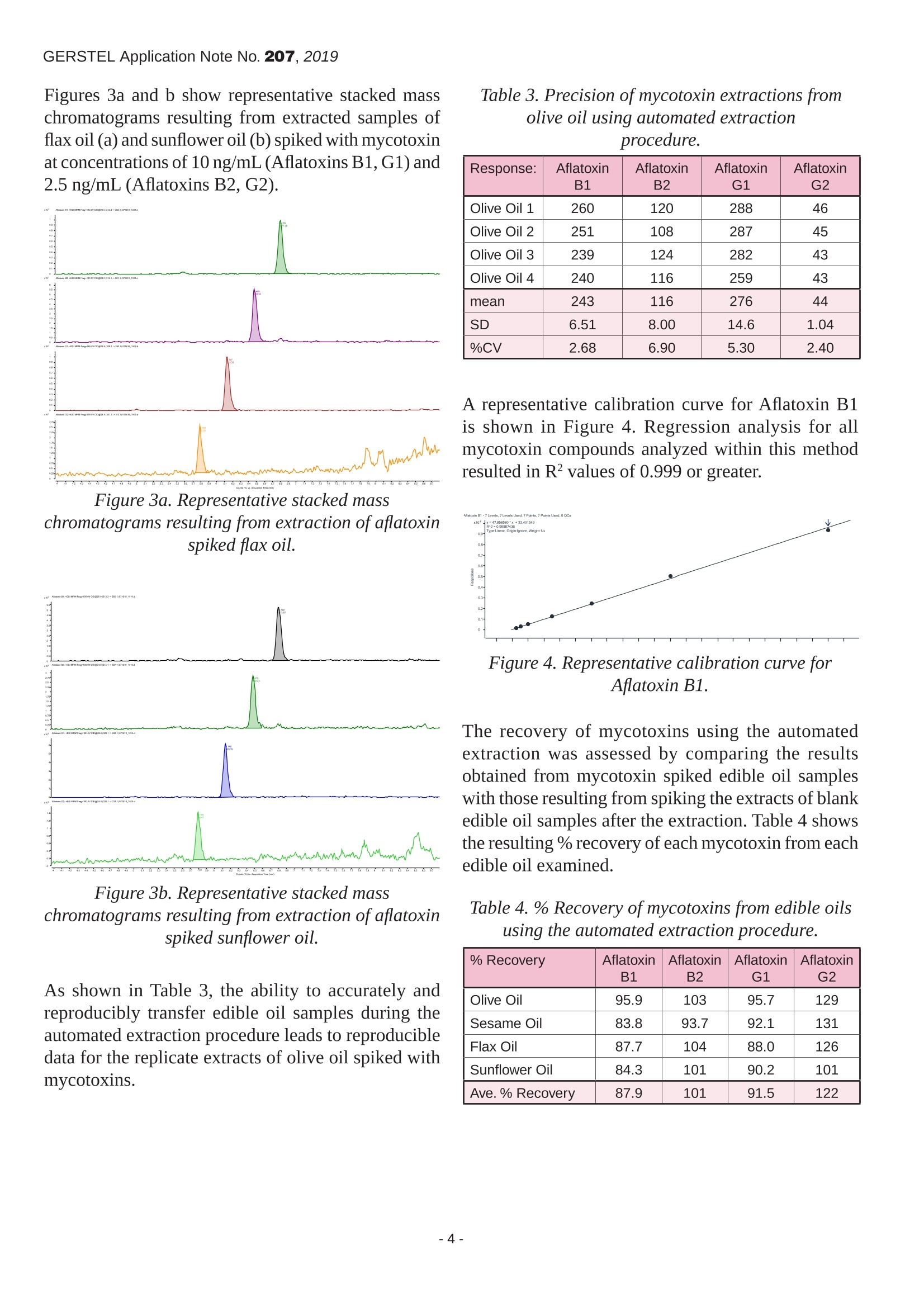
GERSTEL Application Note No. 207, 2019 Automating the Accurate Transfer of Viscous Samplesfor the Completely Automated Extraction of Mycotoxinsfrom Edible Oils Fredrick D. Foster, John R. Stuff, Laurel A. Vernarelli, Jacqueline A. WhitecavageGERSTEL, Inc., 701 Digital Drive, Suite J, Linthicum, MD, 21090, USA KEYWORDS Sample Preparation, High Throughput Lab Automation,Food Safety, Mycotoxins, Edible Oils,LC/MS/MS ABSTRACT The manual transfer of liquid samples is part of dailyactivities throughout the analytical laboratory. Theaccurate and precise transfer of liquid samples can becritical to the analytical results. Liquid samples withhigh viscosities pose several challenges to achievingaccurate and precise delivery of desired volumes.Automating the accurate transfer of such viscousliquids would lend support to the quality of theanalytical procedure, help ensure the high quality ofthe resulting data, and free the analyst from performinga tedious manual task. A robotic autosampler commonly used for sampleintroduction in GC or HPLC can be used to performa wide variety of sample preparation techniques. Thesampler can be configured as part of a GC or LCsystem or as a stand-alone bench-top workstation. Ananalytical balance can be included to provide weightverification of liquid transfers. In this report, a new heated liquid syringe toolthat allows viscous liquid samples to be accuratelytransferred is described and its performance examined.Resulting weight verification data from the performanceassessment for example edible oil samples areprovided. Good accuracy and precision for transferringviscous samples is demonstrated. Data is provided to demonstrate that the new heated liquid syringetool enables completely automated extraction ofmycotoxins from edible oils and LC/MS/MS analysisof the extract using a single automated analysis setupunder integrated control software. INTRODUCTION Due to their potential therapeutic or health-promotingproperties, edible oils extracted from plant seeds havegained popularity compared with animal-based fats[1]. However, adverse growth or storage conditionscan lead to fungal growth resulting in contamination.As a result, a major food safety challenge for edibleoils is the presence of mycotoxins, including but notlimited to those produced by Aspergillus Flavus andAspergillus Parasiticus molds, which are among thefungi that produce the toxic secondary metabolitescollectively known as aflatoxins. Several mycotoxinsare known to be human carcinogens. Contaminationof oil seeds by toxigenic molds can lead to the seedsand the oil extracted from the infected seeds becomingunfit for consumption. Mycotoxin levels in food and animal feed areregulated in most countries so there is great interestin a fast, sensitive, and selective analytical method.However, determining mycotoxin concentrationsat trace levels in the presence of large amounts ofviscous oil matrix is a challenging task. The accuracyand precision of the analytical results depend on the extraction and cleanup methods used to isolate themycotoxins from the complex food matrices, but alsoon accurate transfer of amounts of viscous liquids. As a result of this study, we were able to showthat a liquid-liquid extraction of mycotoxins fromedible oil samples, including accurate transfer ofthe initial oil sample, was successfully automatedusing the GERSTEL MPS robotic sampler. Based onthe presented method, analytes can be rapidly andreproducibly isolated from edible oil samples basedon an automated procedure that includes subsequentLC/MS/MS analysis using the Agilent Ultivo triplequadrupole mass spectrometer. EXPERIMENTAL Materials. A stock solution containing aflatoxins B1,B2, G1, and G2 was purchased from Romer Labs(Biopure, Mycotoxin Mix 1,002021).Ahigh calibrationstandard was prepared by making appropriate dilutionsof the mycotoxin mix stock solution using (1:1)water: methanol. Calibration standards were thenprepared using a dilution ratio strategy from the highconcentration sample of 1:2:2:2:2.5:2:2. Extra virgin olive oil (cold pressed), sesame oil(pure),flax oil (organic, pure, unrefined, cold pressed),and sunflower oil (virgin, cold pressed), were purchasefrom local markets. A range of aflatoxin spiked edibleoil samples were prepared by making appropriatedilutions of the mycotoxin mix stock solution. A (95:5) acetonitrile: formic acid (v:v) extractionsolution was prepared by combining 190 mL ofacetonitrile (Sigma Aldrich, 34998) with 10 mLof formic acid (Sigma Aldrich, 695076). All otherreagents and solvents used were reagent grade. Instrumentation. All automated PrepSequences wereperformed using a GERSTEL MPS robotic/roboticPROdual head sampler with the GERSTEL CF-200centrifuge, balance (weighing option) , mVAP, quickMIX, 5-position dilutor option, a heated agitator, andthe GERSTEL Heated Liquid Syringe Module (HLM)as shown in Figure 1. All subsequent analyses wereperformed using an Agilent 1260 HPLC, outfittedwith an Agilent Poroshell 120 EC-C18 column (3.0x 50 mm, 2.7 um), coupled to an Agilent Ultivotriple quadrupole mass spectrometer with jet streamelectrospray source. Sample injections were madeusing a GERSTEL roboticPRO sampler with the LCMStool into a 6 port (0.25 mm) Cheminert C2V injectionvalve outfitted with a 2 uL stainless steel sample loop. Figure 1. MPS robotic/roboticPRo sampler configuredwith GERSTEL automated sample preparationoptions. Automated Prep Sequence. A manual method forliquid-liquid extraction of mycotoxins from edible oils[2] was automated using the MPS robotic/roboticPROdual head sampler. The automated steps performed arelisted below (steps 2-13). 1, User places edible oil sample into a 10 mL vialand places the vial onto the MPS. 2..。MPS moves vial into incubator at 60°C for 10minutes. 3 MPS transfers 1.5 mL of the edible oil sampleusing HLM (at 65°℃) into an empty 10 mL vial. 4. MPS adds 7.5 mL (95:5) acetonitrile: formic acid(v/v). 5. MPS mixes the vial content by agitation for 10minutes at 2000 rpm. 65MPS centrifuges vial for 5 minutes at 2000 g.77 MPS transfers 4 mL of supernatant into aclean,empty, 10 mL vial. 83MPS adds 4 mL hexane.99 MPS mixes the vial content by agitation for 10minutes at 2000 rpm. 10. MPS centrifuges vial for 5 minutes at 2000 g. 11. MPS transfers 2.5 mL of the lower layer into anempty, round bottom, 4 mL vial. 12. MPS evaporates the extract to dryness at 45C. 13. MPS reconstitutes using 500 pL (1:1) methanol:water. 14. MPS injects (or, optionally, filters through a 0.2um filter then injects) the reconstituted extract intothe LC-QQQ. Analysis conditions LC Pump: gradient (800 bar),flowrate =0.2 mL/min Mobile Phase: A -0.1% formic acid in waterB -0.1% formic acid in methanol Gradient: Run time: Injection volume: 2.0 uL (loop over-fill technique)Column temperature: 30℃ Analysis conditions MS Operation: electrospray positive mode Gas temperature: 250°C Gas flow (N,): 8 L/min Nebulizer pressure:30 psi Sheath gas heater: 350°C Sheath gas flow (N,): 11 L/min Capillary voltage: 4000V Nozzle voltage: 500 V Delta EMV: 0V Mass spectrometer acquisition parameters are shownin Table 1 with qualifier ions. Table 1. Mass spectrometer acquisition parameters. CompoundName Precursorion[m/z1 ProductIon [m/z] Frag.Voltage [V] CollisionEnergy[V] Aflatoxin G2 331.1 313.1 115 190 190 24 80 Aflatoxin G1 329.1 311.1 243.1 180 180 20 28 Aflatoxin B2 315.1 287.1 259.1 190 190 24 28 Aflatoxin B1 313.3 285.1 241.1 190 190 20 40 RESULTS AND DISCUSSION Raising the temperature of a viscous sample decreasesits viscosity. The ability to control the temperature ofboth the sample and the syringe being used to transferthe sample is important in order to achieve reliableand accurate transfer of viscous samples using syringebased autosamplers. Figure 2 shows how increasingthe temperature of propylene glycol, (cP=42 at 27°C)leads to an improvement in transfer volume accuracyfor the viscous liquid standard. Figure 2.% Accuracy ofpropylene glycol transferusing the GERSTEL Heated Liquid Syringe Module. Identical volumes of olive oil (cP=40 at38°C), sesameoil (cP=41 at 35℃), flax oil (cP=29 at 38°C) andsunflower oil (cP=49 at25°℃) were placed into theheated agitator at 60°C for 10 minutes and replicatealiquots of each were then transferred to individualvials using the Heated Liquid Syringe Module (65°C).Table 2 shows the resulting precision and accuracy datafor replicate transfers of each edible oil. Table 2. Precision and accuracy ofedible oiltransfer using the Heated Liquid Syringe Module. Replicate Olive Oil[g] SesameOil[g] FlaxOil [g] Sunflower Oil[g] 1 1.3199 1.3305 1.343 1.3281 2 1.3192 1.3298 1.3431 1.3298 3 1.3189 1.3312 1.3438 1.3301 4 1.3180 1.3276 1.3439 1.3284 mean 1.3190 1.3298 1.3435 1.3291 SD 0.000787 0.00156 0.000465 0.000997 % CV 0.0597 0.1172 0.0346 0.0750 % Diff from The0. -5.45 -4.68 -3.70 -4.72 Figures 3a and b show representative stacked masschromatograms resulting from extracted samples offlax oil (a) and sunflower oil (b) spiked with mycotoxinat concentrations of 10 ng/mL(Aflatoxins B1,G1) and2.5 ng/mL (Aflatoxins B2, G2). Figure 3a. Representative stacked mass chromatograms resulting from extraction of aflatoxinspiked flax oil. Figure 3b.Representative stacked masschromatograms resulting from extraction ofaflatoxinspiked sunflower oil. As shown in Table 3, the ability to accurately andreproducibly transfer edible oil samples during theautomated extraction procedure leads to reproducibledata for the replicate extracts of olive oil spiked withmycotoxins. Table 3. Precision of mycotoxin extractions fromolive oil using automated extractionprocedure. Response: Aflatoxin Aflatoxin Aflatoxin Aflatoxin B1 B2 G1 G2 Olive Oil 1 260 120 288 46 Olive Oil 2 251 108 287 45 Olive Oil 3 239 124 282 43 Olive Oil 4 240 116 259 43 mean 243 116 276 44 SD 6.51 8.00 14.6 1.04 %CV 2.68 6.90 5.30 2.40 A representative calibration curve for Aflatoxin B1is shown in Figure 4. Regression analysis for allmycotoxin compounds analyzed within this methodresulted in R² values of 0.999 or greater. Figure 4. Representative calibration curve forAflatoxin B1. The recovery of mycotoxins using the automatedextraction was assessed by comparing the resultsobtained from mycotoxin spiked edible oil sampleswith those resulting from spiking the extracts of blankedible oil samples after the extraction. Table 4 showsthe resulting %recovery ofeach mycotoxin from eachedible oil examined. Table 4. %Recovery ofmycotoxins from edible oilsusing the automated extraction procedure. % Recovery AflatoxinB1 AflatoxinB2 AflatoxinG1 AflatoxinG2 Olive Oil 95.9 103 95.7 129 Sesame Oil 83.8 93.7 92.1 131 Flax Oil 87.7 104 88.0 126 Sunflower Oil 84.3 101 90.2 101 Ave.% Recovery 87.9 101 91.5 122 CONCLUSIONS As a result of this study, we were able to show: ● An extraction procedure for mycotoxins in edibleoils was readily automated using the GERSTELMPS roboticPRO sampler, including introductionof the extract to LC/MS/MS and analysisbased on Agilent Ultivo triple quadrupole massspectrometer. Viscous edible oil samples can be transferredaccurately and precisely using the GERSTELHeated Liquid Syringe Module. Mycotoxins can be reproducibly extracted fromedible oil samples using an automated extractionprocedure with an average precision of 4.32 %(range: 2.40%-6.90%RSD). The recovery of mycotoxins from edible oilsamples using the automated extraction procedureand LC/MS/MS analysis averaged 101 % (range:87.9%-122%). REFERENCEs [1] Bhat,R and Reddy,K.R.N,“Challenges and issuesconcerning mycotoxins contamination in oil seedsand their edible oils: Updates from last decade.",Food Chemistry, 215 (2017),425-437. [2] Eom, T., et al.,“Multiclass mycotoxin analysis inedible oils using a simple solvent extraction methodand liquid chromatography with tandem massspectrometry.",Food Additives & Contaminants:Part A, 2017,Vol. 34, No. 11,2011-2022. GERSTEL GmbH & Co. KG Eberhard-Gerstel-Platz 1 45473 Mulheim an der Ruhr Germany +49(0)208-7 65 03-0 +49 (0)208-7 6503 33 gerstel@gerstel.com www.gerstel.com GERSTEL Worldwide GERSTEL LLP Information, descriptions and specifications in this Publication are subject to change without notice.GERSTEL,GRAPHPACK and TWISTER are registered trademarks of GERSTEL GmbH & Co.KG. -- -- 黄曲霉毒素黄曲霉毒素(AFT)是黄曲霉和寄生曲霉等某些菌株产生的双呋喃环类毒素,属于真菌毒素。其衍生物有约20种,分别命名为B1、B2、G1、G2、M1、M2、GM、P1、Q1、毒醇等。其中以B1的毒性最大,致癌性最强。1993年,黄曲霉毒素被世界卫生组织(WHO)癌症研究机构划定为一类天然存在的致癌物,是毒性极强的剧毒物质。黄曲霉毒素主要污染粮油及其制品,各种植物性与动物性食品也能被污染。世界各地都对食品和动物饲料中的霉菌毒素水平进行了规定,因此人们对快速,灵敏和选择性的分析方法产生了极大的兴趣。但是困难在于,存在大量粘性油基质的情况下确定痕量水平(PPb水平)的霉菌毒素浓度是一项艰巨的任务。分析结果的准确性和精确度取决于用于从复杂食品基质中分离霉菌毒素的提取和净化方法,还取决于准确转移粘性液体的量。哲斯泰自动化解决方案哲斯泰有好方法:使用自动化液液萃取从食用油样品中提取霉菌毒素,然后使用LC-MS/MS来检测。以下示意图就是我们的整体解决方案。应用文献可以点击原文阅读来获取。哲斯泰自动化提取食用油中霉菌毒素的样品前处理平台。关键模块:快速振荡机、多位真空蒸发站、天平、离心机、孵育搅拌器、2米双头样品前处理平台。分析设备:配备Agilent Poroshell 120 EC-C18色谱柱(3.0 x 50 mm,2.7μm)的 Agilent 1260 HPLC与配有喷射流电喷雾源的Agilent Ultivo三重四极杆质谱仪联用。HPLC是近几年发展起来的检测黄曲霉毒素的方法,主要是用荧光检测器检测。黄曲霉毒素经柱后电化学衍生化后,能发射特征性荧光,被荧光检测器捕获后而得到检测。自动化流程:将食用油样品放入10 mL小瓶中,然后将小瓶放在MPS上(唯一手动步骤)将小瓶移至60°C的培养箱中10分钟使用加热针(在65°C下)将1.5 mL食用油样品转移到一个空的10 mL小瓶中添加7.5 mL(95:5)乙腈:甲酸(v / v)搅拌10分钟 (2000 rpm)离心5分钟 (2000 g)将4 mL上清液转移到干净的空10 mL小瓶中添加4 mL己烷搅拌10分钟(2000 rpm)离心5分钟(2000 g)将2.5 mL的下层样品转移到一个空的,圆底的4 mL样品瓶中将提取物在45°C蒸发至干添加500μL(1:1)甲醇:水重构。将重构的提取物注入LC(或可选地通过0.2μm过滤器进行注入)。自动化分析结果为了验证自动化结果的准确性,我们使用四种黄曲霉毒素标样B1、B2、G1、G2来验证。以下是四种目标化合物的质谱分析的采集参数。自动化方法的线性非常好,黄曲霉毒素的校正曲线的R2 大于0.999,下图为B1的标准曲线。下图为添加了10 ng/mL (Aflatoxins B1, G1) 和2.5 ng/mL (Aflatoxins B2, G2)的亚麻籽油的色谱图,峰形好并且信噪比理想。在四种食用油的样品中,四种黄曲霉毒素的平均加标回收率为89.7%、101%、91.5% 和 122%。自动化的可重复性高,4次重复检测的结果RSD % (%CV)小于6.90 %。哲斯泰的解决方案总结:高效准确的自动提取食用油中的霉菌毒素结合LC-MS/MS分析可重复性高,平均RSD为 4.32% 四种黄曲霉毒素的平均回收率为101%线性高,R2 大于 0.999黄曲霉毒素的最高允许含量:联合国世界卫生组织(WHO)和粮农组织(FAO)1975年规定食品中Aflatoxin B1的最高允许含量为15 µg/kg。欧洲法规的最高允许含量:Aflatoxin B1: 8.0 µg/kg (花生), 0.1 µg/kg (婴儿食品); Aflatoxins B1, B2, G1 and G2总量: 15 µg/kg (花生), 4.0 µg/kg (粮食)
确定



还剩4页未读,是否继续阅读?
GERSTEL(哲斯泰)为您提供《食用油中真菌毒素检测方案(样品前处理)》,该方案主要用于食用植物油中真菌毒素检测,参考标准--,《食用油中真菌毒素检测方案(样品前处理)》用到的仪器有GERSTEL自动进样器 MPS robotic
推荐专场
相关方案
更多
该厂商其他方案
更多