方案详情
文
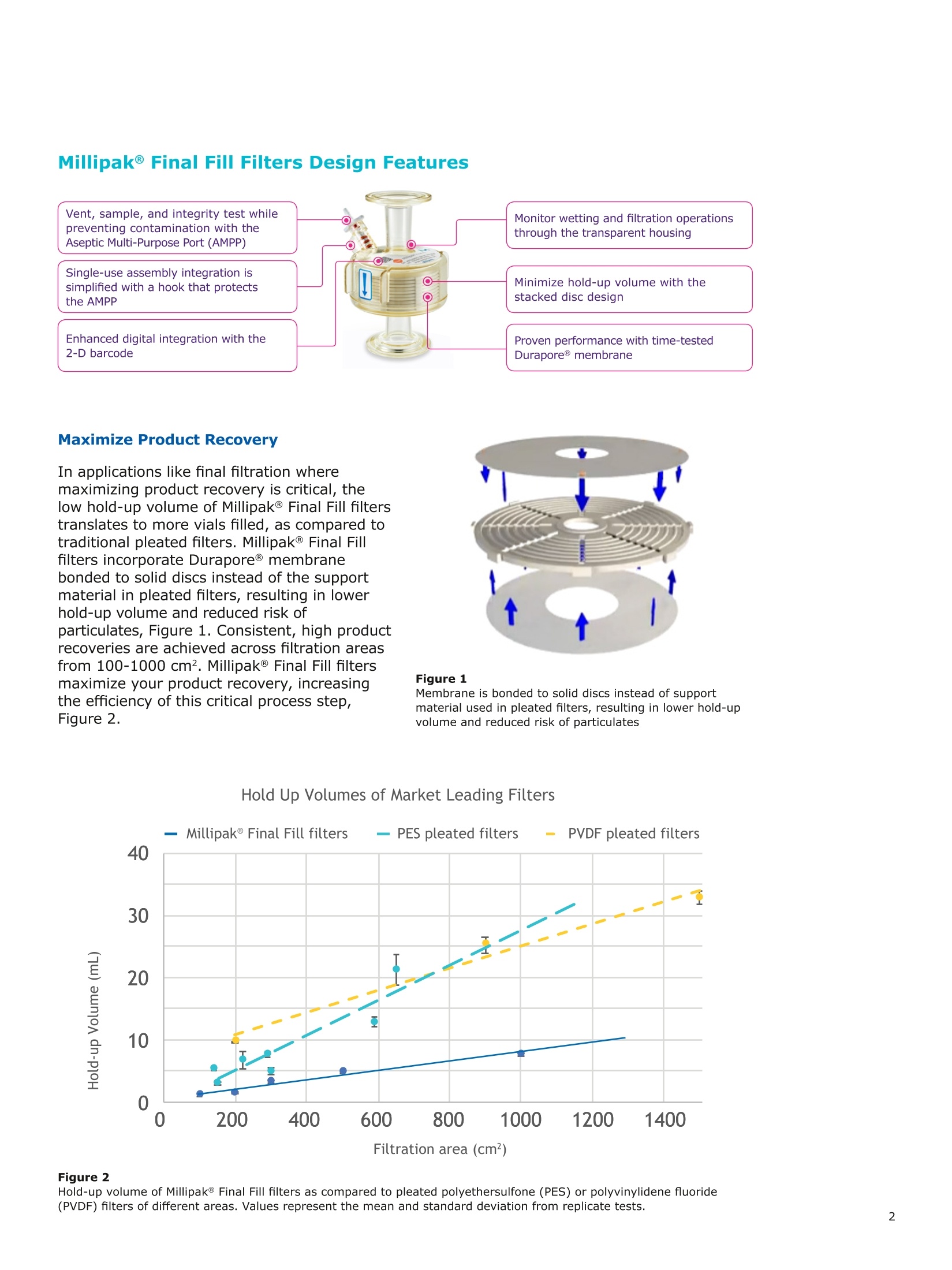
Millipak® Final Fill过滤器的特殊层叠式设计可最大程度地减少残留体积,从而增加产品回收。过滤器吹扫亦可有效降低折叠膜和层叠式过滤器使用后的残留量。
方案详情

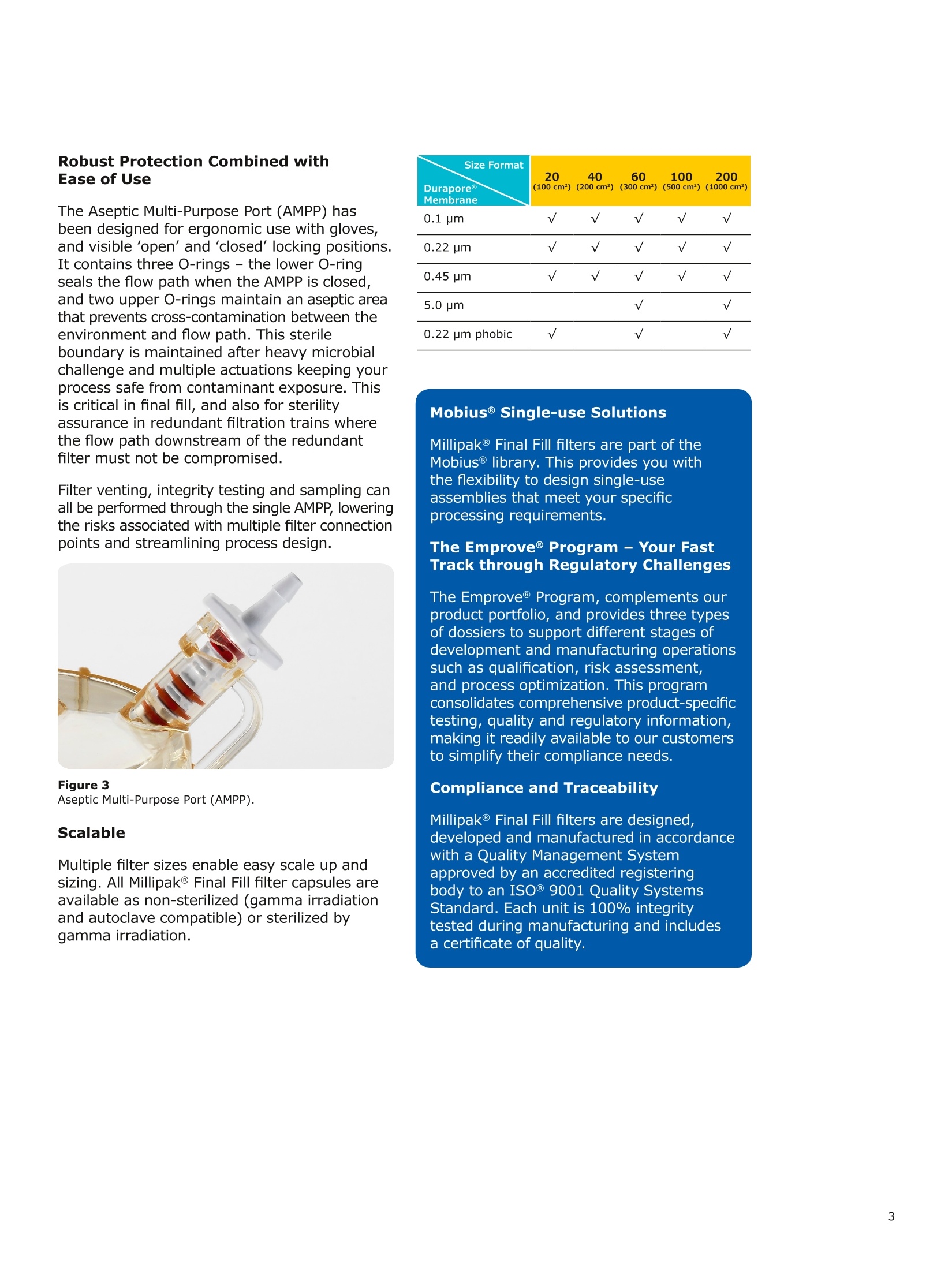
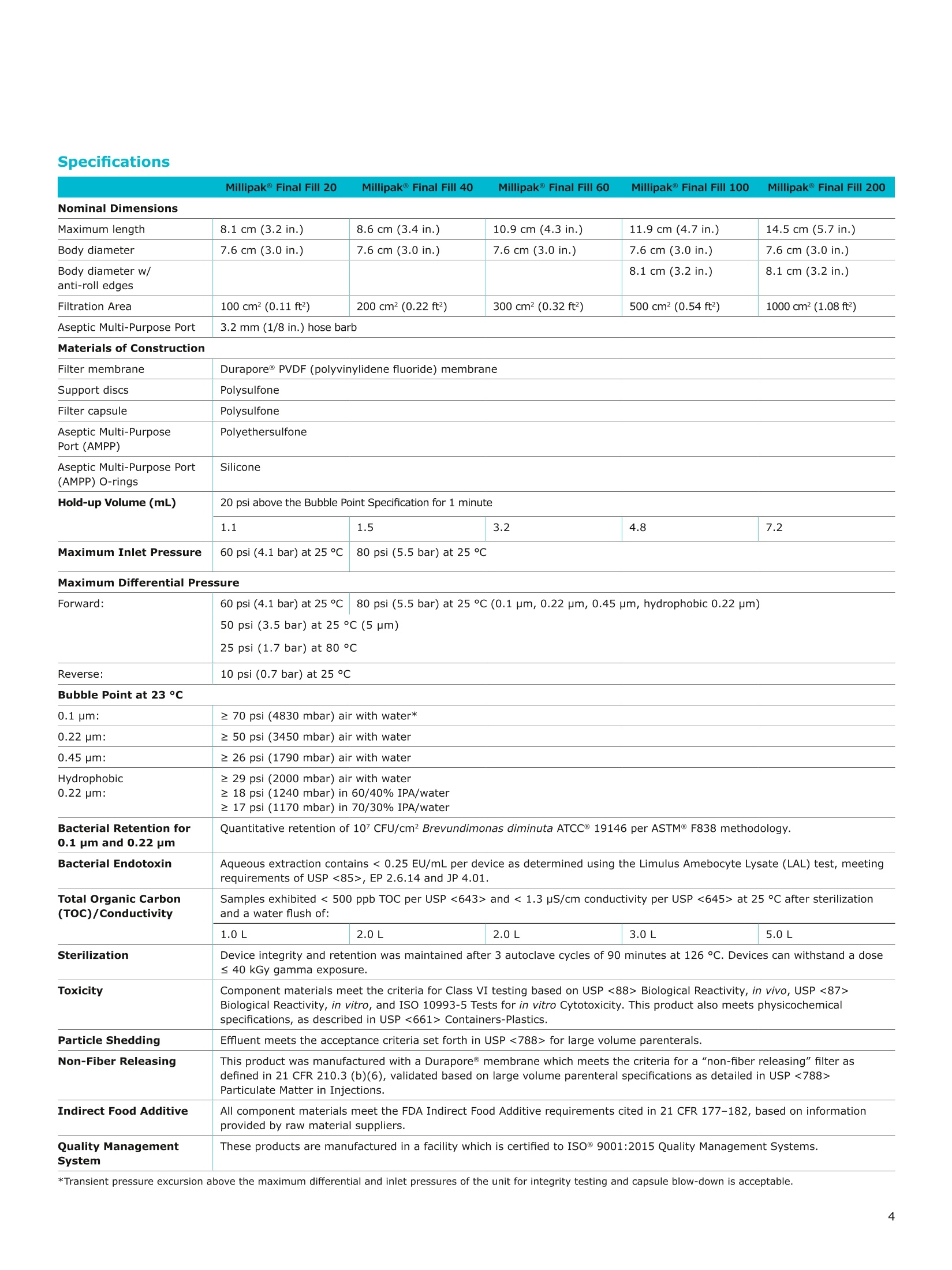
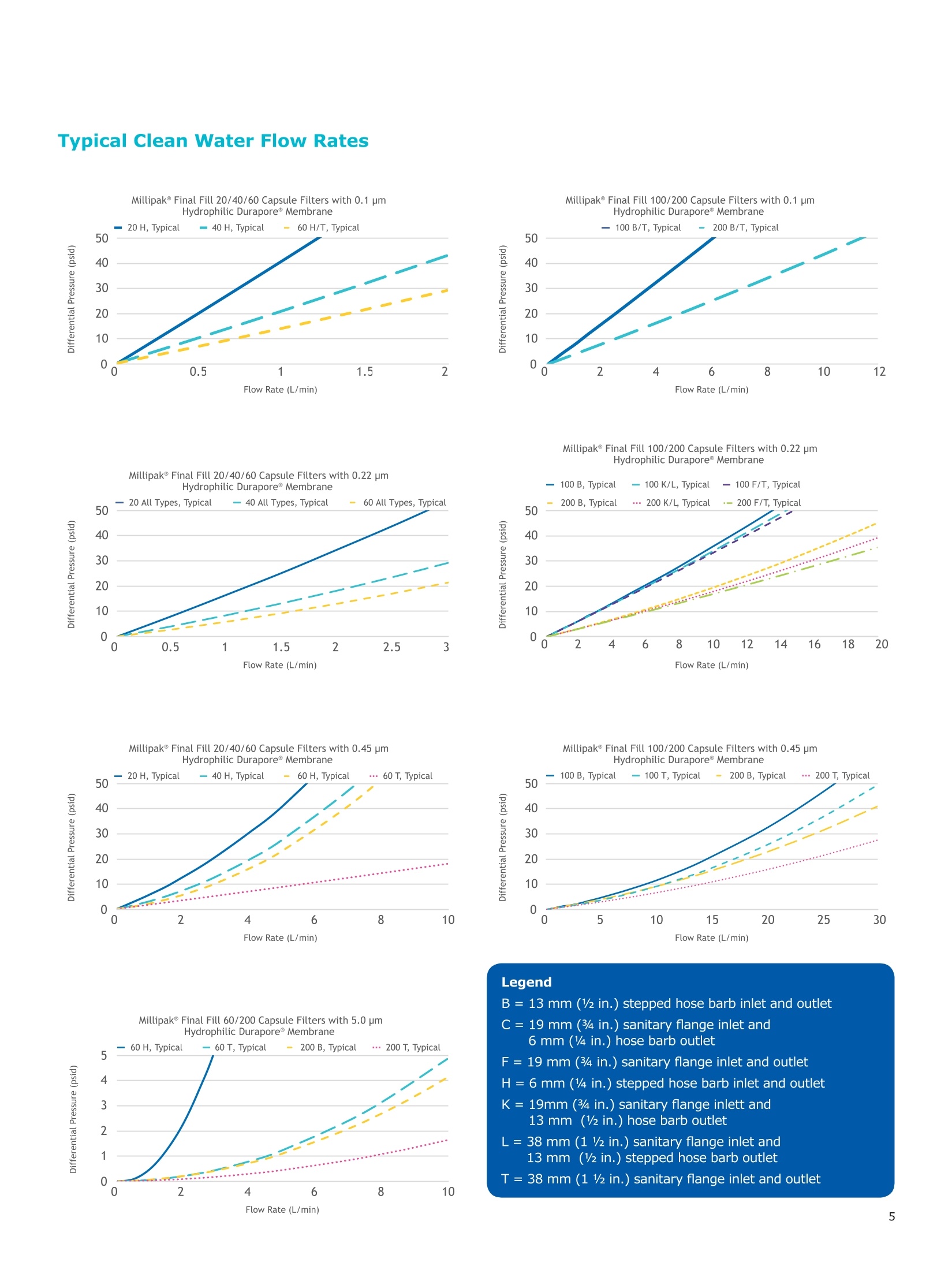
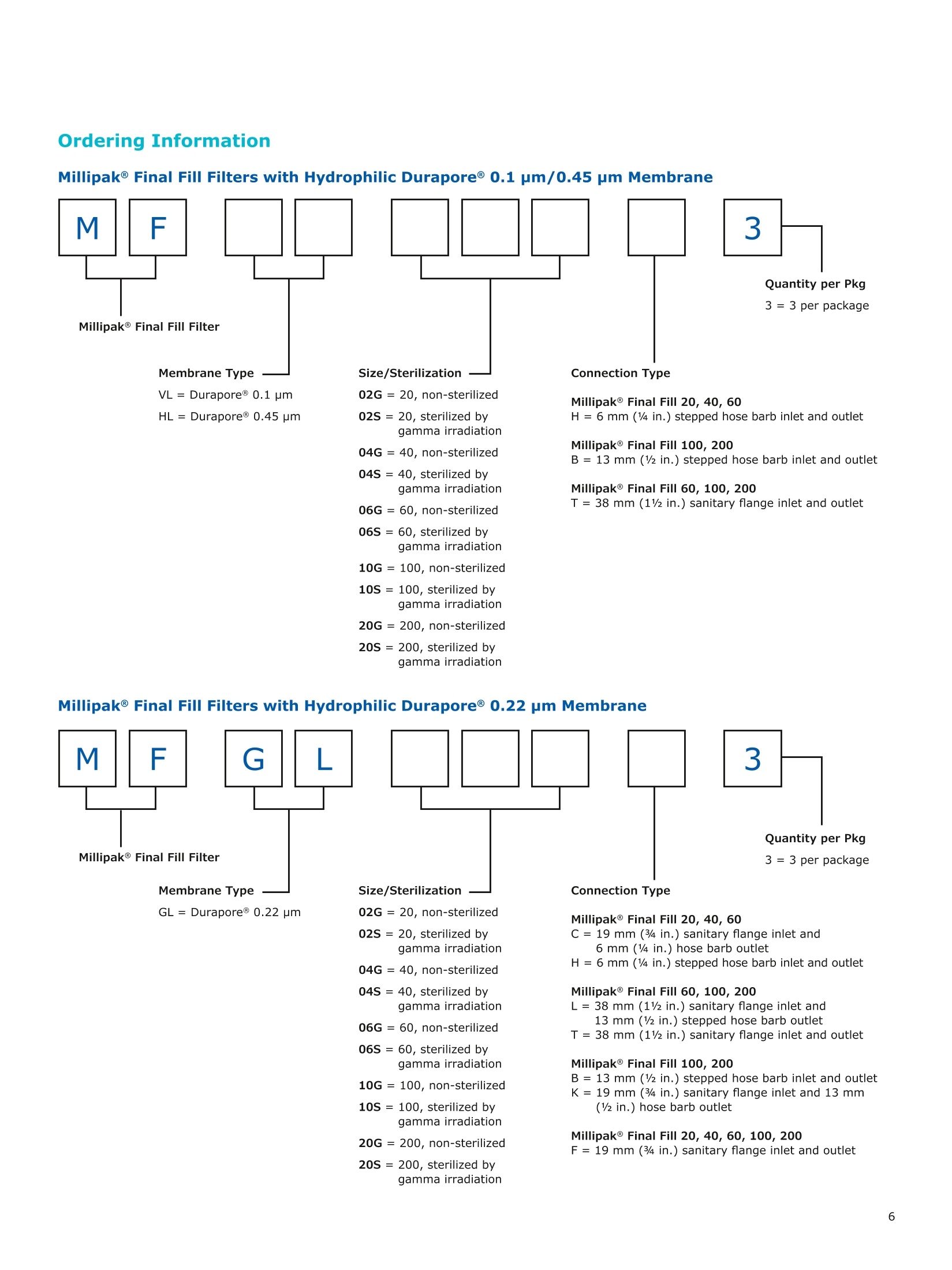
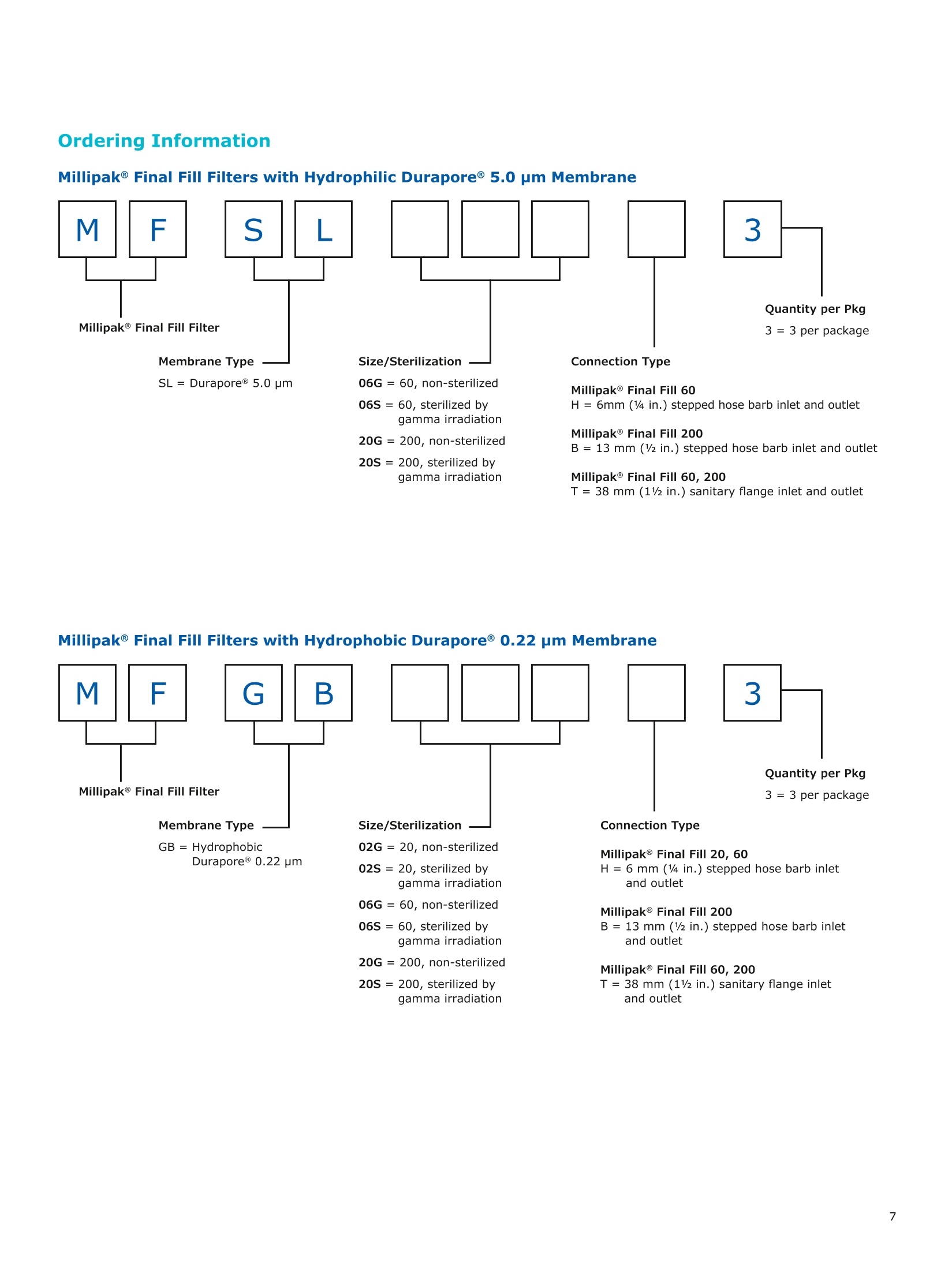
MilliporeaPreparation, Separation,Filtration & Monitoring Products Benefits ·Maximizes product recovery in final and highvalue filtration ·Simplifies operation and reduces risk of microbialand particulate contamination ·Contains Durapore@ membrane for high flowrates, low binding and extractables, and broadchemical compatibility · Improves integration into single-use assemblies Membrane Pore Sizes Available with particulate removal, bioburdenreduction and sterilizing-grade Durapore@polyvinylidene fluoride (PVDF) membranesfor both liquid and solvent applications. · Hydrophilic Durapore@ membrane: 0.1 pm,0.22 pm, 0.45 pm, 5.0pm Maximize Product Recovery In applications like final filtration wheremaximizing product recovery is critical, thelow hold-up volume of Millipak Final Fill filterstranslates to more vials filled, as compared totraditional pleated filters. Millipak Final Fillfilters incorporate Durapore@ membranebonded to solid discs instead of the supportmaterial in pleated filters, resulting in lowerhold-up volume and reduced risk ofparticulates, Figure 1. Consistent, high productrecoveries are achieved across filtration areasfrom 100-1000 cm². Millipak Final Fill filtersmaximize your product recovery, increasingthe efficiency of this critical process step,Figure 2. Figure 1 Membrane is bonded to solid discs instead of supportmaterial used in pleated filters, resulting in lower hold-upvolume and reduced risk of particulates Hold Up Volumes of Market Leading Filters 工 Filtration area (cm²) Hold-up volume of Millipak Final Fill filters as compared to pleated polyethersulfone (PES) or polyvinylidene fluoride(PVDF) filters of different areas. Values represent the mean and standard deviation from replicate tests. Robust Protection Combined withEase of Use The Aseptic Multi-Purpose Port (AMPP) hasbeen designed for ergonomic use with gloves,and visible 'open'and 'closed'locking positions.It contains three O-rings - the lower O-ringseals the flow path when the AMPP is closed,and two upper O-rings maintain an aseptic areathat prevents cross-contamination between theenvironment and flow path. This sterileboundary is maintained after heavy microbialchallenge and multiple actuations keeping yourprocess safe from contaminant exposure. Thisis critical in final fill, and also for sterilityassurance in redundant filtration trains wherethe flow path downstream of the redundantfilter must not be compromised. Filter venting, integrity testing and sampling canall be performed through the single AMPP, loweringthe risks associated with multiple filter connectionpoints and streamlining process design. Figure 3 Aseptic Multi-Purpose Port (AMPP). Scalable Multiple filter sizes enable easy scale up andsizing. All Millipak Final Fill filter capsules areavailable as non-sterilized (gamma irradiationand autoclave compatible) or sterilized bygamma irradiation. Size Format 20 40 60 100 200 Durapore (100 cm²) (200 cm²)(300 cm²) (500 cm²) (1000 cm²) Membrane 0.1 pm √ √ √ √ √ 0.22 pm √ √ √ √ √ 0.45 pm √ √ √ √ √ 5.0 pm √ √ 0.22 pm phobic √ √ √ Mobius@ Single-use Solutions Millipak Final Fill filters are part of theMobius@ library. This provides you withthe flexibility to design single-useassemblies that meet your specificprocessing requirements. The Emprove@ Program - Your FastTrack through Regulatory Challenges The Emprove@ Program, complements ourproduct portfolio, and provides three typesof dossiers to support different stages ofdevelopment and manufacturing operationssuch as qualification, risk assessment,and process optimization. This programconsolidates comprehensive product-specifictesting, quality and regulatory information,making it readily available to our customersto simplify their compliance needs. Compliance and Traceability Millipak Final Fill filters are designed,developed and manufactured in accordancewith a Quality Management Systemapproved by an accredited registeringbody to an ISO 9001 Quality SystemsStandard. Each unit is 100% integritytested during manufacturing and includesa certificate of quality. Specifications Millipak" Final Fill 20/40/60 Capsule Filters with 0.1 pm Millipak Final Fill 20/40/60 Capsule Filters with 0.22 pmHydrophilic Durapore@ Membrane Millipak Final Fill 20/40/60 Capsule Filters with 0.45 um Millipak Final Fill 60/200 Capsule Filters with 5.0 pmHydrophilic Durapore@ Membrane Millipak" Final Fill 100/200 Capsule Filters with 0.1 pmHydrophilic Durapore Membrane Millipak Final Fill 100/200 Capsule Filters with 0.22 pmHydrophilic Durapore@ Membrane Millipak Final Fill 100/200 Capsule Filters with 0.45 pm Legend B = 13 mm (V2 in.)stepped hose barb inlet and outlet C = 19 mm (34 in.) sanitary flange inlet and 6 mm (14 in.) hose barb outlet F = 19 mm (34 in.) sanitary flange inlet and outlet H = 6 mm (14 in.) stepped hose barb inlet and outlet K = 19mm (34 in.) sanitary flange inlett and 13 mm (V2in.) hose barb outlet L= 38 mm (1 V2 in.) sanitary flange inlet and 13 mm (V2 in.) stepped hose barb outlet T=38 mm (1 V2in.) sanitary flange inlet and outlet Millipak Final Fill Filters with Hydrophilic Durapore@ 0.22 pm Membrane M F G L 3 Quantity per Pkg Millipak Final Fill Filter 3 = 3 per package Connection Type Millipak Final Fill 20, 40, 60 C = 19 mm (34 in.) sanitary flange inlet and 6 mm (14 in.) hose barb outlet H = 6 mm (14 in.) stepped hose barb inlet and outlet Millipak Final Fill 60, 100, 200 L= 38 mm (1V2 in.) sanitary flange inlet and 13 mm (Vz in.) stepped hose barb outlet T=38 mm (1V2 in.) sanitary flange inlet and outlet Millipak Final Fill 100, 200 B=13 mm (Vz in.) stepped hose barb inlet and outlet K = 19 mm (34 in.) sanitary flange inlet and 13 mm (V2 in.) hose barb outlet Millipak Final Fill 20, 40, 60, 100, 200 F = 19 mm (34 in.) sanitary flange inlet and outlet Ordering Information Millipak Final Fill Filters with Hydrophilic Durapore@5.0 pm Membrane Millipak Final Fill Filters with Hydrophobic Durapore@ 0.22 pm Membrane Membrane Type GB= HydrophobicDurapore@ 0.22 pm Merck KGaAFrankfurter Strasse 25064293 Darmstadt Germany MeRCKThe life science business of Merck operates asMilliporeSigma in the U.S.and Canada Figure 对一系列市售包含折叠膜的PES和PVDF除菌级囊式过滤器的残留体积进行对比。在重力排空或使用超出泡点20psi的压力吹扫1分钟后,将折叠膜囊式过滤器的残留体积与Millipak® Final Fill过滤器进行比较。在所有测试中,残留量是排干的过滤器与过滤器干重之间的重量差。
确定


还剩6页未读,是否继续阅读?
默克工艺解决方案为您提供《高附加值的注射剂产品中Millipak® Final Fill过滤器及吹扫步骤达到最大化产品收率检测方案(其它生化设备)》,该方案主要用于其他中Millipak® Final Fill过滤器及吹扫步骤达到最大化产品收率检测,参考标准--,《高附加值的注射剂产品中Millipak® Final Fill过滤器及吹扫步骤达到最大化产品收率检测方案(其它生化设备)》用到的仪器有
相关方案
更多








