方案详情
文
诱导期的显着下降干燥的蛤tissue组织在高温下显示出较高的氧化水平,较差的氧化稳定性和货架期的缩短。
因此,OXITEST方法被证明是一种估计热风干燥蛤脂质氧化水平的有效工具。
方案详情

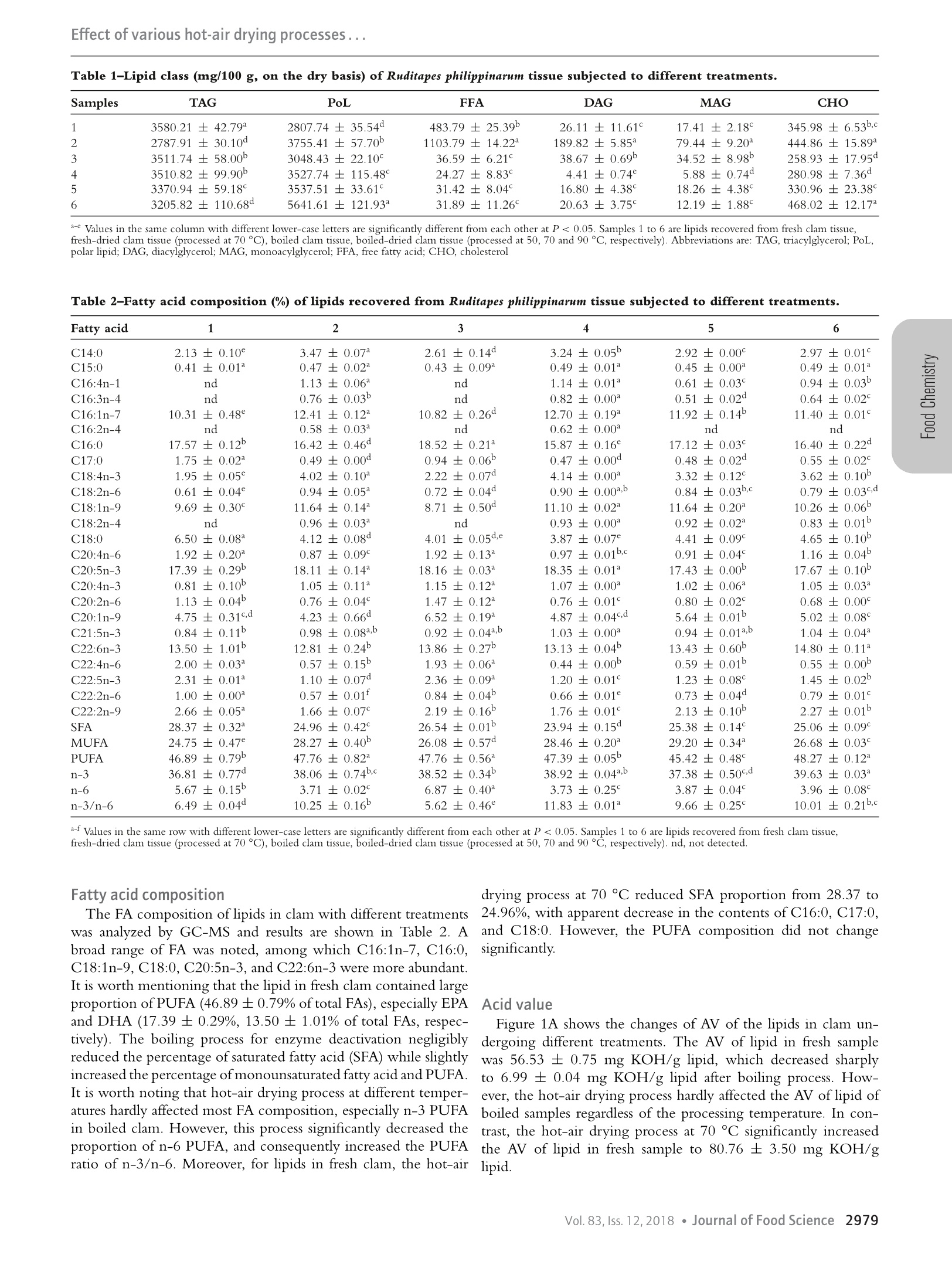
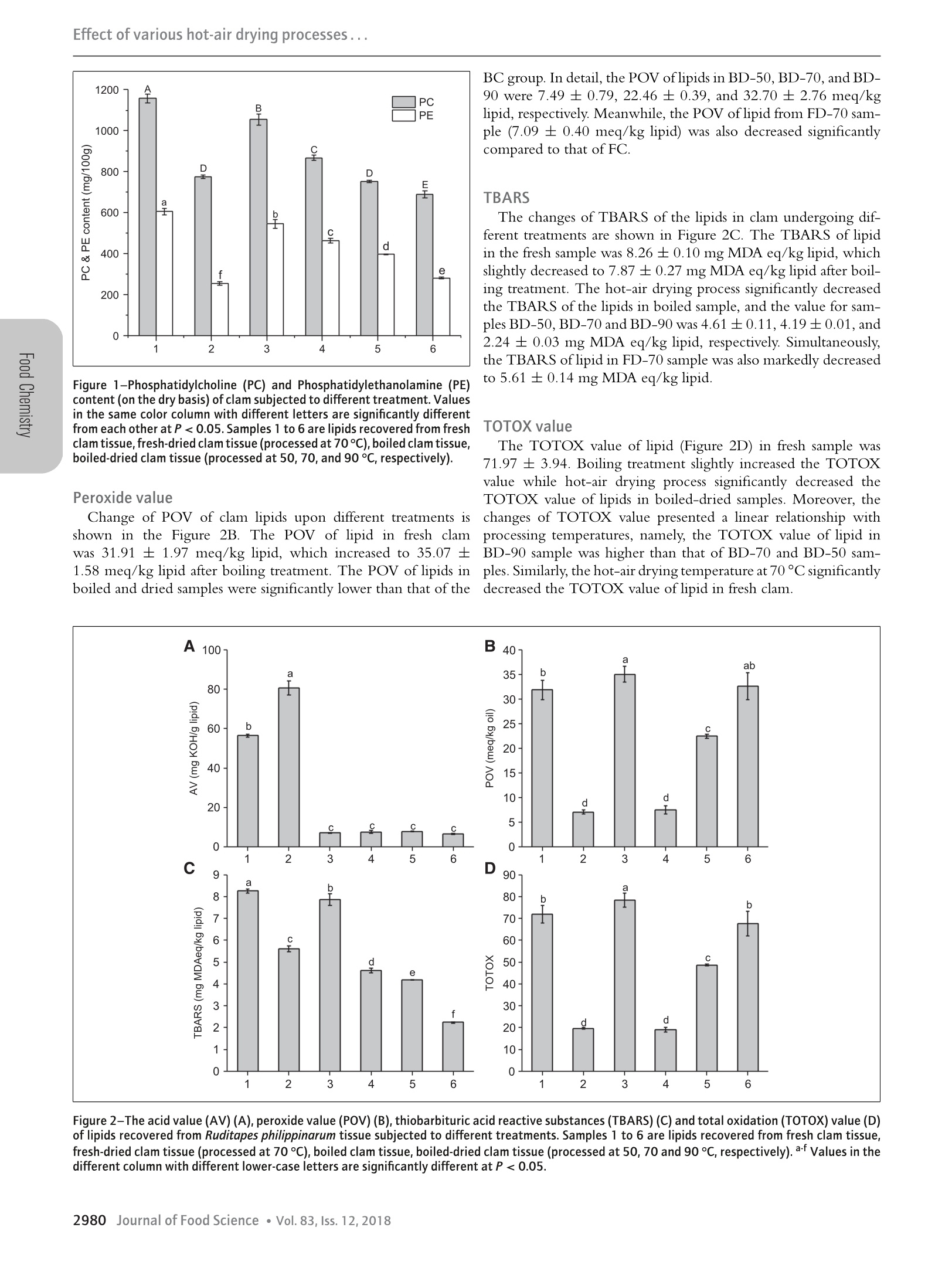
Effect of various hot-air drying processes... Effect of Various Hot-Air Drying Processes on Clam Ruditapes philippinarum Lipids: CompositiorrChanges and Oxidation Development Zhong-Yuan Liu, Da-Yong ZhouD, Xin Zhou, Fa-Wen Yin, Qi Zhao, Hong-Kai Xie, De-Yang Li, Bei-Wei Zhu, Tong Wang,and Fereidoon ShahidiiD Abstract: Clam Ruditapes philippinarum was processed by hot-air drying and the changes of its lipids were evaluated byanalyzing lipid classes, phospholipid classes, fatty acids, as well as oxidation parameters including peroxide value (POV),thiobarbituric acid-reactive substances (TBARS) value, total oxidation value (TOTOX), and oxidation test (OXITEST).The hot-air drying process reduced the contents of triacylglycerol, phosphatidylcholine, and phosphatidylethanolamine,indicating the hydrolysis of lipids. Meanwhile, the hot-air drying process significantly decreased the proportion of n-6polyunsaturated fatty acid (PUFA), and consequently increased the PUFA ratio of n-3/n-6. Interestingly, the POV,TBARS and TOTOX decreased after the hot-air drying process. However, significant decline of the induction period forthe dried clam tissue at elevated temperatures indicated their higher oxidation level, poor oxidative stability and reductionof shelf-life. Therefore, OXITEST method turned out to be an effective tool for estimating the level of lipid oxidationfor hot-air dried clam. Keywords: clam, hot-air drying, lipid hydrolysis, lipid oxidation, OXITEST Introduction Clam, an abundant marine bivalve, is cultivated and harvestedworldwide. Its global production was nearly 6.2 million tons in2016, which ranks first in production among shellfish species(FAO, 2018). As a delicacy that favored by consumers, simulta-neously, clam also serves as a rich source of bioactive componentsincluding peptides, proteins, minerals, vitamins, and essentialamino acids, which are critical for human nutrition and health(Choi & Park, 2016; Xie et al., 2012). Moreover, our previousstudies indicated that clams are rich sources of phospholipid (PL)that carries omega-3 long chain polyunsaturated fatty acids (n-3 LC-PUFA) (Liu et al., 2017; 2018), especially eicosapentaenoicacid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3).Recently, n-3 LC-PUFA in the PL form have captured significantattention due to their superior bioavailability (Cook et al., 2016),higher tissue-delivery capacity (Liu et al., 2014), and better healthpromoting effects (Ramprasath, Eyal, Zchut, & Jones, 2013)compared to that contained in triacylglycerol (TAG). Therefore,clam could be regarded as a “healthy”food. ( J FDS-2018-0697 Submitted 5/16/2018, Ac c epted 9/13/2018. Authors Li u , D - Y. Zhou, X. Z h ou, Yi n , Zhao, Li, an d Zhu are wit h the Sch o ol of F ood Sci e nceand T echnology, Dalian P olytechni c U niv, Dalian, 116034, P R China. Au t hors D -Y. Z hou, Z h ao, an d Zhu are wit h the Natl Engineering Res ea rch Cen t er ofSeafood, Dalian, 1 1 6034, P R C h ina. Authors Xie a n d Zhu are wi t h the BeijingA d vanced Innovation C e ntre of Food Nutrition and Human Health, China Agric u lturalUniv, Beijing, 1 00083, C h ina. Author Zhu is als o with Ti a n jin Food Saf e ty &Low C arbon Manufacturing Collaborative Innovation C enter, T ianjin, 300457, PRChina. A u thor W a ng is with the D ept. of Food S cience and Human N u trition, IowaState Univ, Ames, IA, 50011-1 0 61, U S A . Au t hor Shahidi i s with t he D ept.of B iochemistry, Memorial Univ. of Newfoundland, St. John's , NL, A1B3 × 9 ,Canada. Direct inquiries to auth o rs D-Y. Zhou and Zhu (E-mai ls : zdyzf1@16 3 .com, z hubeiwei@163.com). ) in great quantities, and the nutrient components tend to degradevia complex biochemical reactions, which lead to a significant lossof quality, shorter shelf-life and lower commercial value (Akonor,COfori, Dziedzoave, & Kortei, 2016). To overcome this problem,various drying techniques including sun drying, hot-air drying,freeze drying, microwave drying as well as hybrid drying wereutilized to decrease water activity and extend shelf-life of seafood(Chaijan, Panpipat, & Nisoa, 2017; Duan, Jiang, Wang, Yu, &VWang, 2011; Lee, Cho, Cho, Lee, & Shim, 1998; Murphy, Mann,& Sinclair, 2003). Among them, hot-air drying is extensively usedbecause of the better quality of resultant products, simple operationand low investment cost (Wang, Zhang, & Mujumdar, 2011). Generally, lipids in seafood possess high levels of PUFA,which are prone to oxidation (Selmi, Bouriga, Cherif, Toujani, &Trabelsi, 2010). Moreover, the active endogenous enzymes includ-ing lipases and phospholipases, hydrolyze the lipids, and release freefatty acid (FFA) that highly susceptible to oxidation (Aubourg,Tabilo-Munizaga, Reyes, Rodriguez, & Perez-Won, 2010;Hoehne-Reitan, Okland, & Reitan, 2007). Therefore, hydrolysisWhile as the moisture-rich organisms, seafood such as calm is- and oxidation of lipids occurring during hot-air drying process ofprone to rapid microbial spoilage, when they appear on the marketseafood impact nutritive value and quality of products (Chaijanet al., 2017; Shah, Tokunaga, Kurihara, & Takahashi, 2009).Previous research related to the effects of hot-air drying on foodlipids have mainly focused on marine fish species (Ali, Ahmadou,Mohamadou, Saidou, & Tenin, 2011; Shah et al., 2009; Wu &Mao, 2008). However, little information is available about the im-pact of drying process on the lipids in shellfishes, especially clams. Therefore, the present study intended to evaluate the hydrolysisand oxidation of lipids in clam Ruditapes philippinarum uponhot-air drying. To fulfill this goal, lipid content, lipid classes,PC and PE absolute content, fatty acid composition, acidvalue (AV), peroxide value (POV), thiobarbituric acid-reactivesubstances (TBARS), and total oxidation (TOTOX) value inclam tissue undergoing different treatments were determined.Furthermore, oxidation (OXITEST) method was also used for the first time to evaluate the oxidation level and stability of driedclam. Materials and Methods Materials Fresh clam Ruditapes philippinarum was purchased from a localmarket in Dalian, Liaoning, China. Reagents including methanol,hexane and isopropanol (high performance liquid chromatog-raphy, HPLC grade) were purchased from Spectrum ChemicalMfg. Corp. (Gardena, CA, USA). Glacial acetic acid and triethy-lamine were purchased from Aladdin Reagent Co., Ltd. (Shanghai,China). All other reagents were of analytical grade from KemiouChemical Reagent Co., Ltd. (Tianjin, China). Drying process After hulling the shell manually, the clam sample was dividedinto two parts, which were fresh sample and 15-min boiled (todeactivate endogenous enzyme) sample. A part of the fresh samplewas dried at 70°C (FD-70), and the rest was used as the freshcontrol (FC). The boiled sample was divided into four groups,three of which were dried at 50, 70 and 90℃ (BD-50, BD-70and BD-90, respectively), the rest was left as the control group(BC). Materials of known weight were dried under differenttemperatures with PH-050(A) hot-air oven (Yiheng Science andTechnology Co., Ltd., Shanghai, China), the drying process wasterminated when the water content was below 22% according toChinese national standard(NY/T 1712-2009) about aquatic prod-ucts processing. The BC group for OXITEST was lyophilized by2KBTES-55 freeze-dryer (VirTis Co., Gardiner,NY, USA). Thedried clam samples were smashed using DFY-500C pulverizer(Dalin machinery Co., Ltd., Wenling, Zhejiang, China). Lipid extraction Total lipids of the fresh clams and hot-air dried clams wereextracted based on the procedures described in Bligh and Dyer(1959). Lipid class composition analysis Lipid class compositions were determined using IatroscanMK-6S Thin Layer Chromatography-Flame Ionization Detec-tor (TLC-FID) (Iatron Inc., Tokyo, Japan) (Yin et al., 2015).Lipid sample solution of 10 to 20 mg/mL in chloroform wasspotted onto the silica-coated quartz rods (SIII Chromarods; Ia-tron Inc.), and developed with mixture of n-heptane/diethylether/formic acid (v/v/v, 42:28:0.3) for 20 min in developingtank. The chromarods were taken out, evaporated completely thesolvents, and scanned with the FID to detect and quantify thecompounds separated on the rods. For FID, the hydrogen flowrate was 160 mL/min, the air flow rate was 2 L/min and thescanning speed was 30s per chromarod burned. Then the nor-malization method was utilized to calculate the proportion of eachcomponent. - Fatty acid composition analysis FA compositions were determined using Agilent 7890A GasChromatography/5977A Mass Spectrometer (GC-MS) system(Palo Alto, CA, USA) (Yin et al., 2015). Briefly, 100 mg of lipidsample were mixed with 2.5 mL of KOH (500 g/L) and 5 mLof 95% ethanol in a tightly closed Teflon lined screw-capped vial.After incubation in a water bath at 60°C for 2 hrs with mildstirring, the mixture was cooled quickly to room temperature. The unsaponifiables were removed by extracting with hexane forsix times with 3 mL of hexane each time. Then the system wasadjusted to acid condition with 6 M HCl, and then the FFA wasextracted for six times with 3 mL ofhexane each time. The hexaneextracts were combined and flow dried with nitrogen at 35 °C.The residue was dissolved in hexane to 10 mg/mL, and methy-lated with freshly prepared reagent (0.1% H2SO4 in HPLC-grademethanol) in a vial. After interaction in a water bath at 70 °Cfor 1 hr, the mixture was cooled, and 1 mL of distilled waterwas added. The upper layer containing FA methyl esters was col-lected and transferred into another new vial with some anhydroussodium sulfate. After storage at 4°C overnight, the upper layerwas collected, filtered through a 0.22 um filter membrane and an-alyzed by GC-MS. The column temperature profile was the initialtemperature of 50°C maintained for 1 min, raised to 170°C at arate of 50°C/min, raised to 300 C at a rate of 4C/min, raisedto 320 °C at a rate of 40 °C/min and kept at 320°C for 3.6 min.The injection volume was 1 uL with a split ratio of 50:1. TheMS was in EI mode (70 eV) with a 2.0 scan/s interval over a 50to 550 m/z range. Solvent delay was 4 min. The identificationof fatty acid methyl esters was performed by comparison of rela-tive retention times and mass spectra with authentic standards (37Component FAME Mix; Supelco Inc., Bellefonte, PA, USA) andNIST08 mass spectral database. Phospholipid analysis The absolute content of major PL components (PC and PE) wasdetermined by a Shimadzu LC-20AD Prominence HPLC system(Shimadzu Corp.,Kyoto,Japan) combined with an Alltech ELSD6000 detector (Alltech, Deerfield, IL, USA) according to the pre-vious literature (Xie et al., 2018). The gas flow rate was 2 L/minand ELSD tube temperature was 70 °C. PC and PE were separatedon an Agilent Zorbax RX-SIL (4.6×250 mm, 5 um) column ata flow rate of 1.0 mL/min, and the mobile phase was (A) methylalcohol: water: glacial acetic acid: triethylamine (85:15:0.45:0.05,v/v) and (B) n-hexane: isopropanol: A (20:48:32, v/v). The elu-tion gradient was as follows: 90% to 70% B, 0 to 20 min; 70%to 5% B, 21 to 35 min; 90% B, 36 to 41 min. The lipid sam-ple concentration was 2 mg/mL, and the injection volume was10 uL. The PC and PE contents were calculated by standard curvemethod with GPCho (C16:0/18:1) and GPEtn (C16:0/18:1) asstandards. Acid value The AVWasislddetermined. according totheAmericanOil Chemists’ Society (AOCS) official method Cd 3d-63: AcidValue (AOCS, 2009a) with some modifications. Briefly, 0.20 gof lipid was dissolved in mixture of 50 ml of isopropanol anddiethyl ether (1:1; v/v). After thorough mixing, the sample wastitrated with 0.01 M potassium hydroxide (KOH) using automaticpotentiometric titrator (INESA Scientific Instrument Co., Ltd,Shanghai, China). The end point of titration was determined bythe abrupt pH change. The AV was calculated according to volumeof KOH (v) consumed and mass of lipid (m) with the followingformula and expressed as mg KOH/g lipid. Peroxide value The POV was measured according to the AOCS officialmethod AOCS Cd 8b-90: Peroxide Value, Acetic Acid-IsooctaneMethod (AOCS, 2003). Briefly, 0.20 g lipid sample was dissolved in 50 mL of mixture of isooctane and glacial acetic acid (2:3; v/v).After an addition of 1 mL saturated potassium iodide, the solutionwas stirred for 60 s, followed by an addition of 30 ml deionizedwater. Subsequently, the mixture was titrated immediately with0.005 M sodium thiosulphate using above used automaticpotentiometric titrator. The end point of titration was approachedwhen an abrupt potential change was observed. The POV werecalculated according to volume of sodium thiosulphate (v) andweight of lipid sample (m) by using the following formula andexpressed as mmol/kg reactive oxygen in lipid sample, which canbe converted to meq/kg lipid with constant coefficient directly. Thiobarbituric acid value The TBARS assay, mainly for the secondary oxidation productas malondialdehyde (MDA) equivalent, was performed accordingto John et al. (2005) with minor modification. Briefly, 0.10 glipid was mixed with 2.5 ml stock solution of 0.375% TBA, 15%trichloroacetic acid and 0.25 M hydrochloric acid. The mixturewas heated for 10 min in boiling water bath to develop a pink colorfollowed by cooling with tap water and centrifuged at 2500 ×gfor 10 min to allow phase separation. Subsequently, the super-natant was measured spectrophotometrically at 532 nm using anInfinite 200 auto multifunction microplate reader (Tecan (Shang-hai) trading Co.Ltd, Shanghai,China). The TBARS, expressedas mg MDA equivalents/kg lipid, was calculated according to thestandard curve constructed with stock solutions ofMDA. Total oxidation value The TOTOX value, an indicator of both primary and sec-ondary oxidation products, was calculated according to the fol-lowing equation: which was from Wanasundara and Shahidi (1995), TBARS re-placed para-anisidine value (p-AnV), a marker of secondary ox-idation products, because determination of p-AnV is not alwaysfeasible especially for marine lipids Statistical analysis Measurement of the oxidative stability by OXITEST methodThe oxidative stability evaluation of boiled clam Ruditapes philip- PC and PE contentspinarum was conducted with OXITEST reactor (Velp Scientifica, According to our previous study, PC and PE stand out as theUsmate, Milan, Italy) according to the method described byypredominant components of the PL from clams (Liu et al., 2018).Caruso et al.(2017). The dried clam powder (4.5 g) was placed In the current study, the absolute contents of PC and PE wasin oxidation chambers and performed at constant temperature of1157.24 ±20.58 and 605.44 ±16.28 mg/100 g clam (on the90 °C with an initial pure oxygen pressure of 6 bar. Each ac- dry basis), respectively (Figure 1). After boiling treatment, thecelerated OXITEST was conducted in duplicate. The OXITESTcontents of PC and PE slightly decreased to 1054.15 ±27.77response of induction period (IP) was automatically calculateddand 545.79 ±21.36 mg/100 g, respectively. Then after hot-airfrom the oxidation curve (by the two tangent method) with theedrying process at 50, 70, and 90C, the PC content decreased toOXISoft program. 868.31±12.60,753.50±5.55, and 689.16±16.27 mg/100 g,respectively, while the corresponding values for PE were 464.58 ±11.61,396.87±2.60, and 282.10±5.55 mg/100 g, respectively.Experiments for determination of lipid profiles and lipid oxida-TThus, the absolute contents of PC and PE were both significantlytion indices were conducted in triplicates unless otherwise spec-decreased after the boiling and hot-air drying process, especially forified. Data were presented as mean ± standard deviation (SD).).the higher-temperature-treated samples. As for the clam withoutStatistical analysis was performed using SPSS 16.0 software (SPSSthe boiling procedure for enzyme deactivation, the hot-air dryingInc., Chicago, IL, USA). Differences between means were eval--process at 70°C undoubtedly decreased contents of PC and PEuated by one-way analysis of variance (Student-Newman-Keuls to 776.28±9.26 and 256.08±8.23 mg/100 g, respectively.post hoc test). Comparisons that yielded P values <0.05 wereMoreover, compared to PC, the reduction of PE was far moreregarded as significant. serious (Figure 1). Results Lipid content Drying time for FD-70, BD-50, BD-70 and BD-90 group sam-c ples were 42, 40, 32, and 20 hrs, respectively, and the water contentOof samples were 7.20 ±0.19%,5.65±0.32%,4.05±0.08%, and4.15±0.12%, respectively. Moreover, the lipid extraction ratefor the FC, FD-70, BC, BD-50, BD-70, and BD-90 group sam-ples were 6.71 ±0.19%,7.76±0.19%,6.42±0.07%,6.94±0.02%, 7.01±0.16%, and 8.99 ±0.09% of total tissue weight,respectively. Lipid class composition TLC-FID analysis indicated that the fresh clam contained3580.21 ±42.79 mg/100 g TAG (49.36 ±0.59% of total lipid),2807.74±35.54 mg/100 gpolar lipids (PoL) (38.71 ±0.49%oftotal lipid), 483.79±25.39 mg/100 g FFA (6.67±0.35% of totallipid), 26.11±11.61 mg/100 g diacylglycerol (DAG) (0.36 ±0.16% of total lipid), 17.41±2.18 mg/100 g monoacylglycerol(MAG) (0.24±0.03% of total lipid), and 345.98 ±6.53 mg/100 gcholesterol (CHO)(4.77±0.09% of total lipid) (Table 1). Boiling of shellfish prior to drying is usually required in theindustry. After 15 min of boiling, the contents of PoL, DAG, andMAG increased to 3048.43±22.10,38.67±0.69, and 34.52 土8.98 mg/100 g, respectively, and the contents of TAG, FFA, andCHO decreased to 3511.74±58.00,36.59±6.21, and 258.93±17.95 mg/100 g, respectively. The following hot-air drying processat 50,70, and 90°C reduced the absolute contents of TAG, DAG,MAG, and FFA but increased the contents of PoL and CHOin boiled samples (Table 1). For example, the content of TAGdecreased to 3510.82 ±99.90,3370.94±59.18,and 3205.82±110.68 mg/100 g, respectively, while the content of PoL in-creased to 3527.74 ±115.48,3537.51±33.61, and 5641.61 ±121.93 mg/100 g (Table 1). Obviously, the changes of lipid classescorrelated with processing temperature. With increase of dryingtemperature, the reduction of TAG and the increase of PoL weremore prominent. Meanwhile, for the clam with the absence ofboiling process, hot-air drying process at 70 °C also significantlyreduced the content of TAG and increased the contents of PoL,FFA,DAG, and MAG in clam tissue (Table 1). Table 1-Lipid class (mg/100 g, on the dry basis) of Ruditapes philippinarum tissue subjected to different treatments. Samples TAG PoL FFA DAG MAG CHO 1 3580.21±42.79 2807.74±35.54° 483.79土25.39 26.11±11.61° 17.41±2.18° 345.98±6.53b,c 2 2787.91±30.10 3755.41±57.70° 1103.79±14.22 189.82±5.85 79.44±9.20 444.86±15.89 3 3511.74±58.00 3048.43±22.10° 36.59±6.215 38.67±0.69 34.52±8.98 258.93±17.95° 4 3510.82±99.90° 3527.74±115.48° 24.27±8.83° 4.41±0.74° 5.88±0.74° 280.98±7.36d 5 3370.94±59.18° 3537.51±33.61° 31.42±8.04° 16.80±4.38° 18.26±4.38° 330.96±23.38° 6 3205.82±110.68° 5641.61±121.93 31.89±11.26° 20.63±3.75° 12.19±1.88° 468.02±12.17 a-e Values in the same column with different lower-case letters are significantly different from each other at P <0.05. Samples 1 to 6 are lipids recovered from fresh clam tissue,fresh-dried clam tissue (processed at 70°C), boiled clam tissue, boiled-dried clam tissue (processed at 50, 70 and 90°C, respectively). Abbreviations are: TAG, triacylglycerol; PoL,polar lipid; DAG, diacylglycerol; MAG, monoacylglycerol; FFA, free fatty acid; CHO, cholesterol Table 2-Fatty acid composition (%) of lipids recovered from Ruditapes philippinarum tissue subjected to different treatments. a-f Values in the same row with different lower-case letters are significantly different from each other at P <0.05. Samples 1 to 6 are lipids recovered from fresh clam tissue, Fatty acid composition The FA composition of lipids in clam with different treatmentswas analyzed by GC-MS and results are shown in Table 2. Abroad range of FA was noted, among which C16:1n-7, C16:0,C18:1n-9, C18:0, C20:5n-3, and C22:6n-3 were more abundant.It is worth mentioning that the lipid in fresh clam contained largeproportion of PUFA (46.89 ±0.79% of total FAs), especially EPAand DHA (17.39 ±0.29%,13.50±1.01% of total FAs, respec-tively). The boiling process for enzyme deactivation negligiblyreduced the percentage of saturated fatty acid (SFA) while slightlyincreased the percentage of monounsaturated fatty acid and PUFA.It is worth noting that hot-air drying process at different temper-atures hardly affected most FA composition, especially n-3 PUFAin boiled clam. However, this process significantly decreased theproportion of n-6 PUFA, and consequently increased the PUFAratio ofn-3/n-6. Moreover, for lipids in fresh clam, the hot-air drying process at 70°C reduced SFA proportion from 28.37 to24.96%, with apparent decrease in the contents of C16:0, C17:0,and C18:0. However, the PUFA composition did not changesignificantly. Acid value Figure 1A shows the changes of AV of the lipids in clam un-dergoing different treatments. The AV of lipid in fresh samplewas 56.53±0.75 mg KOH/g lipid, which decreased sharplyto 6.99 ±0.04 mg KOH/g lipid after boiling process. How-ever, the hot-air drying process hardly affected the AV of lipid ofboiled samples regardless of the processing temperature. In con-trast, the hot-air drying process at 70 °℃ significantly increasedg Pthe AV of lipid in fresh sample to 80.76 ±3.50 mg KOH/glipid. BC group. In detail, the POV oflipids in BD-50, BD-70, and BD-90 were 7.49±0.79,22.46±0.39, and 32.70 ±2.76 meq/kglipid, respectively. Meanwhile, the POV of lipid from FD-70 sam-ple (7.09 ± 0.40 meq/kg lipid) was also decreased significantlycompared to that of FC. TBARS The changes of TBARS of the lipids in clam undergoing dif-ferent treatments are shown in Figure 2C. The TBARS of lipidin the fresh sample was 8.26 ±0.10 mg MDA eq/kg lipid, whichslightly decreased to 7.87 ± 0.27 mg MDA eq/kg lipid after boil-ing treatment. The hot-air drying process significantly decreasedthe TBARS of the lipids in boiled sample, and the value for sam-ples BD-50,BD-70 and BD-90 was 4.61±0.11,4.19±0.01, and2.24 ±0.03 mg MDA eq/kg lipid, respectively. Simultaneously,the TBARS of lipid in FD-70 sample was also markedly decreasedto 5.61 ±0.14 mg MDA eq/kg lipid. Change of POV of clam lipids upon different treatments isshown in the Figure 2B. The POV of lipid in fresh clamwas 31.91±1.97 meq/kg lipid, which increased to 35.07 ±1.58 meq/kg lipid after boiling treatment. The POV of lipids inboiled and dried samples were significantly lower than that of the Figure 2-The acid value (AV) (A), peroxide value (POV) (B), thiobarbituric acid reactive substances (TBARS) (C) and total oxidation (TOTOX) value (D)oflipids recovered from Ruditapes philippinarum tissue subjected to different treatments. Samples 1 to 6 are lipids recovered from fresh clam tissue,fresh-dried clam tissue (processed at 70 ℃), boiled clam tissue, boiled-dried clam tissue (processed at 50, 70 and 90C, respectively). af Values in thedifferent column with different lower-case letters are significantly different at P <0.05. The OXITEST monitors the oxygen uptake of the lipid presentin food samples to evaluate the oxidative stability under acceleratedoxidation conditions. The IP value, a parameter representing thetime needed to reach the initial point of lipid oxidation, wasdetermined by calculating the inflection point of oxygen pressurecurve. In the current study, the corresponding value of IP forfreeze-dried boiled clam, hot air-dried clam at 50 C, and hotair-dried clam at 90 °C was 160.6 ±10.1,135.5±5.2, and80.5 ± 0.3 hrs, respectively. Obviously, the hot-air drying processdecreased the IP value of clam. More specifically, the IP value ofthe dried clam decreased with the increasing drying temperature. f Discussion Hot-air drying is widely used in processing of seafood to pre-vent quality deterioration caused by microbial spoilage and nutri-ents degradation, thus effectively extending shelf-life of products.Before drying, a boiling treatment is usually required to deacti-vate microorganisms and enzymes thus improving the stability andquality of the final product (Wang et al., 2011). For the boiled clam, the hot-air drying process decreased thecontents of TAG, PC, and PE, which clearly indicate the hydrol-ysis of lipids. At the same time, the more serious decline of TAG,PC, and PE for the fresh clam that caused by hot-air drying at70 °C further confirmed the hydrolysis of clam lipids during dry--ing process. The above results suggest that the 15 min boiling didnot completely inactivate lipase and phospholipase in clam. Theevidence that lipase from Staphylococcus saprophyticus retained 30%of its activity after 15 min of boiling may support this hypoth-esis (Sakinc, Kleine, & Gatermann, 2007). Xie et al. (2018) alsoDreported that clam Mactra chinensis Philippi retain 22% to 24% of ;lipolytic enzymes activity after boiling process. Interestingly, thesharp increase of PoL and the reduction of TAG were both ob-served in lipids of boiled clam after 90 °C drying, which couldbe ascribed to the polymerization and/or oxidation of TAG athigher temperatures and consequently form products with greaterpolarity (Chen et al., 2017). The hydrolysis of lipids was demonstrated in the boiled anddried clams by the reduction of TAG and the major componentsof PL, namely PC and PE. However, the FFA content and AVwere not increased to the same extent. It was speculated that FFAsreleased from TAG and PL during hot-air drying were oxidizedand degraded because they are more susceptible to oxidation thanTAG and PL (Miyashita, Nara, & Ota, 1994). Shah et al. (2009)also similarly reported that the releasing FFAs from herring during drying process were greatly oxidized. Interestingly, the PUFAproportions in the clam lipids were hardly changed after dryingprocess. This result was supported by two previous studies aboutgrass carp and silverside, in which the PUFA proportion wereslightly changed after hot-air drying (Selmi et al., 2010; Wu &Mao, 2008). Henna-Lu, Nielsen, Timm-Heinrich, and Jacobsen(2011) reported that most PUFA in clam lipids are preferentiallyesterified to PL than TAG. Generally, PUFA in PL are moreresistant to oxidation than those in TAG from the same source(Henna-Lu et al., 2011). Carotenoids in the clam like fucoxanthinmight also exhibit potent antioxidant effect which prevents PUFAfrom oxidation (Maoka, 2011). Moreover, lipase is typically sn-1,3 specific, while PUFA are predominantly esterified to sn-2position of seafood lipid, thus the TAG was also “protected." POV, an indicator of the amount of lipid hydroperoxide, sharply-decreased for all hot-air dried samples. Similarly, Wu and Mao(2008) also reported that the POV of grass carp lipid decreased-from 7.62 to 3.76 meq/kg after hot-air drying process due tothe breakdown of peroxides into the carbonyl components. Thisconfirmed a significant decomposition of hydroperoxide to sec-In the present study, boiling process slightly increased the POVondary oxidation products at higher temperature. Moreover, aof lipids in clams, which indicated the low level of lipid oxida- positive linear correlation existed between POV and drying tem-tion. Accordingly, boiling treatment caused negligible effect onperature in the present study, which indicates a higher generationthe FA composition especially PUFA composition. Previous stud-of more heat stable hydroperoxides than their decomposition of-ies have also demonstrated slight lipid oxidation during boiling厂the hydroperoxide at higher temperatures (Tan, Che Man, Jinap,process of shrimp and grass carp (Becerra et al., 2014; Zhang et al.,& Yusoff, 2002). On the other hand, TBARS, the main represen-2013). However, no obvious changes of FA composition were ob-tative of secondary oxidation products, also decreased dramaticallyserved during boiling process for shrimp (Becerra et al., 2014). after hot-air drying process. A similar decrease in TBARS of alba-Meanwhile, boiling treatment brought about a sharp reductioncore oil after sterilization was also reported by Aubourg, Gallardo,in FFA content and AV value. Similar results have been observedand Medina (1997). Gatellier, Sante-Lhoutellier, Portanguen, andupon boiling of shrimp, which could be ascribed to the leachingKondjoyan (2009) also observed the decrease of TBARS whenof FFAs into hot water during the process (Perez-Santin, Calvo,bovine meat was subjected to high temperature cooking. ThisLopez-Caballero, Montero, & Gomez-Guillen, 2013). Moreover,observation could be explained by the volatilization and loss ofthe remarkable decline of PC and PE contents indicate the hy-aldehydes during hot-air drying at high temperature. In addition,drolysis of PL during boiling process. This could be supportedthese aldehydes can react with amine groups of proteins or freeindirectly by the result of Hoehne-Reitan et al. (2007) that lipaseamino acid and form Schiff bases under relatively high temperatureand phospholipase A2 were abundant in bivalve shellfish (Gatellier et al., 2009; Harkouss et al., 2015; Xiong & Decker,1995). Therefore, the oxidation indices like POV and TBARS-could not offer persuasive information about the degree of lipidoxidation in the drying of clam. The IP obtained by OXITEST method is expressed as“stabil-ity time”before initiation of lipid oxidation and it correspondsto a drop of oxygen pressure due to the consumption of oxygenby the sample (Caruso et al., 2017). The higher IP value corre-sponds with better stability against oxidation and indicates a longershelf-life (Andreou et al., 2017). In this study, significant declineof IP for the hot-air dried clam at elevated temperatures indicatesa higher oxidation level, poor oxidative stability and a reductionof shelf-life (Andreou et al., 2017; Caruso et al., 2017; Verardoet al., 2013). Therefore, a more convincing conclusion could bedrawn that hot-air drying compromises the stability of lipids. Fur-thermore, higher temperature and shorter drying period cause ahigher degree of lipid oxidation than the lower temperature andlonger drying time. These results are of great importance to theseafood processing industry. As a result, the OXITEST methodmay serve as an effective and simple tool for estimation of lipiddeterioration and for evaluating the shelf-life of the hot-air driedseafood. Conclusion Lipid hydrolysis and oxidation both occurred during the hot-airdrying process of clam Ruditapes philippinarum. The hydrolysis of the lipids in clam undergoing hot-air drying process was reflectedby a reduction of TAG content and wastage of major PL includingPC and PE. However, the traditional oxidation measurement ofAV, POV, TBARS or even TOTOX fail to comprehensively reflectthe level of lipid oxidation in clam upon long period hot-air dryingprocess. In contrast, OXITEST method seemed to be an effectivetool for estimating the level of lipid oxidation for hot-air driedclam. Furthermore, high-temperature short-time drying processcaused more serious oxidation degree and reduction of shelf-lifethan the lower-temperature longer-time processing. Acknowledgments This work was financially supported by “Public Science andTechnology Research Funds Projects of Ocean (201505029),”“Project of Distinguished Professor of Liaoning Province (2015-153),”“Program for Liaoning Excellent Talents in University(LR2015006),”“Liaoning Provincial Natural Science Foundationof China (2015020781),” and Program for“Dalian High-LevelInnovative Talent (2015R0007). Author Contributions Zhongyuan Liu carried out the experiment and prepared themanuscript. Xin Zhou, Deyang Li, and Hongkai Xie helped todeal with the sample for experiment. Fawen Yin and Qi Zhao con-tributed with date processing and valuable discussions. Both Day-ong Zhou and Beiwei Zhu (corresponding author) conducted theexperimental design. Tong Wang and Fereidoon Shahidi helpedto revise the paper carefully. References ( Akonor, P . T., Ofori, H ., D ziedzoave, N . T ., & K o rtei, N . K . ( 2 016). Drying characteris t ics a nd physical and nutritional properties of shrimp meat as affected by diffe r ent traditional drying t echniq u es. International Journal of Food Sci e nce, 2016, htt p s://d o i.org/10.1155/2016/7879097. ) ( A li, A., Ahmadou, D. , Mohamadou, B., S a ido u , C., & Te n in, D. (20 1 1) . Inf l ue n ce of tradit i onal d rying and s m oke-dr y ing on the quality of t h ree fish species (Tilapia nilotica, Silurus g l anis andA r ius p arkii ) from Lagdo L ake, C ameroon. Journal of Animal an d Vete r inary A d vances, 10(3), 301- 3 06. ) ( Andreou, V, D i m opoulos, G. , Ale x andr a kis, Z . , K at saros, G., Oi konomou, D., T oe pf l, S. , . . . T a oukis , P . ( 2 0 17). S h elf - li f e e v aluation o f v i r g in o l ive o i l extract e d f r om ol i ve 1 0 s s ubjected t onon-thermal pretreatm e nts for yield increase. Inn o vative Foo d Science 8 E m e rg i n g Technologies, . 40 , 52 - 57. ) AOCS. (2003). Official method Cd 8b-90. Sampling and analysis ofcommercial fats and oils: Peroxide value, acetic acid-isooctane method. Champaign-Urbana. Illinois: American Oil Chemists’Society. ( AOCS. (2 0 09a). Of f icial method Cd 3d - 63. Sampl i ng and analysi s o f com m er c ial fats a nd oils: A cid v a lue. C hampaign-Urb a na. Il l inois: A merican Oil Chemists’Soc i ety. ) ( Aubourg, S.P, Gallard o ,J. M. , & M e dina, I.(19 9 7 ) . Chan g es in lipids during different steril i zingc o nditions in ca n ning albacore (Thu n nus alalunga) in oil. In t ernati o nal Journal of F o od Science 8 T echnology, 3 2(5), 4 2 7-431. ) ( A ubourg, S . P, Tabilo- M unizaga, G . , Reyes, J. E . , Rodriguez, A ., & P erez- W on, M. (2010). E ffec t of high-pressure treatment o n microbial act i vity and l ipid oxidation in chill e d coho s almon. European Journal of Lipid Scien c e a nd T e chnology, 112(3) , 362- 3 7 2. ) ( B ecer r a,J . A . H ., Fl o res, A . A . O., Valerio-Alfaro, G., S oto-Rodriguez, I ., Ro d riguez-Estrada, M . T, & G a rcia , H . S . (2014). C holester o l o xidation a nd a staxanthin degradation in shrimpduring sun drying and storage. F ood C hemist r y, 1 45, 832-839 ) ( Bligh, E. G . , & D yer, W . J. ( 1959). A rapid method of total l i pid e xtrac t ion a nd puri f ic a tion. C a n adian Journal of B i ochemistry and Physiology, 37(8), 9 11- 9 17. ) ( Caruso , M . C . , G a l g ano, F, Co l angelo, M. A., Con d elli, N., S carpa, T ., To lv e, R., & F av at i , F ( 2017). E valua t ion of the oxidativ e stabilit y of ba k ery produ c t s b y OXI T EST me thod ands e nsory analysis. European Food Research and Technolog y ,243(7) , 118 3- 1191. ) ( Chaijan, M ., P anpipat, W ., & N i soa, M . ( 2 017 ) . C h e m ical de t erioration and di s colorat i on o f semi- d ried tilapia processed by sun drying and microwave dr y ing. D r y ing Technology, 35 (5 ),642-649. ) ( C h e n, H., Cao, P, Li , B., S u n, D . , L i , J, & L i u, Y. (2017). H igh sensitive and efficient d etectionof edible oils adulterated with used f rying oil by electron s pin r esonance. Fo od Con t rol, 7 3 , 5 40- 5 45. ) ( Choi, E ., & Park , Y. ( 2016). Th e a s sociatio n be t ween th e consumption of f i sh / shellfish and therisk of osteoporosis i n men and postmenopausal women ag e d 50 years or older. N u t rients, 8 8((3) , 113 . ) ( Cook, C . M . , H allaraker, H . , S e bo , P. C . , I n n is, S . M . , Ke l ley, K . M., S a noshy, K . D., & Maki, K . C . ( 2 01 6 ). B i oavailability of long c h ain o mega-3 polyunsaturated f atty a c ids f r om phospholipid-r i ch h e rring roe oil i n men an d wom e n with mildly elevated triacylglycerols. Prostaglandins L eukotriene s and Essential F a tty Acids, 1 1 1 , 17 - 24. ) ( Duan, Z., Jiang, L ., Wang,J, Y u, X. , & Wa n g, T.(2 0 1 1 ). Drying and quality characteristics of t ilapia fish fi l le t s dried wit h hot-ai r -microwave heating. F o od an d Bi o products Processing, 89(4) , 4 72- 4 76. ) ( F A O . (2 0 18). FI GI S list of species f or fishery glo b al production statisti c s. Retrieved from URL h ttps: // www.fao.org/f i shery/statistics/en A c c esse d 1 8. 0 8. 24. ) ( Ga t ellier, P, Sante - Lhoutellier, V , Port a nguen, S . , & K o ndjoyan, A. (2 0 09). Use o f m ea t f luore s cence e m ission as a m arker of oxidation promoted by c ooking. Meat Science, 83 (4 ),651-656 . ) ( H arkouss, R . , A stru c , T ., Le bert, A. , G atell i er, P . , L o ison, O. , Safa, H ., M irade, P .S . ( 2015). Quantitative study of the relationships among proteolysi s, lipid oxidation, s t ruct u re and texture throug h out the dry-cured ham process. Food Che m istry, 166,522-530 . ) ( H enna-L u , F H . , N i e lsen, N . S., T imm-He i nrich, M . , & Jacobsen, C. (2 0 11). Oxidative sta b ility o f marine p h ospholipi d s in the l i posomal form and their a p plications. Lipids, 46 ( 1),3 -2 3 . ) ( Hoehne-Re i tan, K., Ok l and, S. N ., & Rei t an, K. I. ( 2 0 0 7). Ne u t ral lipase and pho s pholipase a ctivities in sc a llop j uveniles (Pecten maximus) and dietary algae. Aqu a c u lture Nutri ti on, 13(1), 45-49. ) ( J ohn, L . , C ornforth, D. , Ca r penter, C . E . , S o rheim, O. , P e tt e e, B. C. , & W h i ttier, D. R . ( 2005). C o l or and t h iobar b ituric a c i d v a lues of c ooke d t o p sirloin steaks packaged in modifiedatmospher e s o f 80% o x ygen, or 0. 4 % c ar bon mon o xide, or v a cu u m. Meat S c ience, 69( 3 ), 441-449. ) Lee, K. H., Cho, T.Y., Cho, H. S., Lee,J. H., & Shim, K. H. (1998). Lipid oxidation in shellfishunder the different conditions of drying. Korean Journal of Fisheries and Aquatic Sciences, 31(2),143-148. ( Liu, L . , Bartke, N ., Van D aele, H. , L a wrence, P, Qin, X., Par k , H. G., & B renna, J. T. ( 2 0 1 4 ) . H igher effic a cy o f dietary DHA p r o vided as a p h o spholipid than as a t r i g l yceride for b r ain D HA accretion in neonatal pig l ets. Jo u rnal of Lipi d Research, 5 5(3), 5 31-539. ) Liu, Z. Y, Zhou, D. Y, Wu, Z. X., Yin, EW, Zhao, Q., Xie, H. K.,... Shahidi, E (2018).Extraction and detailed characterization of phospholipid-enriched oils from six species ofedible clams. Food Chemistry, 239, 1175-1181. Liu, Z. Y, Zhou, D. Y, Zhao, Q., Yin, F W, Hu, X. P, Song,L.,... Shahidi, F (2017).Characterization of glycerophospholipid molecular species in six species of edible clams byhigh-performance liquid chromatography-electrospray ionization-tandem mass spectrometry.Food Chemistry,219, 419-427. Maoka, T.(2011). Carotenoids in marine animals. Marine Drugs, 9, 278-293. Miyashita, K., Nara, E.,& Ota, T. (1994). Comparative study on the oxidative stability of phos-phatidylcholines from salmon egg and soybean in an aqueous solution. Bioscience, Biotechnologyand Biochemistry, 58(10), 1772-1775. ( M urphy, K . , M a nn, N., & S inc l air, A. ( 2003). Fat t y acid and sterol composition of frozen and f reeze- d ried N ew Ze a lan d green lip p ed mus s el (Perna canal i culus) fr o m three sites in New . Zealand. A s ia Pa c ific J o urnal of C l i n ical N u t rition, 12 ( 1), 50-60 . ) ( Perez- S antin, E., C alvo , M. M., Lopez- C aballero, M. E . , Montero, P, & Gomez-Guillen, M. C . ( 2013 ) . C ompositional pr o perties and bi o active p o tential o f waste m a terial f r om shrimp cooking juice. L WT-Fo o d Science a n d T e c h nology, 5 4(1), 87- 9 4 . ) Ramprasath, V.R., Eyal,I., Zchut, S., & Jones, P.J.(2013). Enhanced increase of omega-3 indexin healthy individuals with response to 4-week n-3 fatty acid supplementation from krill oilversus fish oil. Lipids in Health and Disease, 121(1),178. Sakinc, T, Kleine, B., & Gatermann, S. G. (2007). Biochemical characterization of thetsn: 27urface-associated lipase of Staphylococcus saprophyticus. FEMS Microbiology Letters, 274(2),335-341. Selmi, S., Bouriga, N., Cherif, M., Toujani, M., &Trabelsi, M. (2010). Effects of drying processon biochemical and microbiological quality of silverside (fish) Atherina lagunae. InternationalJournal of Food Science 8Technology, 45(6),1161-1168. Shah, A. A., Tokunaga, C., Kurihara, H., & Takahashi, K. (2009). Changes in lipids and theircontribution to the taste of migaki-nishin (dried herring fillet) during drying. Food Chemistry,115(3),1011-1018. Tan, C. P, Man, Y. C., Jinap, S., & Yusoff, M. S. A. (2002). Effects of microwave heating onthe quality characteristics and thermal properties ofRBD palm olein. Innovative Food Science Emerging Technologies, 3(2), 157-163. ( Verardo, V, R iciputi, Y. , Sorrenti , G . , Ornaghi , P,Marangoni, B . , & Ca bo ni, M. E ( 2 0 13). Effect of n itroge n fertilisation rates on the c onten t of fatty aci d s, s te r ols, tocopherols a n d p h enoliccompounds, and on t he oxidative stability of walnuts. LWT-Foo d S c ie nc e and T e chno l ogy, 50(2) , 732-738. ) ( Wanasundara, U . N. , & Shahidi, E ( 1 995). Sto r age stab i lity of microencapsulated seal blubber oil. Journal of Food Lipids, 2 ( 2) , 73-86. ) Wang, Y., Zhang, M.,& Mujumdar, A. S. (2011). Trends in processing technologies for driedaquatic products. Drying Technology, 29(4), 382-394. ( Wu, T , & Ma o , L. ( 2 00 8). Inf l uences of hot-air drying and microwave drying on nutri t ional a nd odorous pr o perti e s of grass ca r p (Cten o pharyngodon idel l us) fil l ets . F o od Che m istry, 11 0( 3 ), 647-653. ) ( X ie, W ., C h e n, C.,Liu , X . , W a ng, B. , Sun, Y , Ya n , M. , & Z ha n g, X. ( 2 01 2 ). M e retrix meretrix: Active components and thei r bioactivitie s . Life Sc i enc e J o urnal, 9 ( 3 ) , 7 56-762. ) Xie, H. K., Zhou, D. Y, Hu, X. P, Liu, Z. Y., Song, L., & Zhu, B. W. (2018). Changes ( i n lipid profiles of dried c lams (M a ctra c hinensis P h ilippi and Ruditapes ph i lippinarum) duringaccelerated storage and prediction of shelf li f e. Journal of A g ricultural and Food Ch e mistry,66(29), 7764-7774 ) ( X iong, Y . L ., & D e ck e r,E. A . ( 1 9 9 5). Alt e rat i on of mu s cle pr o tein fun c tionality by oxidative a nd antioxidative p roces s es. Journal of Muscle Foods , 6, 139-160. ) ( Y in, E W ., Li u , X . Y., F an, X . R ., Zhou, D. Y . , X u, W. S., Z h u, B. W., & Mur a ta, Y. ( 2 01 5 ). E xtrusion of An t arctic k ri l l (Eup h ausia superba) meal and its e ffect on oi l ex t ract i on. Inter na tional Journal of F o od S cience & T echnolo g y , 5 0(3), 6 33- 6 39. ) ( Zhang, J , W u,D.,Liu , D., Fa n g, Z., Chen,J., Hu, Y., & Y e , X . ( 2 01 3 ). E f fect of cooking styles o n the l ipid o xidation and fatty aci d composition of grass ca r p (Ctenopharynyodon idel l us ) fillet. J ournal of F o od Biochemistry , 37(2),212-21 9 . ) ournal of Food Science·Vol. Iss. Vol. Iss. ·Journal ofFood Science 利用VELP Oxitest方法分析蛤仔在热风干燥过程中的脂质氧化水平:通过热风干燥处理菲律宾蛤仔,并通过分析脂质类别,磷脂类别,脂肪酸以及包括过氧化物值(POV),硫代巴比妥酸反应性物质(TBARS)值在内的氧化参数来评估其脂质的变化,总氧化值(TOTOX)和氧化测试(OXITEST)。热风干燥过程降低了三酰基甘油,磷脂酰胆碱和磷脂酰乙醇胺的含量,表明脂质的水解。同时,热风干燥过程显着降低了n-6多不饱和脂肪酸(PUFA)的比例,因此提高了n-3 / n-6的PUFA比。有趣的是,经过热风干燥后,POV,TBARS和TOTOX下降了。但是,诱导期的显着下降干燥的蛤tissue组织在高温下显示出较高的氧化水平,较差的氧化稳定性和货架期的缩短。 因此,OXITEST方法被证明是一种估计热风干燥蛤脂质氧化水平的有效工具。
确定





还剩5页未读,是否继续阅读?
意大利VELP公司为您提供《蛤仔中油脂氧化检测方案(氧化分析仪)》,该方案主要用于渔业中理化分析检测,参考标准--,《蛤仔中油脂氧化检测方案(氧化分析仪)》用到的仪器有VELP Oxitest 油脂氧化分析仪
推荐专场
相关方案
更多