方案详情
文
对通过烘烤和研磨核仁获得核桃糊,并辅以葡萄皮提取物,以评估其对氧化稳定性和/或抗氧化能力的潜在影响。
方案详情

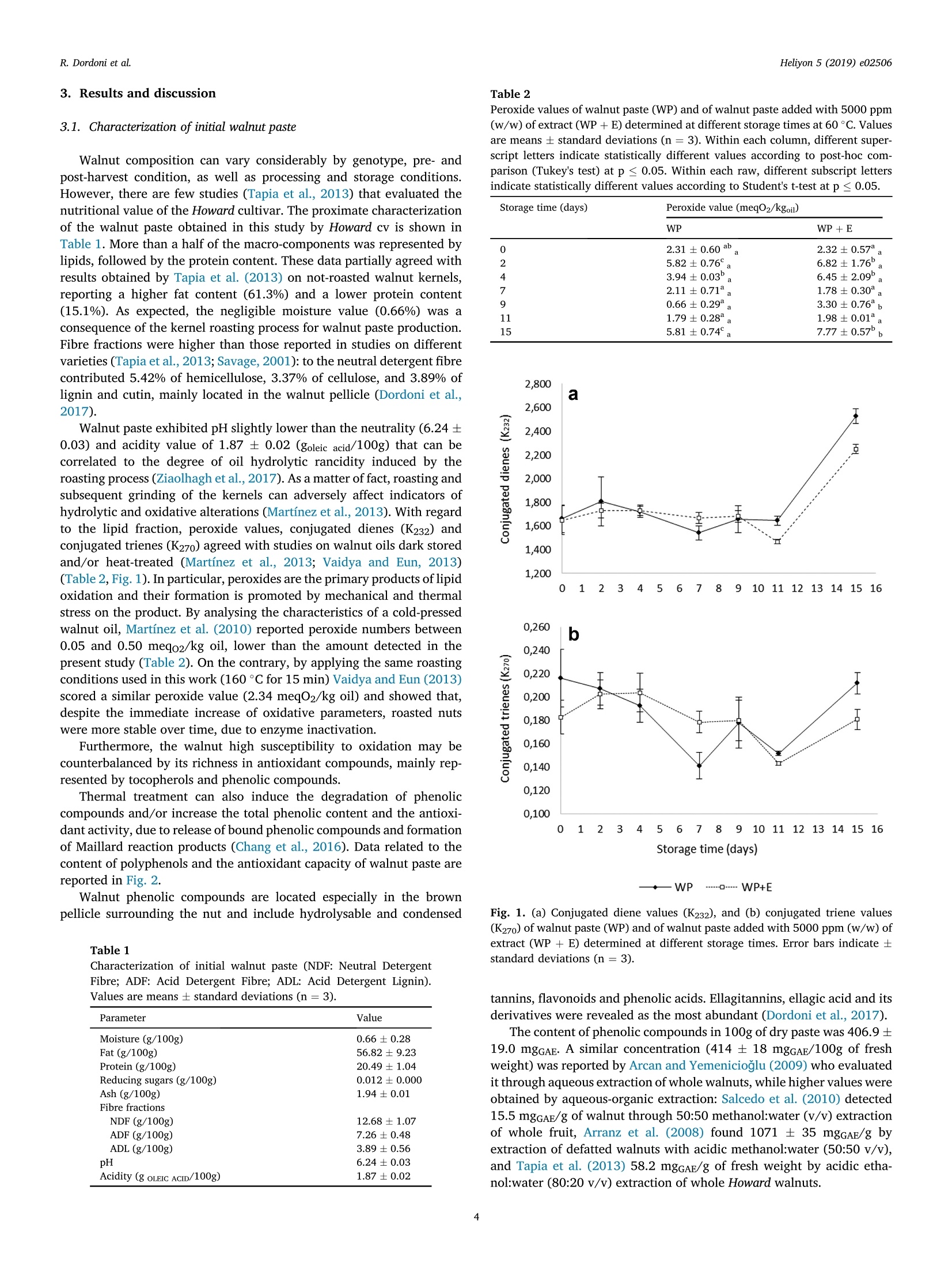
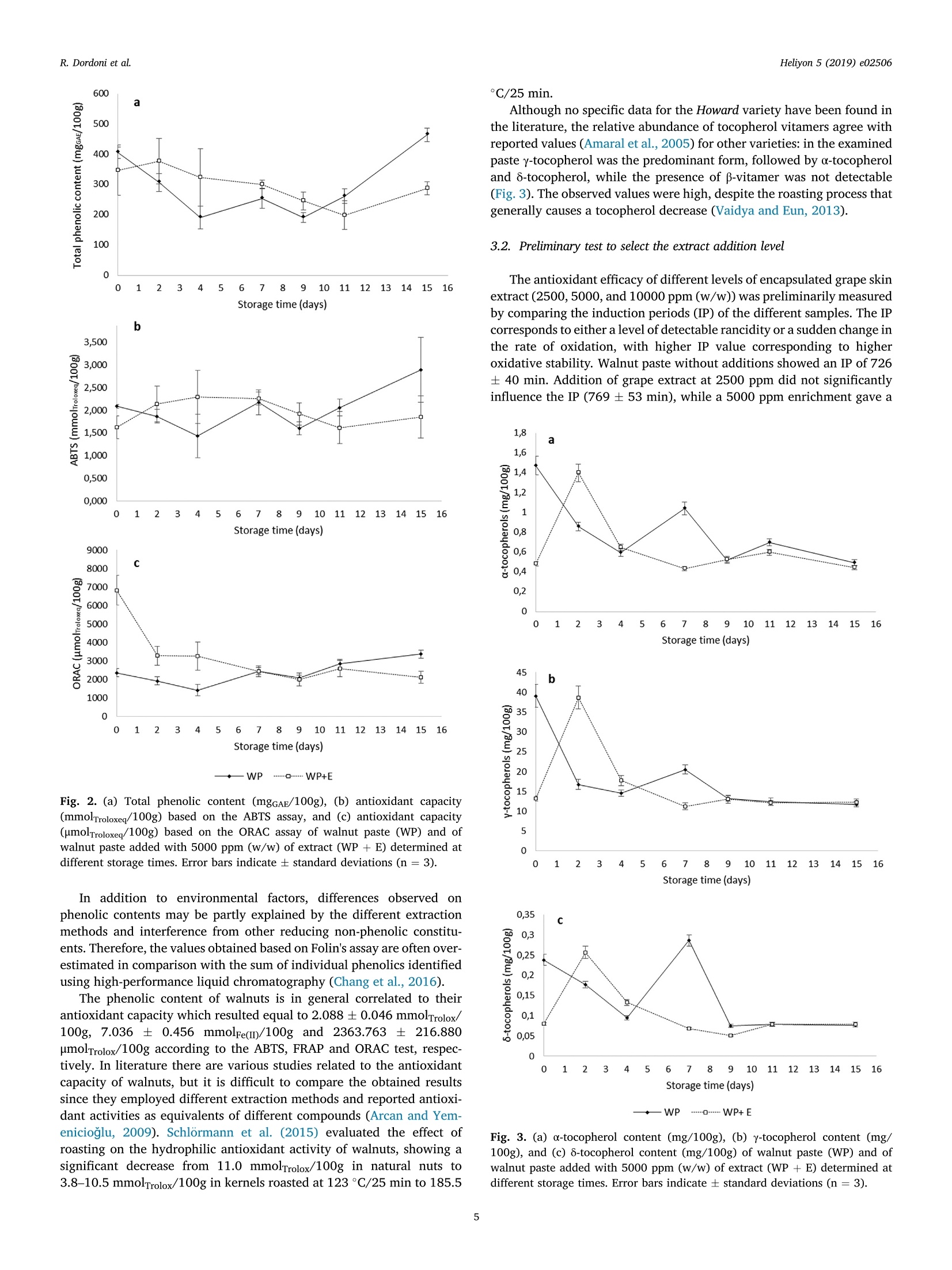
Heliyon 5 (2019) e02506Contents lists available at ScienceDirectjournal homepage: www.heliyon.com R. Dordoni et al.Heliyon 5 (2019)e02506 Heliyon Heliyo n Walnut paste: oxidative stability and effect of grape skin extract addition Roberta Dordoni*, Silvia Cantaboni, Giorgia Spigno Department for Sustainable Food Process, Universita Cattolica del Sacro Cuore, Via Emilia Parmense 84, 29122, Piacenza, Italy ARTICLEINFO A BSTRACT Keywords: Food technology Food processing Food analysis Food composition Food chemistry Walnut paste Grape skin extract Oxidative stability Antioxidant capacity Accelerated storage test Walnut paste, obtained by roasting and grinding of kernels, was characterized and supplemented with encap-sulated grape skin extract aiming to evaluate its potential effect on oxidative stability and/or antioxidant capacity.Based on the oxidation induction period in screening trials 5000 ppm (w/w) extract addition was selected aseffective in inhibiting oxidation processes. Walnut paste with and without 5000 ppm grape skin extract weremaintained for 15 days at 60 °C, simulating 2 year storage at 20 °C, based on an estimated activation energy of80,327 kJ/mol for walnut lipid oxidation. Monitoring of data from peroxides, conjugated dienes and trienes, totalphenolics, ABTS,ORAC, FRAP, and tocopherols values showed the deterioration of walnut paste started at the endof the observed period, even remaining below the threshold of unacceptability. Moreover, 5000 ppm extractaddition did not prove to enhance oxidative stability nor antioxidant properties of the walnut paste. In the future,specific parameters of oxidation kinetics and antioxidant activity in the advanced phase of storage could beinvestigated. 1. Introduction Widely cultivated and consumed all around the world, walnuts haverecently aroused great interest as a promising natural functional food. Infact, several studies demonstrated walnut role in preventing neurode-generative disorders and the inversely correlation between their con-sumption and the occurrence of cardiovascular diseases. Healthproperties of walnuts are related to their chemical composition: since,among nuts, they are the richest in polyunsaturated fatty acids (PUFA),with a favourable n6: ω3 ratio, and show the highest antioxidant capacitydue to their content of polyphenols. They also include other importantbioactive compounds such as melatonin, serotonin, minerals and vita-mins (especially tocopherols) (Tapia et al., 2013; Martinez et al., 2010). Walnuts are commonly commercialized as shelled or unshelled (aswhole or ground kernels), but they also can be processed to obtain in-gredients such as walnut oil, flour and paste. The major constituents ofkernels are lipids (62-74%), with relatively high contents of linoleic acid(~63%) and linolenic acid (~13%), notably susceptible to oxidativedegradation resulting in rancidity development and shorter shelf life.Walnut oil has a low oleic to linoleic acid ratio, ranging between 0.08 and0.57, being therefore highly unstable compared with other plant oils(Gama et al., 2018; Martinez et al., 2010). As known, oxidation rate is correlated to temperature according toArrhenius law (Abedi Gonabad et al., 2015). Ling et al. (2014) performed an accelerated shelf life test on walnut paste, showing that a storage at 35°C for 20 days simulated approximately two years at +4°C or two monthsat room temperature (25C). Furthermore, several researches werecarried out to study the effect of packaging and storage conditions onshelf life of walnut kernels (Bakkalbasi et al., 2012) and oil, stating thatoxidation also depends on both endogenous (walnut variety, activity oflipolytic enzymes) and environmental factors (lightning conditions,temperature, humidity, oxygen concentration, presence of metals). Oxidative stability of food products can also be strongly affected bytechnological processes. Roasting is extensively applied on nut fruits withthe aim of improving both sensory properties and shelf life. After roast-ing, walnuts acquire characteristic colour, favourable taste and flavour,as well as delicate and crispy texture. Moreover, roasted walnuts show alonger shelf life because of water evaporation, lipoxygenase inactivation(Buranasompob et al., 2007), greater retention of tocopherols (Vaidyaand Eun, 2013) and higher antioxidant activity (Vinson and Cai, 2012). According to Arranz et al. (2008), the major contributors to antioxi-dant capacity of walnuts are polyphenols, mainly hydrolysable ellagi-tannins located in their pellicle. The phenolic content of kernels has beeninvestigated by several authors, revealing that it is affected by extractionmethodologies (solvents, temperatures and times), cultivar, harvest year,orchard location, processing steps and storage (Tapia et al., 2013; Arranzet al., 2008). Walnuts also contain tocopherols in the lipid fraction, withy-tocopherol as the prevalent form, followed by 8- and a-, and trace E-mail address: roberta.dordoni@unicatt.it (R. Dordoni). https://doi.org/10.1016/j.heliyon.2019.e02506 Received 5 March 2019; Received in revised form 19 August 2019; Accepted 18 September 2019 2405-8440/O 2019 Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). amounts of p-isomer (Amaral et al., 2005). Despite their radical scav-enging activity, tocopherols seem to contribute to total antioxidant ca-pacity of walnut with less than 5% (Arranz et al., 2008). In order to extend the shelf life of walnut products, antioxidants couldbe added to delay rancidity development. Nowadays there is a great in-terest for obtaining and exploiting antioxidants from natural sources,avoiding the use of synthetic additives, scarcely accepted by consumers.However, there are few studies related to their use for the preservation ofnuts and/or derivatives: rosemary extract proved an efficient additive indelaying oxidation of stored walnut oil (Martinez et al., 2013), or eventhe addition of powdery encapsulated extract from red-grape marc wassuccessfully tested to delay lipid oxidation in hazelnut paste (Spignoet al., 2013). Grape marc is a wine-making by-product of wine industry,rich in phenolic compounds (mainly anthocyanins, flavanols and flavo-nols) that can be recovered to obtain extracts useful in the developmentof new food products as functional ingredients or as natural antioxidantadditives (Lavelli et al., 2016). Based on these premises, the objective of this work was to determinechemical composition and oxidative stability of walnut paste. In addition,accelerated storage tests on walnut paste, with or without added anti-oxidant, were performed in order to investigate the potential protectiveeffect exerted by the addition of a red grape skin phenolic extract on theoxidative status during storage. 2. Materials and methods 2.1. Materials Walnuts in shell (var. Howard, California, USA) were purchased froma local market in Piacenza (Italy). Grape skin extract, encapsulated intomaltodextrin, was obtained from grape pomace of the Barbera varietyaccording to the procedure reported in Dordoni et al. (2019). Briefly,grape skin powder was extracted with 60% aqueous ethanol undercontinuous stirring for 2h at 60°C. The mixture was then centrifuged andthe supernatant recovered. The extract was concentrated 15 times with arotary evaporator (Biichi Rotavapor R-144), diluted with an aqueoussolution of maltodextrins, and spray-dried. Maltodextrins (Glucidex IT 12DE, Roquette Italia S.P.A.) were used at a dosing level of 0.6 M ratioDE/gallic acid equivalents (GAE). Spray-drying operation conditionswere described in Lavelli et al. (2016). The spray-dried extract wascharacterized for moisture content (7.19±0.39g/100g), total phenolics(98.24±3.50 mgGAE/g), ABTS value (790.91 ±50.51 umolTroloxeq/g),FRAP value (2.25±0.15 mmolFe(I)/g),and ORAC value (985.78±59.51umolTroloxeq/g). All standards and chemicals used in the analytical determinationswere purchased from Sigma Aldrich (Milan, Italy). 2.2. Walnut paste preparation After manual shell removal, kernels were roasted in a forced con-vection oven (Tecnoeka, Italy) at 160 °C for 15 min, as reported byVaidya and Eun (2013). Roasted kernels were firstly ground by an elec-tric domestic grinder (La Moulinette, Moulinex) and then reduced topaste using a planetary micro mill (Pulverisette, Fritsch, Germany) at 700rpm for 2 min. The paste was collected in plastic and opaque containersto limit its exposure to light. Before closing, the head space was saturatedwith nitrogen to avoid contact with air. The samples were then stored at-20°C until use. 2.3. Experimental plan The obtained walnut paste was analysed for moisture, fat, protein,reducing sugar, ash, fibre, pH, and total acidity. Preliminary tests were carried out to select the extract concentrationto be used in the next accelerated storage test: three different doses of thespray-dried grape skin extract were handily and thoroughly mixed with the walnut paste. The obtained samples were subjected to acceleratedoxidative stability tests by using an Oxidation Test Reactor (Oxitest)device (see section 2.3.1). Therefore, an accelerated storage test (see section 2.3.2) wasdesigned and performed to compare samples with and without theaddition of the grape extract at the best concentration selected frompreliminary tests. For this purpose oxidative quality indexes weremonitored during storage: peroxide values, conjugated dienes and tri-enes, and tocopherols (determined in the lipid fraction); total poly-phenols content and antioxidant capacity (ABTS and ORAC tests)(evaluated on the defatted matter). Antioxidant capacity by FRAP testswas assessed on defatted samples, at the beginning and end of the test. 2.3.1. Accelerated oxidative stability test (Oxitest) Walnut paste was enriched with the spray-dried grape skin extract atthree different concentrations: 2500, 5000 and 10000 ppm (w/w). Toassess the extract concentration with the greatest antioxidant efficacy,the Oxidation Test Reactor (Oxitest, VELP Scientifica, Milan, Italy) wasemployed. Walnut pastes (10 g), with and without extract addition, weredistributed homogeneously on the instrument sample holders and testconditions were set at 90 °C and 6 bar oxygen pressure. The InductionPeriod (IP in min) was calculated by the instrument software(OXISoftM). 2.3.2. Accelerated storage test In order to evaluate the oxidative stability of walnut paste and theprotective effect exerted by grape skin extract, an accelerated storagestudy was performed on both the walnut paste and the walnut pasteadded with the extract concentration selected from the acceleratedoxidative stability test. According to Abedi Gonabad et al. (2015) an activation energy of80.327kJ/mol was assumed for walnut oil oxidation. In orderto simulatestorage at room temperature (20 °C), the Arrhenius law was applied,finding the acceleration factor of oxidation: k60°=c52.3k20°c A day storage at 60 °C can then be considered equivalent to a mini-mum of 52 days at 20°C. Therefore, in the present study a period of 15days at 60 °C was chosen for the accelerated storage tests, aiming tosimulate a 2 year shelf life at 20 C and notice quality deterioration. Thirty grams of both samples (paste with and without grape extract)were placed in open ceramic vessels and stored at 60 °C for 15 days(Vaidya and Eun, 2013).Samples were analysed after 2, 4, 7,9, 11,and15 days. For each type of walnut paste (with and without extract) onevessel was used at each sampling time. Walnut paste quality was assessedon triplicate at each sampling time by determination of oxidativeindexes. 2.4. Chemical composition Moisture content was determined as stated by the AOAC officialmethod 931.04 (AOAC, 2005). The fat content was assessed according tothe method 948.22 (AOAC, 2005) suitably modified. In detail, the sam-ples were mixed with distilled water and hydrochloric acid (25% w/w),and subjected to 30 min-boiling for hydrolysis. The resulting solutionswere then filtered through a folded filter (Whatman 595 ). The filterscontaining the samples were washed with distilled water and subse-quently dried for 6 h at 100°C. The dried filters were then transferredinto thimbles and extracted for 6 h with 250 mL n-hexane through aSoxhlet apparatus. The solvent was finally evaporated (Rotavapor BuchiR-3) and the residual fat weighed. Official methods of analysis were used to determine ash and totalprotein content (method 950.48) (AOAC, 2005). For the evaluation offree reducing and non-reducing sugar content (glucose, fructose,maltose, lactose and sucrose) the volumetric method of Luff- Shoorl was applied(Egan et al.,1981). Finally, neutral detergent fiber (NDF), acid detergentfiber (ADF), and acid detergent lignin (ADL) contents were determinedon the defatted samples according to the Van Soest et al. (1991) pro-cedure adapted to the ANKOM Fiber Analyzer (ANKOM TechnologyCorporation, Macedon, NY). Total acidity and pH were evaluated on walnut paste samples ac-cording to the methods recommended by the Office International duCacao, du Chocolat et de la Confiserie (OICCC, 1972, 9. In particular,10 gof walnut paste were dissolved in 90 mL of boiling distilled water. The pHwas measured after cooling to 25°C. Total acidity was measured on thesame dispersion added with 20 mL of 0.1 N sodium hydroxide, thentitrated with 0.1 N hydrochloric acid until pH of 7.00, and expressed asgoleic acid/100 g of dry sample. 2.5. Oxidative quality indexes 2.5.1. Defatting of walnut paste Oil fraction and defatted powder were separated by a cold extractionprocedure. Samples were added with hexane at a 1:5 ratio (Belscak et al.,2009), stirred at 180 rpm for 1h at 20 °C (orbital shaker Infors HT), thencentrifuged at 3000 rpm for 15 min at 15 °C (Varifuge 20 RS, HeraeusSepatech). The supernatant mixture was filtered throughout a filter paper(Whatman n°595%) and the free oil was collected after hexane evapo-ration (Rotavapor Buchi R-3). The solid residue was kept for about 8 hunder nitrogen flow, in order to evaporate all the remaining solvent andobtain the defatted powder. Peroxide value, conjugated dienes and trienes, and tocopherols weremeasured on the lipid fraction, whereas total phenolic content andantioxidant capacity (through ABTS, ORAC, and FRAP assays) wereevaluated on defatted matter. 2.5.2. Peroxide value, conjugated dienes and trienes The peroxide values (expressed as meq O2/kgoil) and conjugateddiene and triene systems (expressed as extinction coefficients at 232 and270 nm, K232 and K270) were determined on the oil fraction as describedin the European Union Commission Regulations (1991). 2.5.3. Tocopherols The chromatographic determination of tocopherols was performed ona HPLC system including a Perkin Elmer (Norwalk, CT, USA) 200 Seriespump equipped with a Perkin-Elmer 650-10S fluorescence detector,Jasco LC-Net II/ADC (Oklahoma City,OK, USA) communication moduleand ChromNAV Control Center software. The analysis was carried outaccording to Calvo et al. (2011): a LiChrosorb Si60-5 C18 column 250mm × 4.6 mm, 5 um (Supelco, Bellefonte, PA, USA) was used, the in-jection volume was 20 pL, and the mobile phase washex-ane:isopropanol:ethanol(98.5:1:0.5).Fluorescence detector was set at290 nm excitation and 330 nm emission wavelengths. u-,y-and 8-tocopherols were identified by comparing retention timeswith those of commercial standards. Results were expressed as mg/100gof dry sample for each tocopherol. 2.5.4. Total phenolic content Two grams of defatted sample were added with 5 mL methanol:-water/70:30 and kept in ultrasonic bath for 30 min, as reported byBelscak et al. (2009). Then, the mixture was centrifuged at 3000 rpm for10 min at 15°C (Varifuge 20 RS, Heraeus Sepatech): the supernatant wasrecovered and the solid residue was re-extracted with 5 mL of the samesolvent. Filtered supernatants (Whatman n° 595%) were collected into10 mL flasks and made up to the mark with methanol:water/70:30. Theextracts were stored in the dark, at -20°C, until further analysis. Total phenolic content was determined through the Folin-Ciocalteuassay (Moncalvo et al., 2016). Briefly, an aliquot (50 pL) of extract(diluted as necessary withwater) was mixed with 250 pL ofFolin-Ciocalteau reagent; then 4,7 mL of Na2CO3 2.2% were added and the mixture was brought to 50 mL with distilled water. The samples werekept in a thermostatic bath at 40 °C for 30 min, then the absorbance ofthe samples was measured at 750 nm (Shimadzu UV-1601), against blankwith no extract addition. Results were expressed as equivalents of gallicacid (GAE) per 100 g of paste (dry weight) through a calibration curvemade with a standard of gallic acid (>98%) in the range 100-800 mg/L. 2.5.5. ABTS assay The test assesses the ability of a sample to reduce the ABTS radical(2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)-diammoniumsalt) by measuring its absorbance decrease at 734 nm. A radical solu-tion (7 mM ABTS and 2.45 mM potassium persulfate) was prepared andkept in the dark at room-temperature for 16 h before use. The solutionwas then diluted with ethanol:water/50:50 to an absorbance of 0.700(±0.020) at 734 nm against ethanol:water/50:50 and equilibrated at 30°C. For the analysis,2 mL of the final radical solution were mixed with 20uL of different dilutions (in ethanol:water/50:50) of the phenolic extract.The samples, along with a control (2 mL of the radical solution) and ablank (2 mL of the radical solution mixed with 20 pL of the sample sol-vent) were kept in the dark for 6 min at 30°C and then their absorbancewas read at 734 nm (Vadivel et al., 2016). Antioxidant capacity wascalculated as percentage of absorbance decrease and expressed asmmolTrolox@equivalents/100g or mg Trolox@ equivalents/100g (basedon paste dry weight) by a calibration curve obtained with standardTrolox@ (0.1-3.2 mM final concentration in the cuvette). 2.5.6. ORAC assay The ORAC (Oxygen Radical Absorbance Capacity) assay was per-formed to measure the peroxyl radical-scavenging activity of phenolicextracts based on the method described by Huang et al. (2002). A4 uMfluorescein solution was daily prepared in 75 mM phosphate buffer (pH7.4). Two hundred microliters of fluorescein solution and 33 pL of 0.153M AAPH solution (2,2'-azobis-2-amidinopropane dihydrochloride) wereadded to all working wells. In addition, blank wells received 33 uL of 75mM phosphate buffer (pH 7.4), while standards received 33 pL of Tro-lox@ dilution, and samples received 33 uL of diluted extracts. The fluo-rescence decay of each well was then measured and recorded by afluorescence reader (Bio Tek Synergy HT-Bio-Tek Instruments, Inc.,Winooski, VT): the fluorescence was monitored kinetically with datataken every minute, with a 485 nm excitation filter and a 528 nmemission filter. Results were expressed as umol TroloxR equivalents/100g of dry paste. 2.5.7. FRAP assay The FRAP assay (Ferric Reducing Antioxidant Power) was carried outas described by Vadivel et al. (2016). The FRAP reagent was daily pre-pared with 2.5 mL of 20 mM TPTZ solution (2,4,6-Tris (2-pyr-idyl)-s-triazine) in 40 mM hydrochloric acid, plus 2.5 mL of 20 mMFeCl3·6H2O solution, and 25 mL of 0.3 M acetate buffer (pH 3.6). Analiquot (3.7mL) of this reagent, kept for 15 min at 37 °C, was mixed with360 pL of distilled water and 120 pL of the phenolic extract opportunelydiluted. The samples and the blank were incubated at 37 °C for 30 min;then, the absorbance of the sample was read at 593 nm against the blank.The absorbance was converted into mMFe(II) through a calibration curveprepared with different concentrations of FeSO47H2O solutions, thenexpressed as mmoles of Fe(II) per 100g of dry paste. 2.6. Statistical analysis Chemical analysis were carried out on triplicate; mean values andstandard deviations were calculated for each case. Peroxide values wereanalysed by Student's t-test at p< 0.05. Analysis of variance (ANOVA)followed by Tukey's post-hoc test was performed at the p≤0.05 levelusing statistical software SPSSQ (version 23.0, SPSS Inc., Chicago, IL,USA). 3.1. Characterization of initial walnut paste Walnut composition can vary considerably by genotype, pre- andpost-harvest condition, as well as processing and storage conditions.However, there are few studies (Tapia et al., 2013) that evaluated thenutritional value of the Howard cultivar. The proximate characterizationof the walnut paste obtained in this study by Howard cv is shown inTable 1. More than a half of the macro-components was represented bylipids, followed by the protein content. These data partially agreed withresults obtained by Tapia et al. (2013) on not-roasted walnut kernels,reporting a higher fat content (61.3%) and a lower protein content(15.1%). As expected, the negligible moisture value (0.66%) was aconsequence of the kernel roasting process for walnut paste production.Fibre fractions were higher than those reported in studies on differentvarieties (Tapia et al., 2013; Savage, 2001): to the neutral detergent fibrecontributed 5.42% of hemicellulose, 3.37% of cellulose, and 3.89% oflignin and cutin, mainly located in the walnut pellicle (Dordoni et al.,2017). Walnut paste exhibited pH slightly lower than the neutrality (6.24±0.03) and acidity value of 1.87 ±0.02 (goleic acid/100g) that can becorrelated to the degree of oil hydrolytic rancidity induced by theroasting process (Ziaolhagh et al., 2017). As a matter of fact, roasting andsubsequent grinding of the kernels can adversely affect indicators ofhydrolytic and oxidative alterations (Martinez et al., 2013). With regardto the lipid fraction, peroxide values, conjugated dienes (K232) andconjugated trienes (K270) agreed with studies on walnut oils dark storedand/or heat-treated (Martinez et al., 2013; Vaidya and Eun, 2013)(Table 2, Fig.1). In particular, peroxides are the primary products of lipidoxidation and their formation is promoted by mechanical and thermalstress on the product. By analysing the characteristics of a cold-pressedwalnut oil, Martinez et al. (2010) reported peroxide numbers between0.05 and 0.50 meqo2/kg oil, lower than the amount detected in thepresent study (Table 2). On the contrary, by applying the same roastingconditions used in this work (160 °C for 15 min) Vaidya and Eun (2013)scored a similar peroxide value (2.34 meqO2/kg oil) and showed that,despite the immediate increase of oxidative parameters, roasted nutswere more stable over time, due to enzyme inactivation. Furthermore, the walnut high susceptibility to oxidation may becounterbalanced by its richness in antioxidant compounds, mainly rep-resented by tocopherols and phenolic compounds. Thermal treatment can also induce the degradation of phenoliccompounds and/or increase the total phenolic content and the antioxi-dant activity, due to release of bound phenolic compounds and formationof Maillard reaction products (Chang et al., 2016). Data related to thecontent of polyphenols and the antioxidant capacity of walnut paste arereported in Fig. 2. Walnut phenolic compounds are located especially in the brownpellicle surrounding the nut and include hydrolysable and condensed Table 1 Characterization of initial walnut paste (NDF: Neutral DetergentFibre; ADF: Acid Detergent Fibre; ADL: Acid Detergent Lignin). Values are means ± standard deviations (n=3). Parameter Value Moisture (g/100g) 0.66±0.28 Fat (g/100g) 56.82±9.23 Protein (g/100g) 20.49±1.04 Reducing sugars (g/100g) 0.012±0.000 Ash (g/100g) 1.94±0.01 Fibre fractions NDF (g/100g) 12.68±1.07 ADF (g/100g) 7.26±0.48 ADL (g/100g) 3.89±0.56 pH 6.24±0.03 Acidity (g OLEIC ACID/100g) 1.87±0.02 Peroxide values of walnut paste (WP) and of walnut paste added with 5000 ppm(w/w) of extract (WP+ E) determined at different storage times at 60°C. Valuesare means ± standard deviations (n =3). Within each column, different super-script letters indicate statistically different values according to post-hoc com-parison (Tukey's test) at p≤0.05. Within each raw, different subscript lettersindicate statistically different values according to Student's t-test at p≤0.05. Storage time (days) Peroxide value (meq02/kgoil) WP WP+E 0 2.31±0.60ab 2.32±0.57 2 5.82±0.76°。 6.82±1.76. 4 3.94±0.03a 6.45±2.09. 7 2.11±0.71 1.78±0.30 9 0.66±0.29. 3.30±0.76 11 1.79±0.28 1.98±0.01 15 5.81±0.74°。 7.77±0.57b, —WP…-…. WP+E Fig. 1. (a) Conjugated diene values (K232), and (b) conjugated triene values(K270) of walnut paste (WP) and of walnut paste added with 5000 ppm (w/w) ofextract (WP +E) determined at different storage times. Error bars indicate ±standard deviations (n=3). tannins, flavonoids and phenolic acids. Ellagitannins, ellagic acid and itsderivatives were revealed as the most abundant (Dordoni et al., 2017). The content of phenolic compounds in 100g of dry paste was 406.9 ±19.0 mgGAE. A similar concentration (414±18 mgGAE/100g of freshweight) was reported by Arcan and Yemenicioglu (2009) who evaluatedit through aqueous extraction of whole walnuts, while higher values wereobtained by aqueous-organic extraction: Salcedo et al.(2010) detected15.5 mgGAE/g of walnut through 50:50 methanol:water (v/v) extractionof whole fruit, Arranz et al. (2008) found 1071± 35 mgGAE/g byextraction of defatted walnuts with acidic methanol:water (50:50 v/v),and Tapia et al. (2013) 58.2 mgGAE/g of fresh weight by acidic etha-nol:water (80:20 v/v) extraction of whole Howard walnuts. Storage time (days) Fig. 2. (a) Total phenolic content (mgGAE/100g), (b) antioxidant capacity(mmolTroloxeq/100g) based on the ABTS assay, and (c) antioxidant capacity(umolTroloxeq/100g) based on the ORAC assay of walnut paste (WP) and ofwalnut paste added with 5000 ppm (w/w) of extract (WP +E) determined atdifferent storage times. Error bars indicate ± standard deviations (n=3). In addition to environmental factors, differences observed onphenolic contents may be partly explained by the different extractionmethods and interference from other reducing non-phenolic constitu-ents. Therefore, the values obtained based on Folin's assay are often over-estimated in comparison with the sum of individual phenolics identifiedusing high-performance liquid chromatography (Chang et al., 2016). The phenolic content of walnuts is in general correlated to theirantioxidant capacity which resulted equal to 2.088±0.046 mmolTrolox/100g, 7.036 ±0.456 mmolFe(m)/100g and 2363.763 ± 216.880umolTrolox/100g according to the ABTS, FRAP and ORAC test, respec-tively. In literature there are various studies related to the antioxidantcapacity of walnuts, but it is difficult to compare the obtained resultssince they employed different extraction methods and reported antioxi-dant activities as equivalents of different compounds (Arcan and Yem-enicioglu, 2009). Schlormann et al. (2015) evaluated the effect ofroasting on the hydrophilic antioxidant activity of walnuts, showing asignificant decrease from 11.0 mmolTrolox/100g in natural nuts to3.8-10.5 mmolTrolox/100g in kernels roasted at 123C/25 min to 185.5 Although no specific data for the Howard variety have been found inthe literature, the relative abundance of tocopherol vitamers agree withreportedvalues (Amaral et al., 2005) for other varieties: in the examinedpaste y-tocopherol was the predominant form, followed by a-tocopheroland 8-tocopherol, while the presence of B-vitamer was not detectable(Fig. 3). The observed values were high, despite the roasting process thatgenerally causes a tocopherol decrease (Vaidya and Eun, 2013). 3.2. Preliminary test to select the extract addition level The antioxidant efficacy of different levels of encapsulated grape skinextract (2500,5000, and10000 ppm (w/w)) was preliminarily measuredby comparing the induction periods (IP) of the different samples. The IPcorresponds to either a level of detectable rancidity or a sudden change inthe rate of oxidation, with higher IP value corresponding to higheroxidative stability. Walnut paste without additions showed an IP of 726± 40 min. Addition of grape extract at 2500 ppm did not significantlyinfluence the IP (769±53 min), while a 5000 ppm enrichment gave a Fig. 3. (a) o-tocopherol content (mg/100g), (b) y-tocopherol content (mg/100g), and (c) 8-tocopherol content (mg/100g) of walnut paste (WP) and ofwalnut paste added with 5000 ppm (w/w) of extract (WP + E) determined atdifferent storage times. Error bars indicate ± standard deviations (n =3). longer IP (831±29 min), which did not further increase with 10000 ppmaddition (830±27 min). Taking into account these results and consid-ering that 5000 ppm dosing level also represents the common maximumallowed additives incorporation into foods (Spigno et al., 2013), thisconcentration was selected for the accelerated shelf life study. 3.3. Accelerated storage study Literature reported data on shelf life of walnut stored at temperatureranging between -18 °C and 38°C do not exceed 18 months (Gama et al.,2018) and information on the expiration date of processed walnutderived products such as walnut paste is not available. In the presentstudy, accelerated storage conditions were set in order to simulate a24-month shelf-life (at 20°C), to largely exceed the common commercialexpiration period of 1 year generally assigned to the semi-finishednut-based products (Spigno et al., 2013). Results showed some variations in the measured peroxide values(Table 2) explainable by the heterogeneity nature of the matrix (Linget al., 2014). Contrary to expectations, after 15 days of high temperaturestorage, walnut paste with extract reached a slightly higher value thanthe one without addition, suggesting that grape antioxidant molecules donot exert any protective effect on the lipid fraction. Furthermore, thesemaximum levels were still lower than the value (10 meq02/kg of fat)recommended for avoiding rancidity flavour (Kong and Singh, 2011).Our results are in general in agreement with those reported by Stark et al.(2000) and Ling et al. (2014) who reported good stability of walnut oiland walnut paste during 115 days at 60°C, and 20 days at 35°C storage,respectively. Hydroperoxides accumulation during the initial oxidation periodproved to be correlated with increase of the conjugated dienes. Sincewalnut paste production involved a roasting process, initial K232 of bothnatural and enriched samples (1.662±0.117 and 1.648 ±0.122,respectively) (Fig. 1a) can be considered index of a slight oxidative sta-tus. The conjugated dienes showed a trend similar to that obtained forperoxide values: large increase of the diene conjugation rate occurred atthe end of the storage showing, nevertheless, a slightly higher value inthe sample without extract (Fig. 1a). The production of secondaryoxidation products (conjugated trienes) over time is presented in Figure1b. Their formation is due to reactions of decomposition and lightdegradation of primary oxidation compounds resulting in the generationof a wide variety of different molecules (aldehydes, ketones, alcohols,etc.) (Ferreira et al., 2018). In both samples, the content of trienesgenerally tended to fluctuate over time. Anyway, final values (after 15day storage) remained low even in comparison to reported data forwalnut oil during different storage conditions (Ferreira et al., 2018).Hence, results observed on lipid oxidation indicate that both the testedwalnut pastes have a reasonably long shelf life. As a matter of fact, nosubstantial differences on oxidative parameters were revealed betweensamples with or without grape extract. Total phenolic content of walnut paste samples had a very highvariability during storage test (Fig. 2a). Although a decrease in thecontent of these molecules was observed during storage especially in thewalnut paste with extract, both the samples values were not significantlydifferent between initial and final time. This behaviour might be ascribedto the ability of polyphenols to oxidize and further polymerise to formoligomers with a higher antioxidant activity, since the Folin's assayactually measures the reducing power of samples (Spigno et al.,2013). The same trend was observed in the FRAP and ABTS test (Fig.2b). Infact, the FRAP assay on walnut paste gave 7.036 ±0.456 and 8.803±1.555 mmolFe(I)/100g, at day 0 and 15, respectively, and 5.978±2.596and 5.558 ± 0.970 mmolFe(I)/100g for the grape extract enrichedsample. Similarly ABTS based antioxidant capacity (Fig. 2b) did not varysignificantly during storage in both the samples. On the opposite, ORACtest revealed significantly higher values at time zero (Fig.2c). These dataconfirm the correlation between the Folin's assay and antioxidant activity measured by FRAP, ABTS and other assays based on electron transfer(Huang et al.,2005). Comparing the total polyphenols content in walnut paste with andwithout extract, no significantly differences appeared during the studiedperiod, with the exception of the 9th and, particularly, of the last day,when the sample of walnut paste showed a greater and significantlydifferent phenolic content (465.79± 22.05 mgGAE/100g) than theenriched paste (288.98±21.97 mgGAE/100g) (Fig. 2a). This differencewas noticeably significant also in the ORAC (Fig. 2c) and FRAP results.ABTS capacity appeared slightly higher in the initial walnut paste; afterthat, samples with and without extract could not be considered assignificantly different (Fig. 2b). The presence of grape marc extract had the effect of considerablyincreasing the initial antioxidant capacity of walnut paste only based onthe ORAC assay, but this difference attenuated during the storage, up toannulment at day 7. As already mentioned, at the end of the study,samples showed a slightly higher antioxidant activity in the walnut pastewithout extract (Fig. 2c). Considering added amount (5000 ppm w/w) and antioxidant capac-ity of the grape extract (section 2.1), the measured values in the initialsupplemented walnut paste (day 0) did not correspond to the theoreticalones based on extract addition. In particular, ABTS and FRAP were lowerthan expected by 34% and 28%, respectively, while total phenolic con-tent was 24% lower. The ORAC assay, on the other hand,provided muchhigher results than expected (+139 %). It must be considered thatevaluation of total phenolic content and antioxidant capacity were car-ried out on the defatted walnut paste (as recommended by Arranz et al.,2008). However, it was demonstrated high fat content of walnut caninterfere in the determination of antioxidant capacity and total poly-phenols in the extracts: in some cases high fat content can preventquantification of hydrolysable tannins and their antioxidant capacity inextraction residue (if emulsions are formed), in other cases actual valuesmay be overestimated (Arranz et al., 2008). Nevertheless, on the basis ofresults obtained over time, the grape extract addition did not exert anysignificant and decisive role in terms of enhancing antioxidant capacityof walnut paste. Walnuts are a good source of antioxidants in their lipid fraction and,in fact, o-,y-, and 8-tocopherols, the main lipid soluble compounds withantioxidant activity, were identified both in fresh and roasted walnut oil.Among them, y-tocopherol was reported to be the major homologous(Vaidya and Eun, 2013). Tocopherols can transfer a hydrogen atom at the6-hydroxy group on their chroman ring to lipid peroxy radical andscavenge the peroxy radicals. Lipid peroxy radicals react with tocoph-erols much faster than with lipids: one tocopherol molecule can protectabout 10° to 10° polyunsaturated fatty acid molecules at low peroxidevalue. The effectiveness of tocopherols as antioxidants depends on theisomers and on concentration. Free radical scavenging activity of to-copherols is the highest in 8-tocopherol followed byy -, p-, anda-tocopherol (Choe and Min, 2006). In the present study, y-tocopherol resulted the predominant homol-ogous in all samples, followed by a-and 8-tocopherol (Table 2). The ratioof the isomers remained constant over the observation period (Fig.3a, b,and c). By analysing trend data on the walnut paste, it can be noted thatall the tocopherols underwent a similar and drastic reduction (-60.8%) inthe first 4 days. These results are in disagreement with those reported byVaidya and Eun (2013) who observed a greater retention of y-and 8-to-copherols, and a significantly lower stability of the o-isomer in oil fromroasted walnuts stored at 60 °C: the average decreases of y-, 8-, anda-tocopherols were 0.7%, 2.8%, and 16.4%, respectively, at 6h day ofstorage. In the walnut paste all the tocopherol homologues increased atday 7, then decreased again and stabilized from the 9th to the last day.Although a negative correlation between storage time and tocopherolcontent is known, the tocopherol contents can increase during heatingdue to the release of tocopherols from ruptured membranes and brokenbond between tocopherol and phospholipids or proteins (Vaidya andEun, 2013). Since the added grape extract supposedly does not contain fattyfraction, nor even tocopherols, no substantial differences were expectedin the initial content of the enriched walnut paste. Conversely, thesamples with extract showed on average a tocopherol content equal toone third of the value of the walnut paste without extract (Figs. 3a, 3b,and 3c). Again, the trends were like those observed in not enriched paste.Tocopherols increased at day 2 (reaching values close to those measuredin the initial sample without extract), then decreased until 9th day, andsubsequently stabilized to values comparable to the initial ones. Theprocedure of handily mixing walnut paste with grape extract (withconsequent greater exposure of the sample to light and air) could beresponsible of the initial depletion of tocopherols. They might haveexerted their antioxidant action already at this stage, supporting thebehaviour of enriched samples that seemed to anticipate the trend ofsamples without extract. At the end of the storage, the samples weresimilar for the 8- and y-tocopherol residual content. Only a-isomer wasslightly higher in walnut paste without extract (Fig. 3a). This seems in contrast with the results obtained by the Oxitest in thepreliminary tests. However, forced conditions kept during Oxitest (6 baroxygen pressure and 90 °C temperature) could provide information onoxidative stability of samples not comparable with those obtained duringaccelerated shelf life test at 60°C, open air. Overall, data obtained from the 15 days storage at 60C show that thegrape extract addition to the walnut paste does not improve its oxidativestability. Indeed, natural and/or exogenous antioxidants at high levels(most of 0.01% or less) can behave as pro-oxidants, because involved inthe initiation step of lipid oxidation, and in important phenomena such assynergisms and degradations (Madhavi et al., 1996). The polyphenols contained in the grape extract should to be able todirectly reduce peroxyradicals, but their hydrophilic nature andremoteness from lipophilic radicals could hinder all direct contact re-actions (Spigno et al., 2013). Moreover, edible oil and fat matrices oftencontains multicomponent antioxidants, and interactions among themwere widely demonstrated (Vaidya and Eun, 2013). Alpha tocopheroland phenolic compounds proved to be more effective in preserving oliveoil when used together than used separately (Velasco and Dobarganes,2002); however, a-tocopherol can also exert an adverse effect on theantioxidation of olive oil by phenolic compounds, such as tyrosol oroleuropein, during the period of low hydroperoxide accumulation (Ble-kas et al., 1995). Hence, the joint action of antioxidant compounds,deriving from roasted walnuts and grape pomace extract, may haveinfluenced the antioxidant capacity and/or its evaluation in the exam-ined samples. Over time, foods generally undergo changes that depends on chemi-cal composition and environmental factors (Abedi Gonabad et al., 2015).These aspects interactively affect the oxidation of the lipid fraction and itis not easy to differentiate their individual effect (Choe and Min, 2006)).As temperature is one of the most important external factor affecting thequality and the shelf life of food products, in this paper the acceleratedstorage test was performedby keeping the samples at 60°C to simulatean abuse (Solco,2007). Fifteen-day storage at 60 °C overall resulted in an increase in per-oxides and only a slight raise in conjugated dienes (both primaryoxidation indicators). Tocopherols significantly decreased only in thewalnut paste without extract. However, in sample with extract, the initialvalues were lower, hence the obtained final levels were comparable. Itcan be therefore deduced that walnut paste was in itself a stable product,as the set storage conditions still provided final sample of acceptablequality. Since parameters indicating oxidative process progression (peroxidesand conjugated dienes) started increasing on the 15th day, it is conceiv-able that the quality deterioration of the product started at the end of theobservation period, even though remaining below the threshold ofunacceptability. The activation energy (Ea) of 80,327 kJ/mol (Abedi Gonabad et al.,2015) considered in setting accelerated shelf life test conditions referredto the oxidative kinetics of a fresh walnut oil, while in the present work awalnut paste obtained from whole roasted kernels was studied. Pre-liminary thermal treatment and more complex matrix (richer in antiox-idants and dietary fibre than oil) may have accordingly affected theactivation energy of the oxidative process, increasing it (Solco, 2007).The adoption of a different Ea value than the real one could have led tooverestimating the acceleration factor of oxidation and, as a conse-quence,the corresponding storage at ambient conditions (20°C). Theresults described above are in good agreement with those reported byLing et al. (2014) who showed that the walnut paste from thermal treatedkernels was more stable against oxidation than walnut kernels and coldpressed walnut oils (Ling et al., 2014). In a previous study Vaidya andEun (2013) already supported roasting of walnut kernels as an appro-priate method for extending the oxidative stability of oil during storagein the dark. 4. Conclusions Walnut paste is usuallyobtained by roasting and grinding of kernels;it is used in confectionery and traditional products, and may find appli-cation in innovative foods (e.g. sausages and meat preparations) as fatreplacer and functional component. In the present study, oxidative sta-bility of walnut paste was evaluated during 15-day storage at 60 C.Moreover, the potential efficiency of the incorporation of a grape skinextract at 5000 ppm in improving the shelf life of the walnut paste wasassessed. The experimental results showed that deterioration in quality ofwalnut paste itself started only at the end of the observation period,eventhough remaining below the threshold of unacceptability. The extractaddition did not prove to enhance oxidative stability and/or antioxidantproperties of the walnut paste. Further investigations are needed to identify the specific parametersof oxidation kinetics (i.e. activation energy) and, eventually, to detectany antioxidant activity exerted by the supplemented extracts in the mostadvanced phase of storage, extending the monitoring time. Declarations Author contribution statement Roberta Dordoni: Conceived and designed the experiments; Analyzedand interpreted the data; Contributed reagents, materials, analysis toolsor data; Wrote the paper. Silvia Cantaboni: Performed the experiments; Analyzed and inter-preted the data; Wrote the paper. Giorgia Spigno: Conceived and designed the experiments; Contrib-uted reagents,materials, analysis tools or data; Wrote the paper. Funding statement This work was supported by the Fondazione Cariplo through theproject "ReMarcForFood-Biotechnological strategies for the conversionof Winemaking by-products and their recycling into the food chain:development of new concepts of use" (2016-0740 grant). Competing interest statement The authors declare no conflict of interest. Additional information References Abedi Gonabad, M., Shahidi Noghabi, M., Niazmand, R., 2015. Estimation of kineticparameters of walnut oil using rancimat test. J.Appl. Environ. Biol. Sci 4, 28-32. Amaral, J.S., Alves, M.R., Seabra, R.M.,Oliveira, B.P.P., 2005. Vitamin E composition ofwalnuts (Juglans regia L.):a 3-year comparative study of different cultivars. J. Agric.Food Chem. 53 (13),5467-5472. AOAC, Official Methods of Analysis of AOAC International, 2005. Gaithersburg, MD, USA.Arcan, I., Yemenicioglu, A., 2009. Antioxidant activity and phenolic content of fresh anddry nuts with or without the seed coat. J. Food Compos. Anal. 22 (3), 184-188. Arranz, S., Perez-Jimenez, J.,Saura-Calixto,F., 2008. Antioxidant capacity of walnut(Juglans regia L.): contribution of oil and defatted matter. Eur. Food Res.Technol.227(2), 425-431. Bakkalbasi, E., Yilmaz, O.M., Javidipour, I., Artik, N., 2012. Effects of packagingmaterials, storage conditions and variety on oxidative stability of shelled walnuts.LWTI--Food Sci. Technol. 46 (1), 203-209. Belscak, A., Komes, D., Horzic, D., Ganic, K.K., Karlovic, D., 2009. Comparative study ofcommercially available cocoa products in terms of their bioactive composition. FoodRes. Int. 42 (5-6),707-716. Blekas, G., Tsimidou, M., Boskou, D., 1995. Contribution of a-tocopherol to olive oilstability. Food Chem. 52 (3), 289-294. Buranasompob,A., Tang, J., Powers, J.R., Reyes, J., Clark, S., Swanson, B.G., 2007.Lipoxygenase activity in walnuts and almonds. LWT -Food Sci. Technol. 40 (5),893-899. Calvo, P., Castano, A.L., Hernandez, M.T., Gonzalez-Gomez, D., 2011. Effects ofmicrocapsule constitution on the quality of microencapsulated walnut oil. Eur. J.Lipid Sci. Technol. 113 (10), 1273-1280. Chang, S.K., Alasalvar, C., Bolling, B.W., Shahidi, F.,2016. Nuts and their co-products: theimpact of processing (roasting) on phenolics, bioavailability, and health benefits - acomprehensive review. J. Funct. Foods 26, 88-122. CIChoe, E., Min, D.B., 2006.Mechanisms and factors for edible oil oxidation. Compr. Rev.Food Sci.Food Saf. 5(4), 169-186. Dordoni, R., Duserm Garrido, G., Marinoni, L.,Torri, L., Piochi, M., Spigno, G., 2019.Enrichment of whole wheat cocoa biscuits with encapsulated grape skin extract. Int.J. Food Sci. 2019. Dordoni, R., De Cesare, S., Casali, M., Lambri, M., Spigno,G., De Faveri, D.M., 2017.Stabilization of fat fraction in walnut-based freeze dried products. Chem. Eng. Trans.57. Egan, H., Kirk, R., Sawyer, R., 1981. The Luff Schoorl method. Sugars and preserves. In:Longman Scientific and Technical (8th Edition), Pearson`s Chemical Analysis ofFoods (Pp. 152-153). Harlow, UK. European Union Commission Regulation, 1991. Commission Regulation N. 2568/91 of 11July 1991 on the characteristics of olive oil and olive-residue oil and on the relevantmethods of analysis. Off. J. L 248, 1, 5/9/1991. Ferreira, A.R.V., Bandarra, N.M., Moldao-Martins, M., Coelhoso, I.M., Alves, V.D., 2018.FucoPol and chitosan bilayer films for walnut kernels and oil preservation. LWT -Food Sci. Technol. 91,34-39. ( Gama, T., W a llac e , H. M ., T r ue m an, S.J., Ho s seini-Bai, S., 2 018. Qual i ty and s h elf life of t ree nuts: a review. Sc i . Hortic . 242,116- 1 26. ) Huang, D., Ou, B., Hampsch-Woodill,M., Flanagan, J.A., Prior, R.L., 2002. High- throughput assay of oxygen radical absorbance capacity (ORAC) using amultichannel liquid handling system coupled with a microplate fluorescence readerin 96-well format. J. Agric. Food Chem. 50 (16), 4437-4444. Huang, D., Ou, B., Prior, R.L., 2005. The chemistry behind antioxidant capacity assay.J. Agric. Food Chem. 53 (6), 1841-1856. Kong, F., Singh, R.P., 2011. Advances in instrumental methods to determine food qualitydeterioration.In: Food and Beverage Stability and Shelf Life (Pp. 381-404). ElsevierLtd. Lavelli, V., Sri Harsha, P.S.C., Spigno, G., 2016. Modelling the stability of maltodextrin-encapsulated grape skin phenolics used as a new ingredient in apple puree. FoodChem. 209, 323-331. Ling, B., Hou, L., Li, R., Wang, S., 2014. Thermal treatment and storage condition effectsoTTn walnut paste quality associated with enzyme inactivation. LWT - Food Sci.Technol. 59, 786-793. Madhavi, D.L., Singhal, R.S., Kulkarni, P.R., 1996. Technological aspects of food antioxidants. In: Madhavi, D.L., Deshpande, S.S., Salunkhe, D.K. (Eds.), FoodAntioxidants. Technological, Toxicological, and Health Perspectives. Marcel, Dekker,New York, pp. 159-265. Martinez, M.L., Labuckas, D.O., Lamarque, A.L., Maestri, D.M., 2010. Walnut (Juglansregia L.): genetic resources, chemistry, by-products. J. Sci. Food Agric. 90 (12),1959-1967. Martinez, M.L., Penci, M.C., Ixtaina, V., Ribotta, P.D., Maestri, D.,2013. Effect of naturaland synthetic antioxidants on the oxidative stability of walnut oil under differentstorage conditions. LWT- Food Sci. Technol. 51 (1),44-50. Moncalvo, A., Marinoni, L., Dordoni, R., Garrido, G.D., Lavelli, V., Spigno, G., 2016.Waste grape skins: evaluation of safety aspects for the production of functionalpowders and extracts for the food sector. Food Addit. Contam. A 33 (7), 1116-1126. OICCC Analytical method of the Office International du Cacao et du Chocolat, 1972.Electrometric pH Determination of Cocoa and Chocolate Products, p. 9.E/1972. Salcedo, C.L., Lopez de Mishima, B.A.,Nazareno, M.A., 2010. Walnuts and almonds asmodel systems of foods constituted by oxidisable, pro-oxidant and antioxidantfactors. Food Res. Int. 43 (4), 1187-1197. Savage, G.P., 2001. Chemical composition of walnuts (Juglans regia L.) grown in NewZealand. Plant Foods Hum. Nutr. 56, 75-82. Schlormann, W., Birringer, M., Bohm, V., Lober, K., Jahreis, G., Lorkowski, S., Muiller, A.K., Schone, F., Glei, M., 2015. Influence of roasting conditions on health-related compounds in different nuts. Food Chem. 180, 77-85. Solco, A.K.S., 2007. Accelerated Shelf-Life Test of Alkamides in Echinacea Purpurea Root Aqueous Ethanol Soxhlet Extracts. Retrospective Theses and Dissertations, 14914.Spigno, G., Donsi, F., Amendola, D., Sessa, M., Ferrari, G., De Faveri, D.M., 2013. Nanoencapsulation systems to improve solubility and antioxidant efficiency of agrape marc extract into hazelnut paste.J. Food Eng. 114 (2), 207-214. Stark, C.H., McNeil, D.L., Savage, G.P., 2000. The effect of storage conditions on thestability of peroxide values of New Zealand grown walnuts. Proc. Nutr. Soc. N. Z. 25,143-54. Tapia, M.I., Sanchez-Morgado, J.R., Garcfa-Parra, J., Ramirez, R., Hernandez, T.,Gonzalez-Gomez, D., 2013. Comparative study of the nutritional and bioactivecompounds content of four walnut (Juglans regia L.) cultivars. J. Food Compos. Anal.31(2),232-237. Vadivel, V.,Moncalvo, A.,Dordoni, R., Spigno, G., 2016. Effects of an acid/alkalinetreatment on the release of antioxidants and cellulose from different agro-foodwastes. Waste Manag. 64, 305-314. Vaidya, B., Eun, J.B., 2013. Effect of roasting on oxidative and tocopherol stability ofwalnut oil during storage in the dark. Eur. J. Lipid Sci. Technol. 115 (3), 348-355. Van Soest, P.J., Robertson, J.B., Lewis, B.A., 1991. Methods for dietary fiber, neutraldetergent fiber, and nonstarch polysaccharides in relation to animal nutrition.J. Dairy Sci. 74 (10), 3583-3597. Velasco, J., Dobarganes, C., 2002. Oxidative stability of virgin olive oil. Eur. J. Lipid Sci.Technol. 104 (9-10), 661-676. Vinson, J.A., Cai, Y., 2012. Nuts, especially walnuts, have both antioxidant quantity andefficacy and exhibit significant potential health benefits. Food Funct. 3 (2), 134-140. Ziaolhagh, S.H., Tehrani, M.M., Razavi, S.M.A., Rashidi, H.,2017. Roasting processoptimization of walnut kernels for the preparation of walnut cream using responsesurface methodology.J. Nuts 8 (1), 31-40. 利用OXITEST方法分析核桃糊中添加葡萄皮成分后的氧化稳定性
确定






还剩6页未读,是否继续阅读?
意大利VELP公司为您提供《核桃糊中氧化稳定性检测方案(氧化分析仪)》,该方案主要用于其他食品中食品添加剂检测,参考标准--,《核桃糊中氧化稳定性检测方案(氧化分析仪)》用到的仪器有VELP Oxitest 油脂氧化分析仪
推荐专场
相关方案
更多
该厂商其他方案
更多