方案详情
文
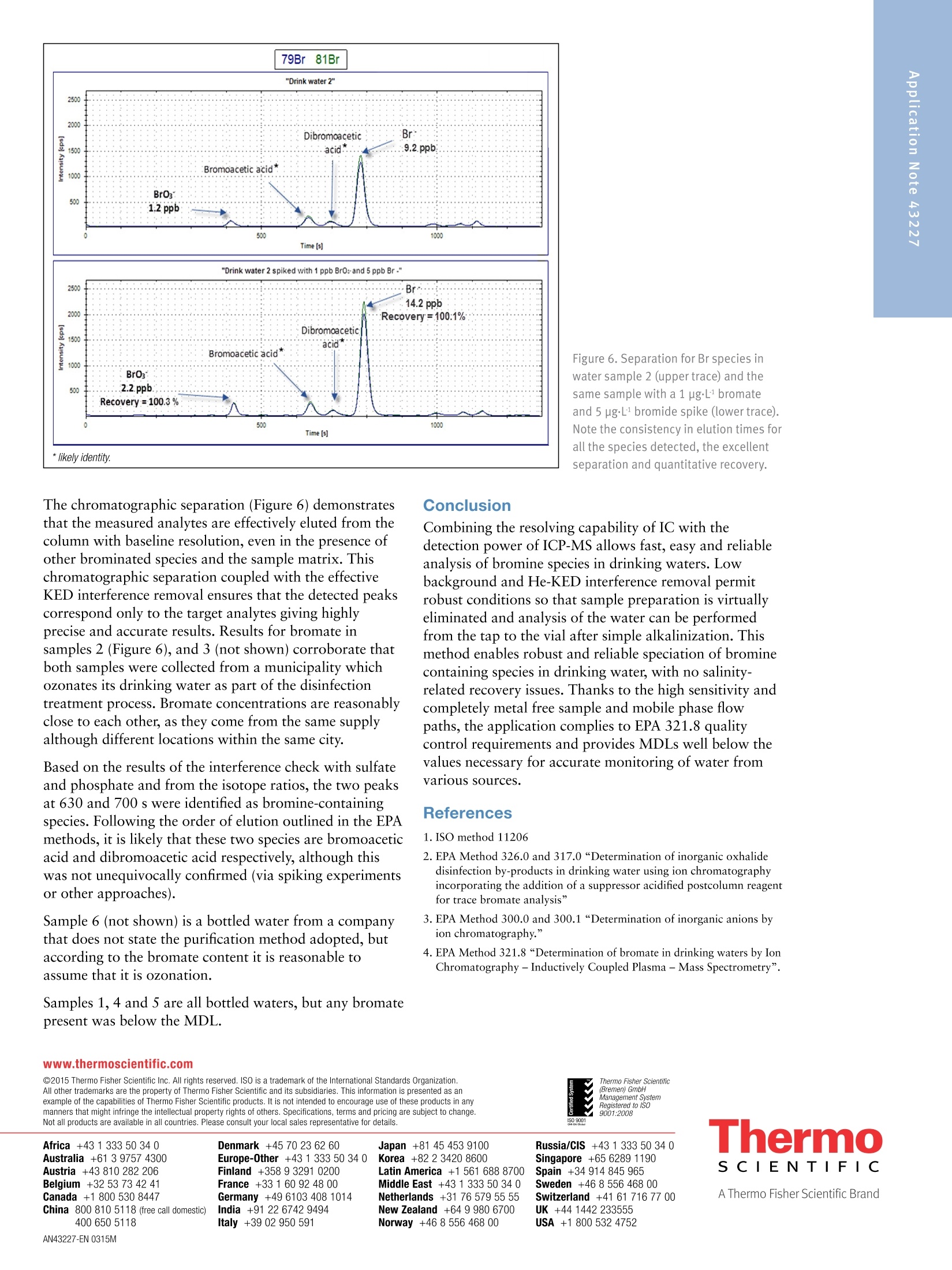
Combining the resolving capability of IC with the detection power of ICP-MS allows fast, easy and reliable analysis of bromine species in drinking waters. Low background and He-KED interference removal permit robust conditions so that sample preparation is virtually eliminated and analysis of the water can be performed from the tap to the vial after simple alkalinization. This method enables robust and reliable speciation of bromine containing species in drinking water, with no salinity-related recovery issues. Thanks to the high sensitivity and completely metal free sample and mobile phase flow paths, the application complies to EPA 321.8 quality control requirements and provides MDLs well below the values necessary for accurate monitoring of water from various sources.
方案详情

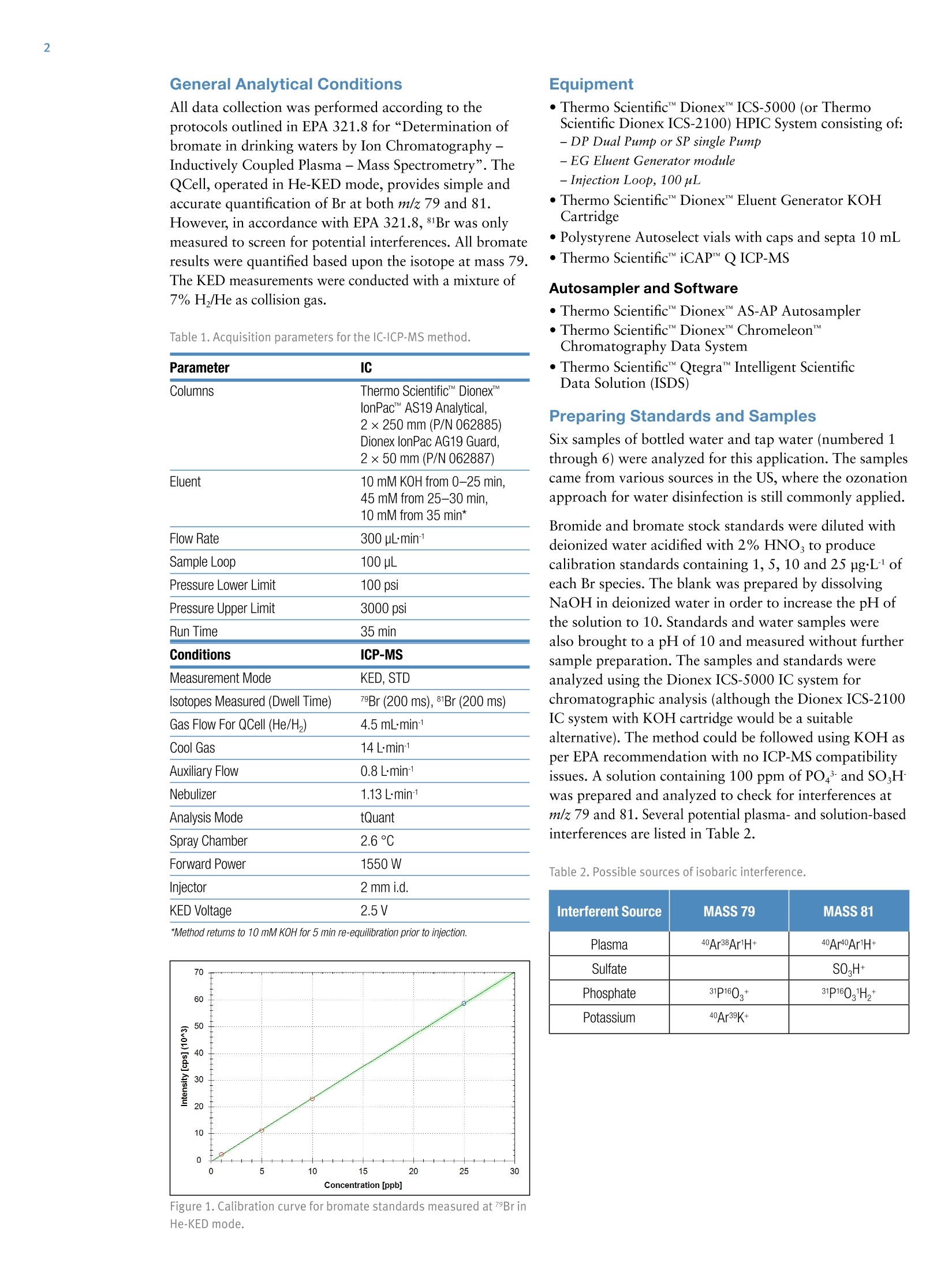
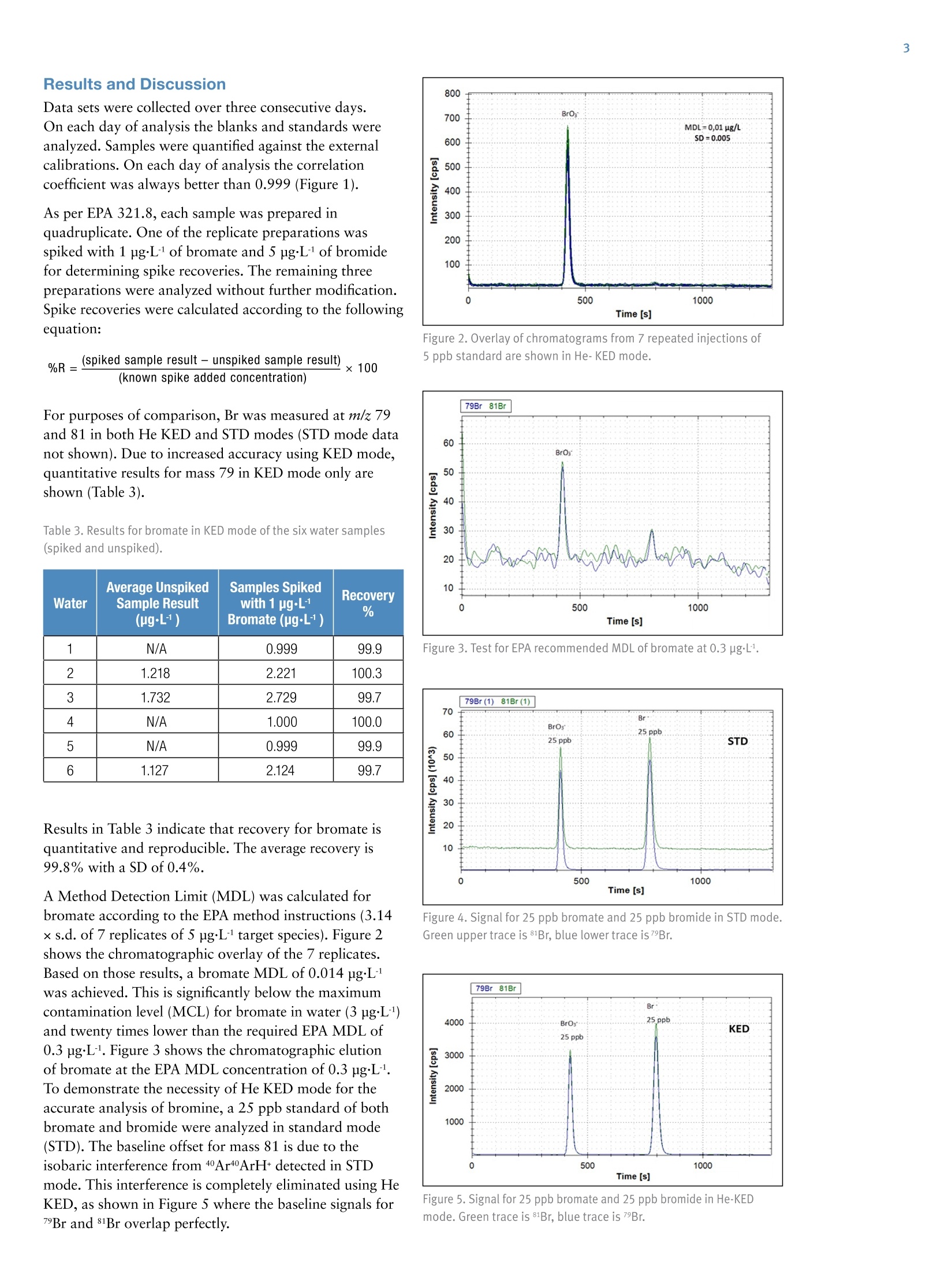
AThermo Fisher Scientific Brand Speciation of Bromine Compounds inOzonated Drinking Water using lonChromatography and Inductively CoupledPlasma Mass Spectrometry Antonella Guzzonato1, Shona McSheehy Ducos1, Daniel Kutscher1, Carl Fisher 1Thermo Fisher Scientific, Bremen, Germany,2Thermo Fisher Scientific, Sunnyvale, CA, USA Bromate, Drinking Water, EPA 321.8, Inductively Coupled Plasma MassSpectrometry, Ion Chromatography, Speciation Speciate and quantify bromine (Br) species in drinking water by means ofion chromatography (IC) coupled with Inductively Coupled Plasma MassSpectrometry (ICP-MS). Applying the Environmental Protection Agency(EPA) method 321.8 for bromate detection in drinking water. Introduction Bromine speciation in drinking water is required worldwideby major regulatory bodies. A maximum contaminantlevel (MCL) of 10 pg·L in the US for bottled drinkingwater and in the EU of 3 pgL. for natural mineral waterand spring water treated with ozonation are stipulated forthe bromate anion. As a result of the ozonation process,a common water disinfection method, bromate can beformed via the oxidation of the naturally occurringbromide. Whereas bromide is non-toxic, bromate istoxic and carcinogenic. Differentiating bromate from bromide is therefore importantdue to the toxicity differences between the two species. Bromine analysis with ICP-MS is challenging because theionization potential of bromine is relatively high and itlowers sensitivity in ICP based techniques. Additionally,there are a number of isobaric interferences on m/z 81 and79 (the two Br stable isotopes) as shown in Table 2. The United States Environmental Protection Agency (EPA)and the International Organization for Standardizationhave developed several methods for bromate determinationin drinking water (EPA 300, 300.1, 302.0,317,326: ISO10304-4 and 15061) all of which have been validated inThermo Scientific"Dionex" application notes (AN167,AN168, AN171,AN184,AN187). The EPA Method 321.8 provides an analytical procedurefor bromate determination in drinking water usingIC-ICP-MS.Various methods for bromate analysis existbut EPA 321.8 is the only method that does not requirethe following: sample pretreatment to remove chlorides,post-column derivatization, and chemical conductivity suppression with dangerous reagents such as sulfuric acid.This method significantly reduces the risk for contamination,eliminates the use of 2-Dimensional IC, has the advantageof a stable and quantitative bromate recovery (Table 1,Figure 6) through the IC column and it tolerates high saltconditions. The method also provides guidance forreducing polyatomic interferences that overlap with thetwo most abundant Br isotopes. In this method, a Thermo Scientific""DionexICS-5000IC was coupled to a Thermo ScientificiCAP""QICP-MS.The iCAP Q ICP-MS is equipped with a collision/reactioncell, the Thermo Scientific"QCell", which enables a singleinterference reduction approach using He-KED (KineticEnergy Discrimination). This measurement mode filtersout polyatomic interferences on bromine and allows forthe accurate quantification of bromide and bromatespecies, all without the need for additional interferencecorrection steps. Additionally, the QCell design maintainshigh transmission and high sensitivity so that quantificationat the sub-ppb levels required by the EPA method can beeasily achieved. All data collection was performed according to theprotocols outlined in EPA 321.8 for“Determination ofbromate in drinking waters by Ion Chromatography-Inductively Coupled Plasma - Mass Spectrometry”. TheQCell, operated in He-KED mode, provides simple andaccurate quantification of Br at both m/z 79 and 81.However, in accordance with EPA 321.8,81Br was onlymeasured to screen for potential interferences. All bromateresults were quantified based upon the isotope at mass 79.The KED measurements were conducted with a mixture of7% H/He as collision gas. Table 1. Acquisition parameters for the IC-ICP-MS method. Parameter IC Columns Thermo ScientificM Dionex lonPac AS19 Analytical, Eluent 2×250 mm (P/N 062885) Dionex lonPac AG19 Guard. 2×50 mm (P/N 062887) 10 mM KOH from 0-25 min. 45 mM from 25-30 min. 10 mM from 35 min* Flow Rate 300 pLmin1 Sample Loop 100 pL Pressure Lower Limit 100 psi Pressure Upper Limit 3000 psi Run Time 35 min Conditions ICP-MS Measurement Mode KED, STD Isotopes Measured (Dwell Time) 79Br (200 ms), 81Br (200 ms) Gas Flow For QCell (He/H,) 4.5 mL·min1 Cool Gas 14 Lmin1 Auxiliary Flow 0.8 Lmin-1 Nebulizer 1.13 L·min-1 Analysis Mode tQuant Spray Chamber 2.6°℃ Forward Power 1550 W Injector 2 mm i.d. KED Voltage 2.5V *Method returns to 10 mM KOH for 5 min re-equilibration prior to injection. ·Thermo Scientific""Dionex""ICS-5000 (or ThermoScientific Dionex ICS-2100) HPIC System consisting of:-DP Dual Pump or SP single Pump -EG Eluent Generator module -Injection Loop, 100 pL ·Thermo Scientific Dionex" Eluent Generator KOHCartridge ·Polystyrene Autoselect vials with caps and septa 10 mL· Thermo Scientific"iCAP QICP-MS Autosampler and Software · Thermo Scientific"DionexAS-AP Autosampler·Thermo Scientific"Dionex ChromeleonMChromatography Data System · Thermo Scientific" Qtegra" Intelligent ScientificData Solution (ISDS) Preparing Standards and Samples Six samples of bottled water and tap water (numbered 1through 6) were analyzed for this application. The samplescame from various sources in the US, where the ozonationapproach for water disinfection is still commonly applied. Bromide and bromate stock standards were diluted withdeionized water acidified with 2%HNO, to producecalibration standards containing 1, 5, 10 and 25 pg·Lofeach Br species. The blank was prepared by dissolvingNaOH in deionized water in order to increase the pH ofthe solution to 10. Standards and water samples werealso brought to a pH of 10 and measured without furthersample preparation. The samples and standards wereanalyzed using the Dionex ICS-5000 IC system forchromatographic analysis (although the Dionex ICS-2100IC system with KOH cartridge would be a suitablealternative). The method could be followed using KOH asper EPA recommendation with no ICP-MS compatibilityissues. A solution containing 100 ppm of POand SOHwas prepared and analyzed to check for interferences atm/z 79 and 81. Several potential plasma- and solution-basedinterferences are listed in Table 2. Table 2. Possible sources of isobaric interference. Interferent Source MASS 79 MASS 81 Plasma 40Ar38Ar1H+ 40Ar40Ar1H+ Sulfate SO,H+ Phosphate 31P160+ 31P1601H,+ Potassium 40Ar39K+ Results and Discussion Data sets were collected over three consecutive days.On each day of analysis the blanks and standards wereanalyzed. Samples were quantified against the externalcalibrations. On each day of analysis the correlationcoefficient was always better than 0.999 (Figure 1). As per EPA 321.8, each sample was prepared inquadruplicate. One of the replicate preparations wasspiked with 1 pgL of bromate and 5 pg·L of bromidefor determining spike recoveries. The remaining threepreparations were analyzed without further modification.Spike recoveries were calculated according to the followingequation: %R=(spiked sample result - unspiked sample result) x 100(known spike added concentration) For purposes of comparison, Br was measured at m/z 79and 81 in both He KED and STD modes (STD mode datanot shown). Due to increased accuracy using KED mode,quantitative results for mass 79 in KED mode only areshown (Table 3). Table 3. Results for bromate in KED mode of the six water samples(spiked and unspiked). Water Average UnspikedSample Result(ug·L) Samples Spikedwith 1 pg·L1Bromate (ug·L) Recovery % 1 N/A 0.999 99.9 2 1.218 2.221 100.3 3 1.732 2.729 99.7 4 N/A 1.000 100.0 5 N/A 0.999 99.9 6 1.127 2.124 99.7 Results in Table 3 indicate that recovery for bromate isquantitative and reproducible. The average recovery is99.8% with a SD of 0.4%. A Method Detection Limit (MDL) was calculated forbromate according to the EPA method instructions (3.14x s.d. of 7 replicates of 5 pgL target species). Figure 2shows the chromatographic overlay of the 7 replicates.Based on those results, a bromate MDL of 0.014 pg·Lwas achieved. This is significantly below the maximumcontamination level (MCL) for bromate in water (3 ugL)and twenty times lower than the required EPA MDL of0.3 ugL1. Figure 3 shows the chromatographic elutionof bromate at the EPA MDL concentration of 0.3 ng·L1.To demonstrate the necessity of He KED mode for theaccurate analysis of bromine, a 25 ppb standard of bothbromate and bromide were analyzed in standard mode(STD). The baseline offset for mass 81 is due to theisobaric interference from 40Ar40ArH+ detected in STDmode. This interference is completely eliminated using HeKED, as shown in Figure 5 where the baseline signals for7Br and 81Br overlap perfectly. Figure 2. Overlay of chromatograms from 7 repeated injections of5 ppb standard are shown in He-KED mode. Figure 3. Test for EPA recommended MDL of bromate at 0.3 ug·L1. Figure 4. Signal for 25 ppb bromate and 25 ppb bromide in STD mode.Green upper trace is 81Br, blue lower trace is 7Br. Br* 79Br 81Br KED The chromatographic separation (Figure 6) demonstratesthat the measured analytes are effectively eluted from thecolumn with baseline resolution, even in the presence ofother brominated species and the sample matrix. Thischromatographic separation coupled with the effectiveKED interference removal ensures that the detected peakscorrespond only to the target analytes giving highlyprecise and accurate results. Results for bromate insamples 2 (Figure 6), and 3 (not shown) corroborate thatboth samples were collected from a municipality whichozonates its drinking water as part of the disinfectiontreatment process. Bromate concentrations are reasonablyclose to each other, as they come from the same supplyalthough different locations within the same city. Based on the results of the interference check with sulfateand phosphate and from the isotope ratios, the two peaksat 630 and 700 s were identified as bromine-containingspecies. Following the order of elution outlined in the EPAmethods, it is likely that these two species are bromoaceticacid and dibromoacetic acid respectively, although thiswas not unequivocally confirmed (via spiking experimentsor other approaches). Sample 6 (not shown) is a bottled water from a companythat does not state the purification method adopted, butaccording to the bromate content it is reasonable toassume that it is ozonation. Samples 1, 4 and 5 are all bottled waters, but any bromatepresent was below the MDL. Conclusion Combining the resolving capability of IC with thedetection power of ICP-MS allows fast, easy and reliableanalysis of bromine species in drinking waters. Lowbackground and He-KED interference removal permitrobust conditions so that sample preparation is virtuallyeliminated and analysis of the water can be performedfrom the tap to the vial after simple alkalinization. Thismethod enables robust and reliable speciation of brominecontaining species in drinking water, with no salinity-related recovery issues. Thanks to the high sensitivity andcompletely metal free sample and mobile phase flowpaths, the application complies to EPA 321.8 qualitycontrol requirements and provides MDLs well below thevalues necessary for accurate monitoring of water fromvarious sources. References ( 1. ISO method 11206 ) ( 2. EPA Method 326.0 and 317.0“Determination of inorganic oxhalide disinfection by-products in drinking water using io n chromatographyincorporating t he addition of a suppressor acidified postcolumn reagent f or trace bromate a nalysis” ) ( 3. EPA Method 300.0 and 300.1“De t ermination of inorganic anions byion chromatography." ) ( 4 . EPA Method 321.8“De t ermination of bromate in drinking waters by IonChromatography-Inductively Co u pled Plasma - Mass Spectrometry”. ) Denmark +45 70 23 62 60 Japan +81 45 453 9100 Europe-Other +43 1 333 50 340 Korea +82 23420 8600 Singapore +65 6289 1190 Finland +358932910200 Latin America +1 561 6888700 France +33160924800 Middle East +43 1 333 50 34 0 Sweden +46 8 556 468 00 Germany +49 6103 408 1014 Netherlands +31 76 579 55 55 India +91 22 6742 9494 New Zealand +64 9 980 6700 Norway +46 8 556 46800 ISO 9001 Russia/CIS +43 1 333 50 34 0 Spain +34 914 845 965 Switzerland +41 61 716 77 00 UK +44 1442 233555 USA +1 800 532 4752 Combining the resolving capability of IC with the detection power of ICP-MS allows fast, easy and reliable analysis of bromine species in drinking waters. Low background and He-KED interference removal permit robust conditions so that sample preparation is virtually eliminated and analysis of the water can be performed from the tap to the vial after simple alkalinization. This method enables robust and reliable speciation of bromine containing species in drinking water, with no salinity-related recovery issues. Thanks to the high sensitivity and completely metal free sample and mobile phase flow paths, the application complies to EPA 321.8 quality control requirements and provides MDLs well below the values necessary for accurate monitoring of water from various sources.
确定

还剩2页未读,是否继续阅读?
赛默飞色谱与质谱为您提供《Drinking Water中Speciation of Bromine Compounds检测方案(离子色谱仪)》,该方案主要用于饮用水中消毒副产物检测,参考标准--,《Drinking Water中Speciation of Bromine Compounds检测方案(离子色谱仪)》用到的仪器有赛默飞Dionex ICS-5000+ CD 电导检测器
推荐专场
相关方案
更多
该厂商其他方案
更多