方案详情
文
内源性硫化氢能够调节血管静张度(血管弹性)。基于钙独立机制和钾离子通道机理研究硫化氢对于肠膜小动脉的松弛研究较少,应用unisense微电极系统监测老鼠肠膜小动脉中测试了硫化氢的含量,讨论了硫化氢的含量对于肠膜小动脉血管的松弛的影响。
方案详情

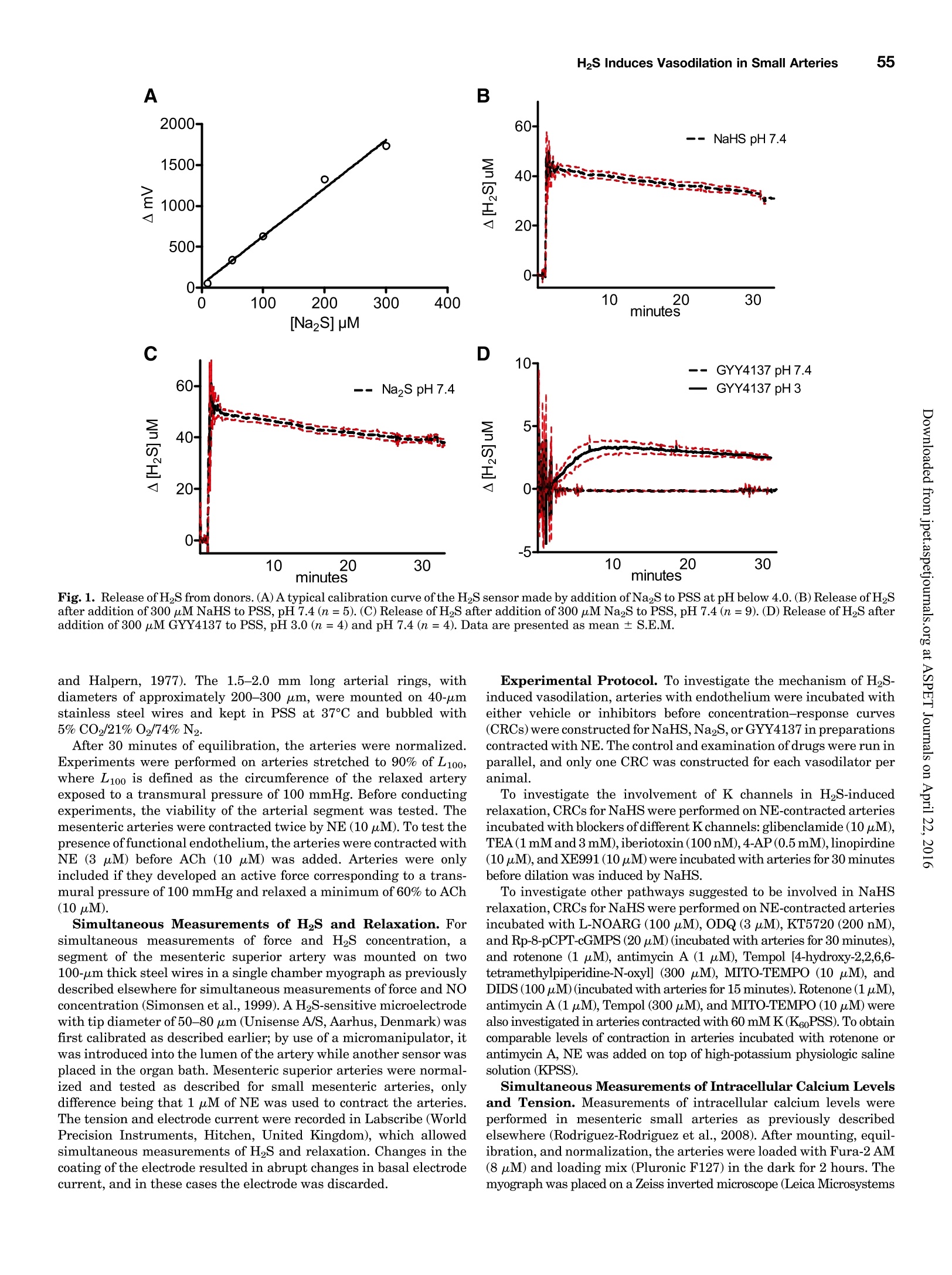
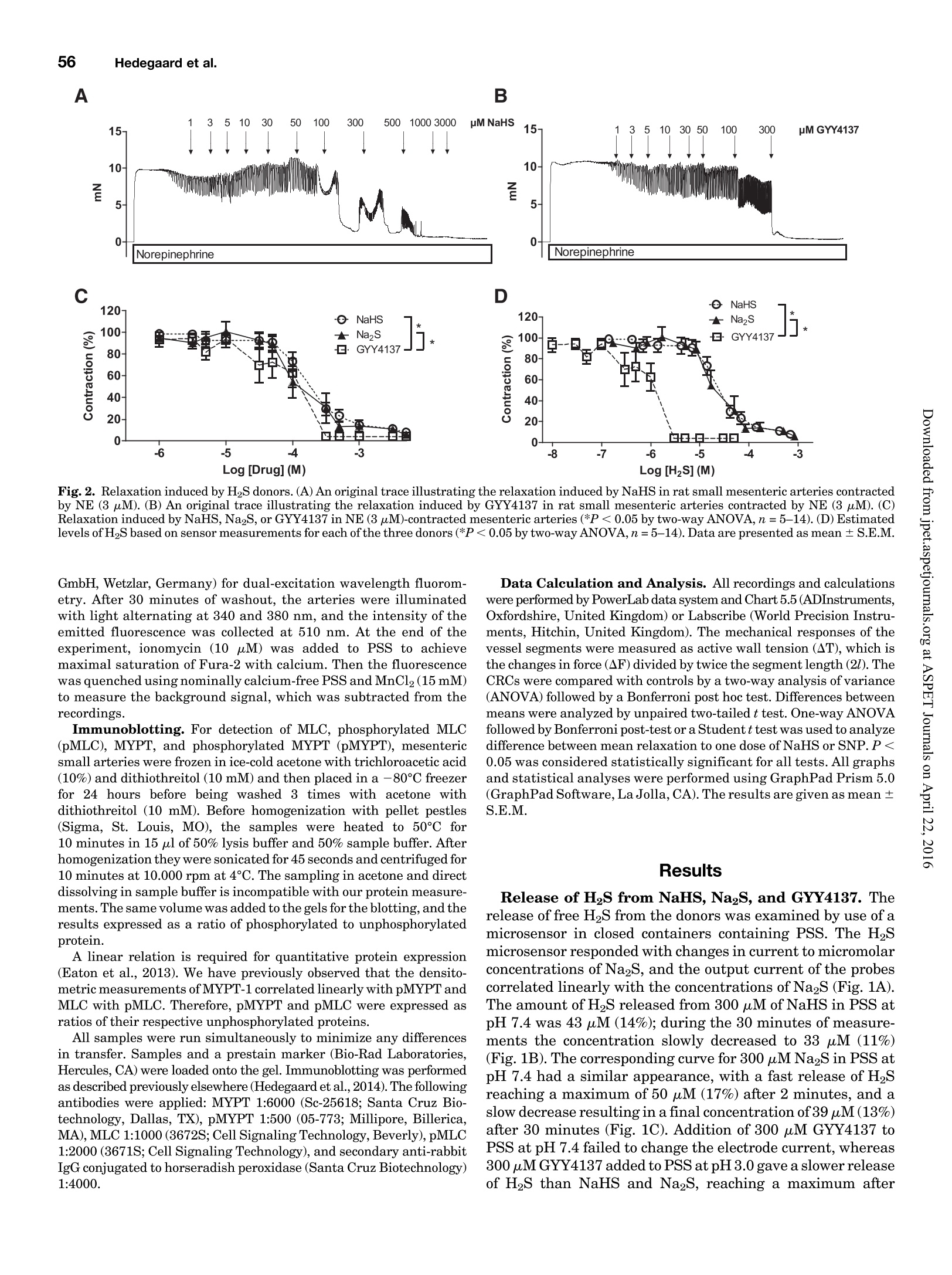
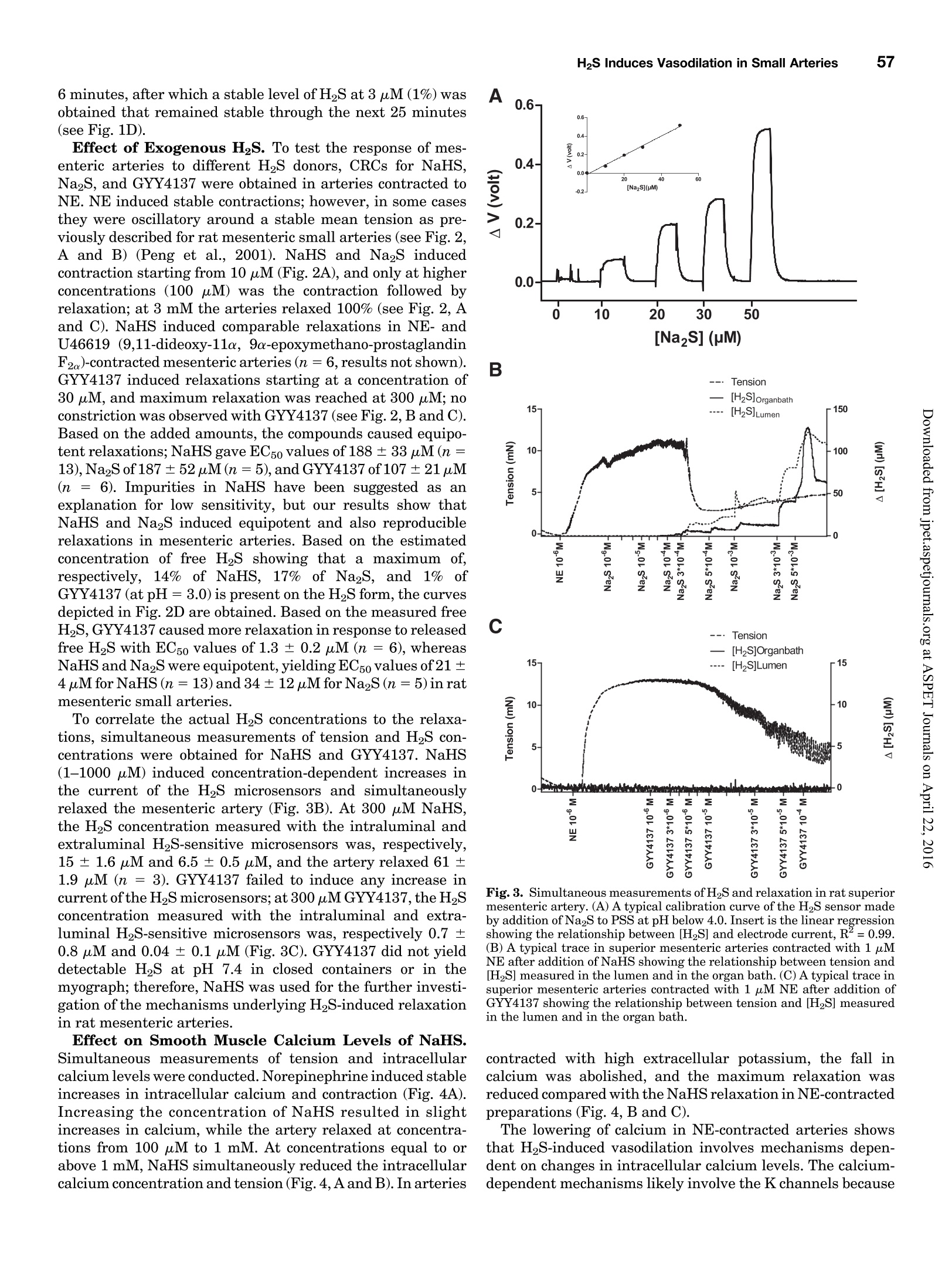
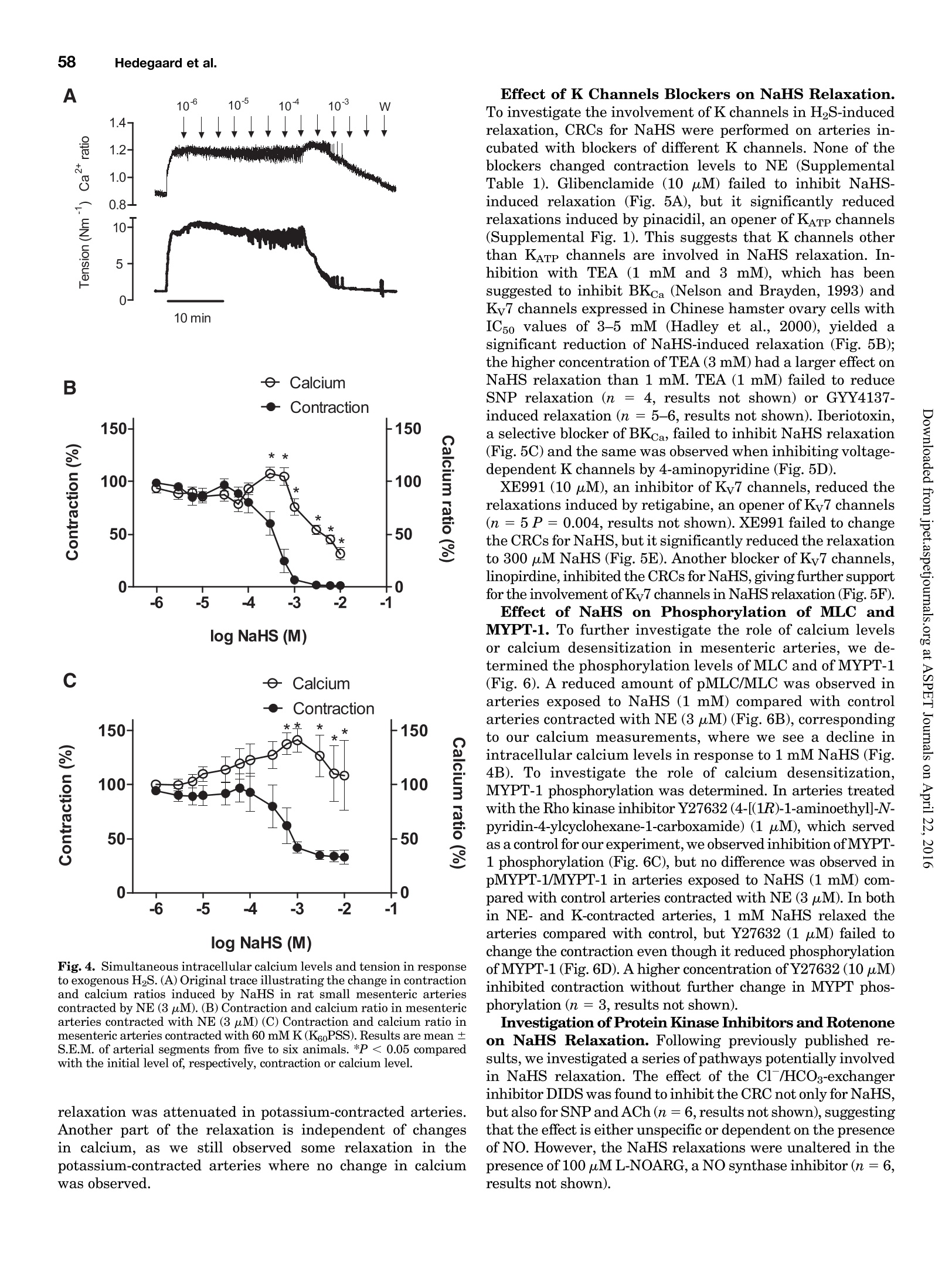
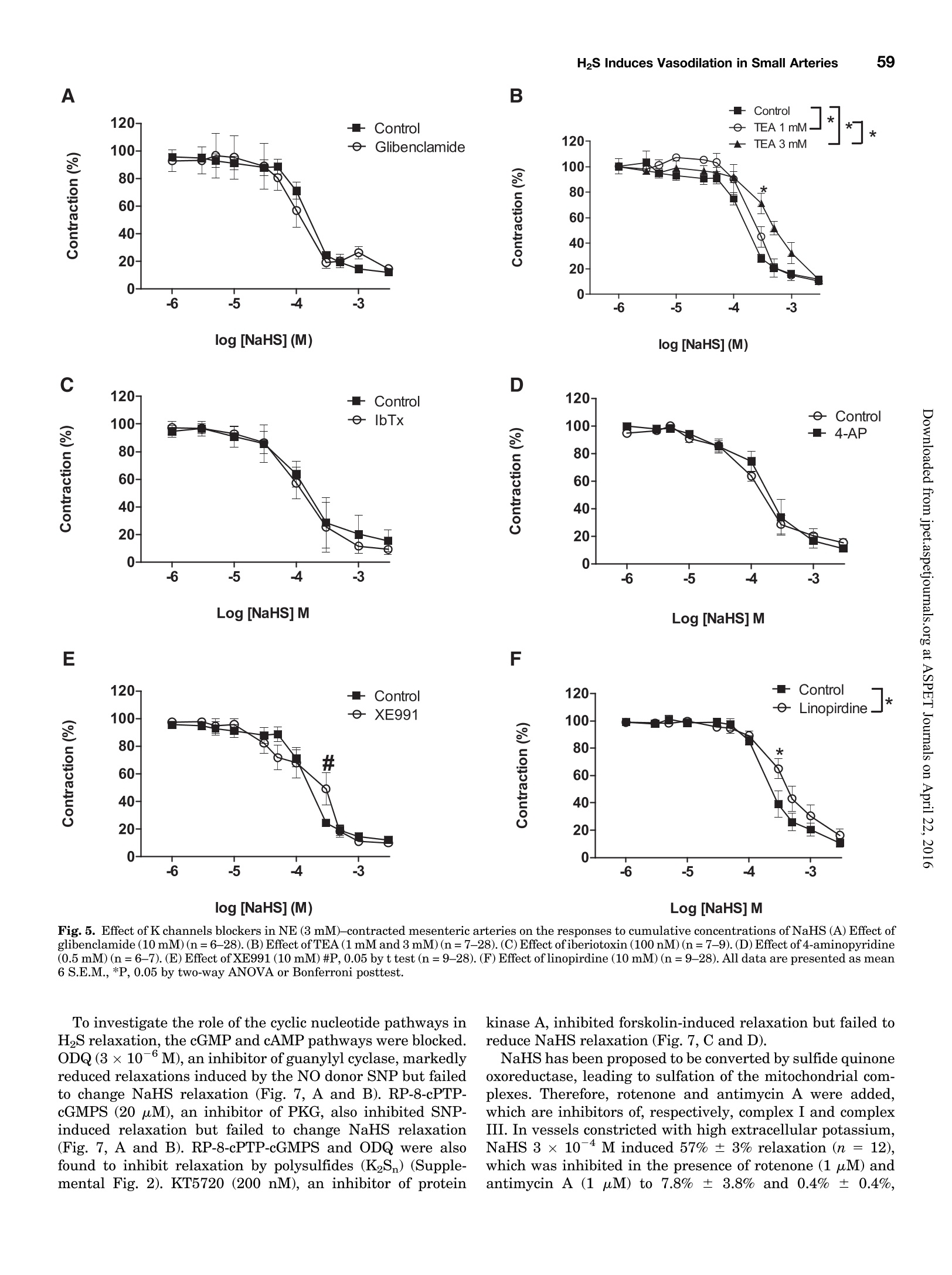
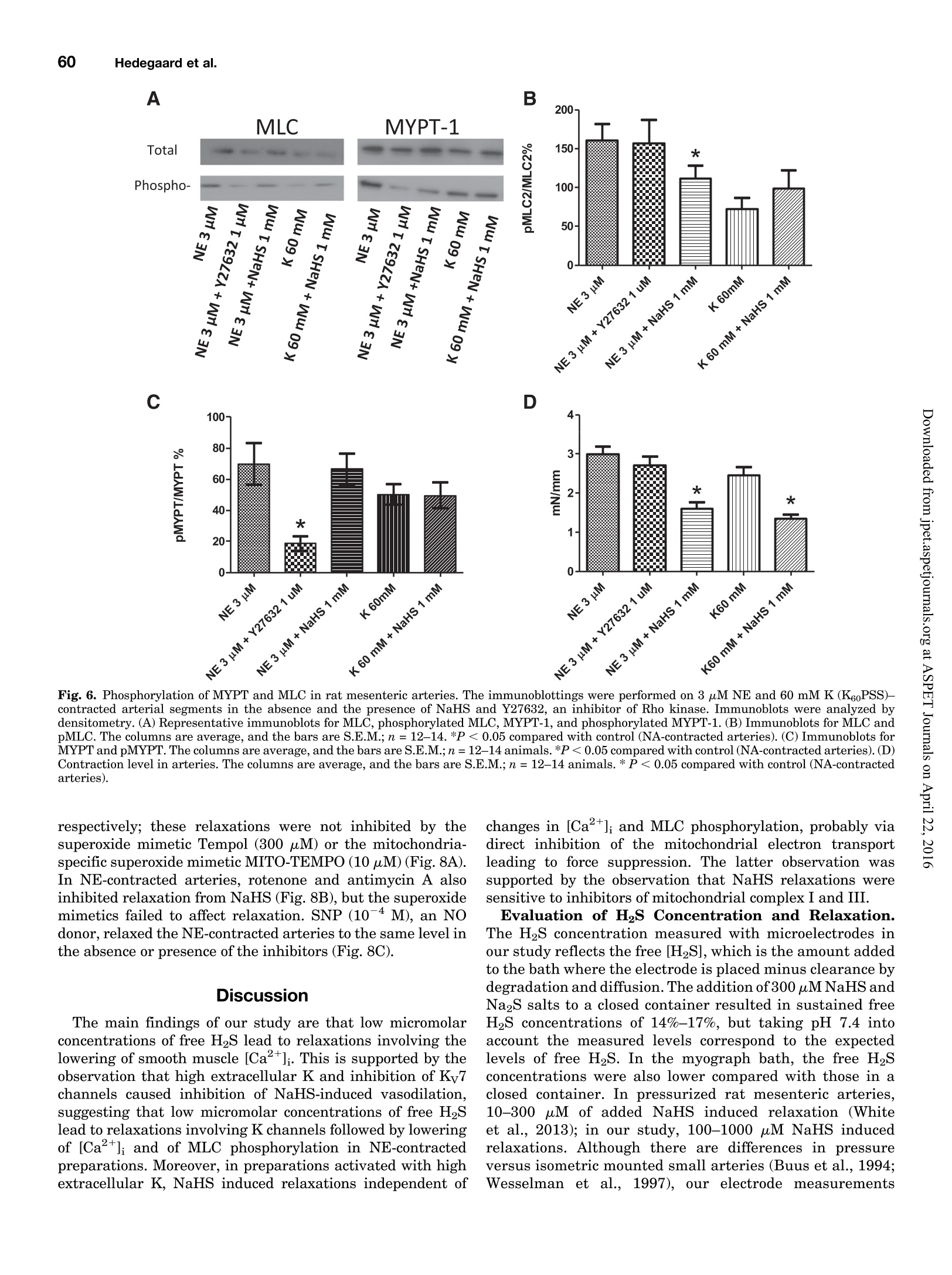
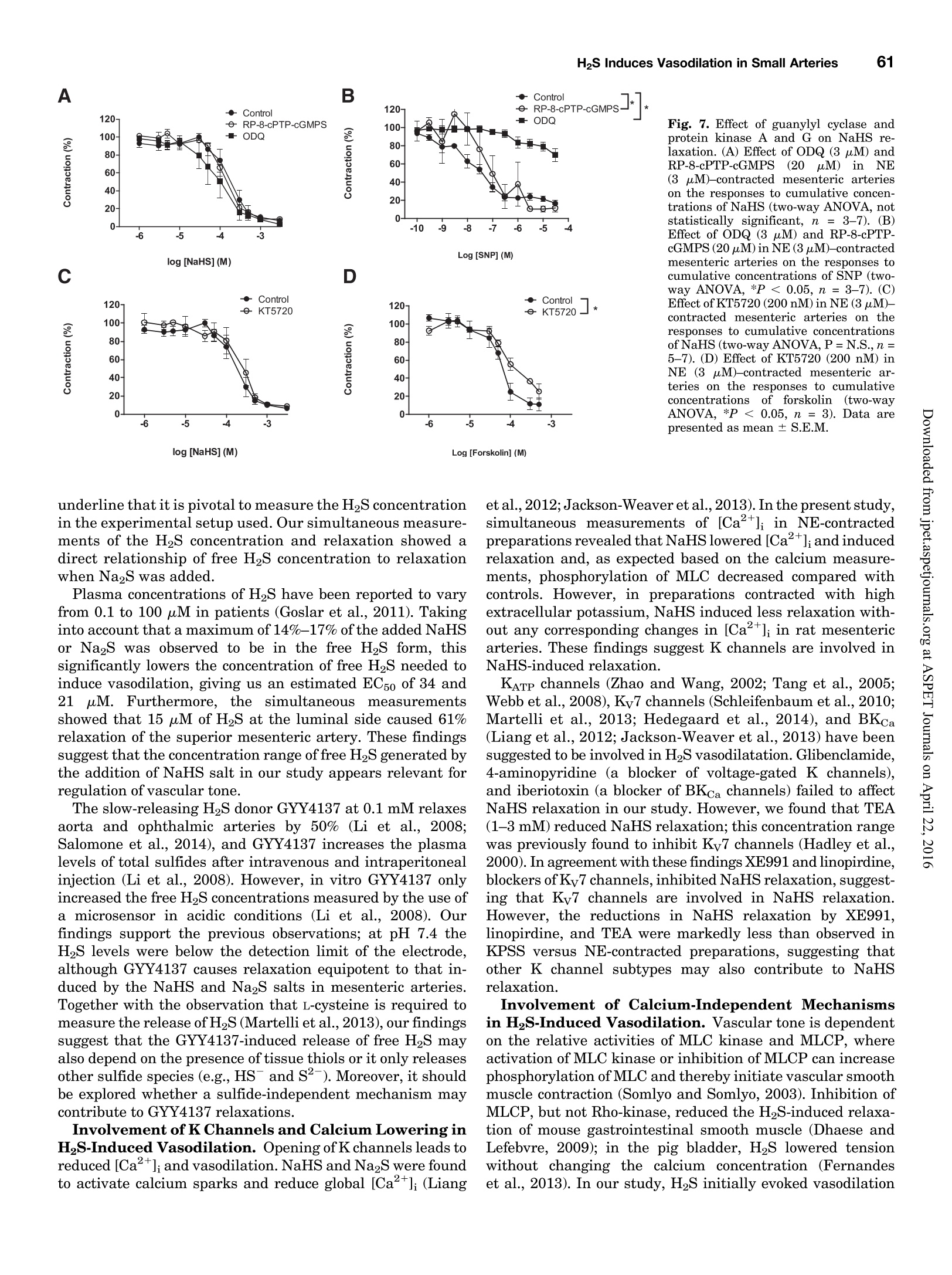
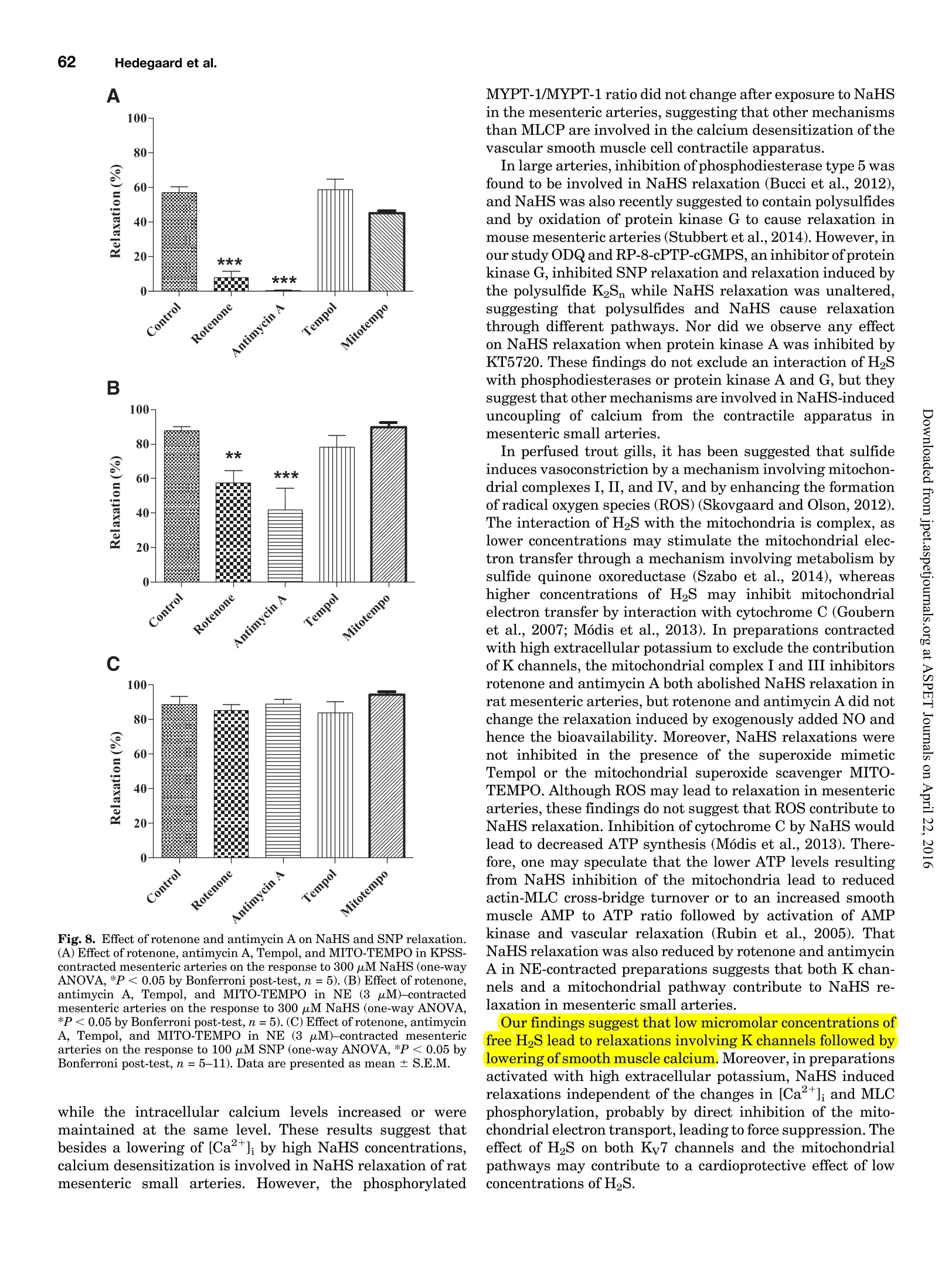
Supplemental material to this article can be found at:http://jpet.aspetjournals.org/content/suppl/2015/10/22/jpet.115.227017.DC1.html1521-0103/356/1/53-63$25.00http://dx.doi.org/10.1124/jpet.115.227017J Pharmacol Exp Ther 356:53-63, January 2016THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICSCopyright C 2015 by The American Society for Pharmacology and Experimental Therapeutics 54Hedegaard et al. 53 Involvement of Potassium Channels and Calcium-IndependentMechanisms in Hydrogen Sulfide-Induced Relaxation of RatMesenteric Small ArteriesS Elise R. Hedegaard, Anja Gouliaev, Anna K. Winther, Daniel D. R. Arcanjo, Mathilde Aalling,Nirthika S. Renaltan, Mark E. Wood, Matthew Whiteman, Nini Skovgaard, and Ulf Simonsen Department of Biomedicine, Pulmonary and Cardiovascular Pharmacology, Aarhus University, Aarhus, Denmark (E.R.H., A.G.,A.K.W., D.D.R.A., M.A., N.S.R., N.S., U.S.); Biosciences, College of Life and Environmental Sciences (M.E.W.), and MedicalSchool, St. Luke’s Campus (M.W.), University of Exeter, Exeter, United Kingdom Received June 24, 2015; accepted October 21,2015 Introduction The three gasses hydrogen sulfide (H2S), nitric oxide(NO),and carbon monoxide have biologic effects at low concentra-tions and are toxic at high concentrations (Szabo,2007;Sunet al., 2011). The reported physiologic levels of H2S vary ( The work was supported by a grant from the Danish Research Council (toA.G. and M.A.), grants from the Villum Kann Rasmussen Foundation, Korning Foundation, and L ’ Oreal (to N.S.); t he Danish Heart Fo u ndation (to E.R.H.), the Korning Foundation, ( t o E .R.H.), and t he K aren Elise Jensen F o undation(to E.R.H.); U.S. is part of the LiPHOS ( L iving Photonics). ) ( E.R.H. a nd A.G. share first authorship. ) ( dx.doi.org/10.1124/jpet . 115.227017. ) Endogenous hydrogen sulfide (H2S) is involved in the regulation smooth muscle calcium and tension revealed that NaHS loweredof vascular tone. We hypothesized that the lowering of calcium calcium and caused relaxation of NE-contracted arteries, whileand opening of potassium (K) channels as well as calcium- high extracellular potassium reduced NaHS relaxation withoutindependent mechanisms are involved in H2S-induced relaxa- corresponding calcium changes. In NE-contracted arteries,tion in rat mesenteric small arteries. Amperometric recordings NaHS (1mM) lowered the phosphorylation of myosin light chain,revealed that free [H2S] after addition to closed tubes of sodium while phosphorylation of myosin phosphatase target subunithydrosulfide (NaHS), Na2S,and GYY4137 [P-(4-methoxyphenyl)- 1 remained unchanged. Protein kinase A and G, inhibitors ofP-4-morpholinyl-phosphinodithioic acid] were, respectively, guanylate cyclase, failed to reduce NaHS relaxation, whereas14%, 17%, and 1% of added amount. The compounds caused blockers of voltage-gated Ky7 channels inhibited NaHS relaxa-equipotent relaxations in isometric myographs, but based on tion, and blockers of mitochondrial complex land IIl abolishedthe measured free [H2S], GYY4137 caused more relaxation in NaHS relaxation. Our findings suggest that low micromolarrelation to released free HS than NaHS and Na2S in rat mesenteric concentrations of free H2S open K channels followed by loweringsmall arteries. Simultaneous measurements of [HS] and tension of smooth muscle calcium, and by another mechanism involvingshowed that 15 uM of free H2S caused 61%relaxation in mitochondrial complex l and Iil leads to uncoupling of force, andsuperior mesenteric arteries. Simultaneous measurements of hence vasodilation. greatly; in both humans and rodents, plasma levels rangingfrom below 1 uM and up to 300 uM have been reported (Olson,2011). Part of this variancecan probably be ascribed to thetechniques used to measure H2S because it is often the totalsulfur pool rather than the free HS that is measured (Olsonet al., 2014). Although it is not well understood which of theforms H2S, HS-, or S- are relevant for the biologic effects,free H2S is thought to mediate most of the effect on vasculartone (Kimura,2014). The precise role of H2S in regulating blood pressure remainsunclarified as knockout of cystathione gamma-lyase (CSE),which is considered the most important H2S producingenzyme in the cardiovascular system, has yielded disparate ( ABBREVIATIONS: ACh, acetylcholine; ANOVA, analysis of variance; BKca, large conductance calcium-activated potassium channels;[Ca *]i, intracellular calcium concentration; CRC, concentration-response curve; CSE, cystathionine gamma-lyase; DIDS, 4,4'-diisothiocyano-2,2'-stilbenedisulfonic aci d ; GYY4137, P-(4-methoxyphenyl)-P-4-morpholinyl-phosphinodithioi c acid ; H2S, hydrogen sulfide ; KATP, ATP-sensitivepotassium channel; KPSS, high-potassium p h ysiologic saline solution ; KT5720, (9S,10S,12R)-2,3,9,10,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3',2',1'-kl]pyrrolo[3,4-i][1,6]-benzo-diazocine-10-carboxylic acid hexyl est e r ; L-NOARG, NC-nitro-L-arginine;MITO-TEMPO, (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium chlor i de monohydrat e ; MLC , myosin l ightchain; MLCP, myosin light chain phosphatase; M YPT-1, m y osin phosphatase target su b unit 1; N E , norepinephrine; N a HS, so d ium hydrosulfide;NO, n itric oxide; ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; PSS, physiologic saline solution ; ROS, radical oxygen species; Rp-8-pCPT-cGMPS, ( Rp )-8-(para-chlorophenylthio)guanosine-3',5'-cyclic monophosphorothioate; SNP, sodium nitr o prusside; TEA , tetr a ethylammonium;Tempol, 4 -hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl; U46619, 9 , 11-dideoxy-11a, 9a - epoxymethano-prostaglandin F2a; XE991 , 10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride;Y27632,4-[(1R)-1 - aminoethyl]-N-pyridin-4-ylcyclohexane-1-carboxamide. ) results. One CSE knockout mouse was found to be normoten-sive (Ishii et al., 2010); another CSE knockout mouse wasreported to have substantially lower levels of plasma H2S aswell as age-dependent hypertension (Yang et al., 2008).Although injection of a H2S salt transiently reduces meanarterial pressure (Ali et al., 2006; Yang et al., 2008), the effecton the vascular tone is complex. In non-mammalian species,H2S induces dose-dependent constriction and/or constrictionfollowed by relaxation (Dombkowski et al., 2005). In arteriesfrom rats, it has been suggested that low H2S concentrationscause contractions whereas high H2S concentrations inducerelaxation of mesenteric,aorta, and gastric arteries as well asofhuman mammary arteries (Ali et al.,2006; Kubo et al., 2007;Webb et al., 2008; d'Emmanuele di Villa Bianca et al., 2011).These studies were based on the application of the H2Ssalts NaHS and Na2S, thought to yield high transientconcentrations of H2S; therefore, the use of slow-releaseH2S donors such as P-(4-methoxyphenyl)-P-4-morpholinyl-phosphinodithioic acid (GYY4137) has been advocated(Papapetropoulos etal., 2015). However,GYY4137 also causesboth vasodilation and vasoconstriction (Li et al., 2008; Salomoneet al., 2014). This suggests that the actual concentrationsreaching the vascular smooth muscle are of major importancefor the observed effect and mechanisms underlying HS-induced vasodilation. A number of other mechanisms underlying H2S-inducedvasorelaxation have been suggested, including involvementof NO (Zhong et al., 2003; Cheang et al., 2010), release ofcalcitonin gene-related peptide from nerve-endings due toactivation of transient receptor potential ankyrin-1 channels(Fernandes et al., 2013; White et al.,2013), and changes inintracellular pH through an effect on a 4.4-diisothiocyano-2.2'-stilbenedisulfonic acid-sensitive Cl-/HCOs-exchanger (Leeet al., 2007; Kiss et al., 2008). Opening of potassium (K)channels leads to hyperpolarization and closing of voltage-dependent Ca+-channels, decreasing the intracellular cal-cium concentration ([Ca +]i), followed by vasodilation. Severalstudies have shown that H2S opens ATP-sensitive potassium-channels (KATp channels), leading to hyperpolarization of thevascular smooth muscle layer (Zhao and Wang, 2002; Tanget al., 2005; Webb et al., 2008; Martelli et al., 2013). Althoughopening of KATp channels has been widely accepted as a mainmechanism underlying H2S-induced vasorelaxation, somestudies have found only a small or no involvement of KATPchannels (Kubo et al., 2007; Kiss et al., 2008; Cheang et al.,2010). Other potassium channels (K channels) have also beenreported to play a role in H2S relaxation such as voltage-gatedK channels (Cheang et al.,2010),Ky7 channels (Schleifenbaumet al., 2010; Martelli et al., 2013; Hedegaard et al.,2014), andlarge conductance calcium-activated potassium channels(BKca) (Jackson-Weaver et al.,2011, 2013; Li et al., 2012).Ithas been shown that the HS donors NaHS and Na2S activatecalcium sparks (Liang et al., 2012; Jackson-Weaver et al., 2013)and also that they reduce global [Ca”+] in cerebral arterioles(Liang et al., 2012). However, so far there have been noattempts to directly correlate vascular smooth muscle cellcalcium to changes in vascular tone by simultaneous measure-ments of [Ca”+]; and relaxation. We hypothesized that lowering of calcium and opening ofK channels as well as calcium-independent mechanisms areinvolved in H2S-induced relaxation. To investigate this hy-pothesis, we performed the following measurements: 1) the release of H2S from NaHS,Na2S,and GYY4137 was examinedby the use of a H2S microsensor; 2) changes in [Ca]i weremeasured simultaneously withn relaxation to determinewhether lowering of [Ca]i contributes to H2S relaxation; 3)the phosphorylation of myosin light chain (MLC) and myosinphosphatase target subunit 1 (MYPT-1) was measured; and 4)the involvement of different Kchannels and the mitochondrialelectron chain complexes was investigated using selectiveblockers in rat small mesenteric arteries. Materials and Methods Solutions and Chemicals. The following drugs were used: nor-epinephrine (NE), acetylcholine (ACh), 4-aminopyridine, antimycin A,NaHS, Na2S, tetraethylammonium (TEA), 4,4'-diisothiocyano-2,2'-stilbenedisulfonic acid (DIDS),linopirdine,MITO-TEMPO [(2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphoniumchloride monohydratel, L-NOARG (N-nitro-L-arginine), retigabine,Rp-8-pCPT-cGMPS[(Rp)-8-(para-chlorophenylthio)guanosine-3',5'-cyclicmonophosphorothioate], KT5720 [(9S,10S,12R)-2,3,9,10,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3',2',1'-kl]pyrrolo[3,4-i][1,6]-benzo-diazocine-10-carboxylic acid hexyl ester],XE991 [10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone dihydrochloride],potassium polysulfide (K2Sn), rotenone, sodium nitroprusside(SNP), and glibenclamide from Sigma-Aldrich (St. Louis, MO).ODQ(1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one) was obtained fromTocris (Bristol, United Kingdom), and Fura-2 AM and pluoronic F127were purchased from Invitrogen (Taastrup, Denmark). Iberiotoxinwas purchased from Latoxan, Valence,France.GYY4137 was synthe-sized as previously described elsewhere (Li et al., 2008). Microvascular Myograph Studies. This study followed therecommendations in the Guide for the Care and Use of LaboratoryAnimals of the U.S. National Institutes of Health and the ARRIVEGuidelines. Wistar rats (10-12 weeks) were euthanized by cervicaldislocation followed by exsanguination, and the mesentery bed wasremoved and placed in ice-cold PSS. Third branch mesenteric arterieswere dissected and mounted on wire myographs (as previouslydescribed elsewhere)for recordings of isometric tension (Mulvany [Na2S]pM D Fig. 1. Release of H2S from donors. (A)A typical calibration curve ofthe H2S sensor made by addition of Na2S to PSS at pH below 4.0. (B) Release of H2Safter addition of 300 uM NaHS to PSS, pH 7.4 (n=5). (C) Release of H2S after addition of 300 uM Na2S to PSS, pH 7.4(n=9).(D) Release of H2S afteraddition of 300 uM GYY4137 to PSS, pH 3.0 (n=4) and pH 7.4(n=4). Data are presented as mean ± S.E.M. and Halpern, 1977). The 1.5-2.0 mm long arterial rings, withdiameters of approximately 200-300 um, were mounted on 40-umstainless steel wires and kept in PSS at 37℃ and bubbled with5%CO2/21% 02/74%N2 After 30 minutes of equilibration, the arteries were normalized.Experiments were performed on arteries stretched to 90% of L100,where L100 is defined as the circumference of the relaxed arteryexposed to a transmural pressure of 100 mmHg. Before conductingexperiments, the viability of the arterial segment was tested. Themesenteric arteries were contracted twice by NE (10 uM). To test thepresence of functional endothelium, the arteries were contracted withNE (3uM) before ACh (10 uM) was added. Arteries were onlyincluded if they developed an active force corresponding to a trans-mural pressure of 100 mmHg and relaxed a minimum of 60% to ACh(10uM). Simultaneous Measurements of HS and Relaxation. Forsimultaneous measurements of force and H2S concentration, asegment of the mesenteric superior artery was mounted on two100-um thick steel wires in a single chamber myograph as previouslydescribed elsewhere for simultaneous measurements of force and NOconcentration (Simonsen et al.,1999). A H2S-sensitive microelectrodewith tip diameter of 50-80 um (Unisense A/S,Aarhus, Denmark) wasfirst calibrated as described earlier; by use of a micromanipulator, itwas introduced into the lumen of the artery while another sensor wasplaced in the organ bath. Mesenteric superior arteries were normal-ized and tested as described for small mesenteric arteries, onlydifference being that 1 uM of NE was used to contract the arteries.The tension and electrode current were recorded in Labscribe (WorldPrecision Instruments, Hitchen, United Kingdom), which allowedsimultaneous measurements of HS and relaxation. Changes in thecoating of the electrode resulted in abrupt changes in basal electrodecurrent, and in these cases the electrode was discarded. Experimental Protocol. To investigate the mechanism of H2S-induced vasodilation, arteries with endothelium were incubated witheither vehicle or inhibitors before concentration-response curves(CRCs) were constructed for NaHS,Na2S,or GYY4137 in preparationscontracted with NE. The control and examination of drugs were run inparallel, and only one CRC was constructed for each vasodilator peranimal. To investigate the involvement of K channels in HS-inducedrelaxation, CRCs for NaHS were performed on NE-contracted arteriesincubated with blockers of different K channels: glibenclamide (10uM),TEA (1mMand3mM),iberiotoxin (100nM),4-AP(0.5mM),linopirdine(10uM),and XE991(10uM) were incubated with arteries for 30 minutesbefore dilation was induced by NaHS. To investigate other pathways suggested to be involved in NaHSrelaxation,CRCs for NaHS were performed on NE-contracted arteriesincubated with L-NOARG(100 uM),ODQ(3uM),KT5720 (200 nM),and Rp-8-pCPT-cGMPS (20 uM) (incubated with arteries for 30 minutes),and rotenone (1 uM), antimycin A (1 uM), Tempol [4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl] (300 uM), MITO-TEMPO (10 pM), andDIDS (100 uM) (incubated with arteries for 15 minutes). Rotenone (1uM),antimycin A (1uM),Tempol (300 uM),and MITO-TEMPO (10 uM) werealso investigated in arteries contracted with 60 mMK(K60PSS).To obtaincomparable levels of contraction in arteries incubated with rotenone orantimycin A, NE was added on top of high-potassium physiologic salinesolution (KPSS). Simultaneous Measurements of Intracellular Calcium Levelsand Tension. Measurements of intracellular calcium levels wereperformed in mesenteric small arteries as previously describedelsewhere (Rodriguez-Rodriguez et al., 2008). After mounting, equil-ibration, and normalization, the arteries were loaded with Fura-2 AM(8uM) and loading mix (Pluronic F127) in the dark for 2 hours. Themyograph was placed on a Zeiss inverted microscope (Leica Microsystems Fig. 2. Relaxation induced by H2S donors.(A) An original trace illustrating the relaxation induced by NaHS in rat small mesenteric arteries contractedby NE (3 uM). (B) An original trace illustrating the relaxation induced by GYY4137 in rat small mesenteric arteries contracted by NE (3uM). (C)Relaxation induced by NaHS, Na2S,or GYY4137 in NE (3pM)-contracted mesenteric arteries (*P <0.05 by two-way ANOVA, n=5-14). (D) Estimatedlevels of HS based on sensor measurements for each of the three donors (*P <0.05 by two-way ANOVA, n=5-14).Data are presented as mean ±S.E.M. GmbH, Wetzlar, Germany) for dual-excitation wavelength fluorom-etry. After 30 minutes of washout,the arteries were illuminatedwith light alternating at 340 and 380 nm, and the intensity of theemitted fluorescence was collected at 510 nm. At the end of theexperiment, ionomycin (10 uM) was added to PSS to achievemaximal saturation of Fura-2 with calcium. Then the fluorescencewas quenched using nominally calcium-free PSS and MnCl2(15mM)to measure the background signal, which was subtracted from therecordings. Immunoblotting. For detection of MLC, phosphorylated MLC(pMLC), MYPT, and phosphorylated MYPT (pMYPT), mesentericsmall arteries were frozen in ice-cold acetone with trichloroacetic acid(10%) and dithiothreitol (10mM) and then placed in a -80°℃ freezerfor 24 hours before being washed 3 times with acetone withdithiothreitol (10 mM). Before homogenization with pellet pestles(Sigma, St. Louis, MO), the samples were heated to 50℃ for10 minutes in 15 ul of 50% lysis buffer and 50% sample buffer. Afterhomogenization they were sonicated for 45 seconds and centrifuged for10 minutes at 10.000 rpm at 4°C. The sampling in acetone and directdissolving in sample buffer is incompatible with our protein measure-ments. The same volume was added to the gels for the blotting, and theresults expressed as a ratio of phosphorylated to unphosphorylatedprotein. A linear relation is required for quantitative protein expression(Eaton et al., 2013).We have previously observed that the densito-metric measurements ofMYPT-1 correlated linearly with pMYPT andMLC with pMLC. Therefore, pMYPT and pMLC were expressed asratios of their respective unphosphorylated proteins. All samples were run simultaneously to minimize any differencesin transfer. Samples and a prestain marker (Bio-Rad Laboratories,Hercules,CA) were loaded onto the gel. Immunoblotting was performedas described previously elsewhere (Hedegaard et al., 2014). The followingantibodies were applied: MYPT 1:6000 (Sc-25618; Santa Cruz Bio-technology, Dallas, TX),pMYPT 1:500 (05-773; Millipore, Billerica,MA), MLC 1:1000 (3672S; Cell Signaling Technology, Beverly), pMLC1:2000 (3671S; Cell Signaling Technology), and secondary anti-rabbitIgG conjugated to horseradish peroxidase (Santa Cruz Biotechnology)1:4000. Data Calculation and Analysis. All recordings and calculationswere performed by PowerLab data system and Chart 5.5(ADInstruments,Oxfordshire, United Kingdom) or Labscribe (World Precision Instru-ments, Hitchin, United Kingdom). The mechanical responses of thevessel segments were measured as active wall tension (AT), which isthe changes in force (AF) divided by twice the segment length (2l). TheCRCs were compared with controls by a two-way analysis of variance(ANOVA) followed by a Bonferroni post hoc test. Differences betweenmeans were analyzed by unpaired two-tailed t test. One-way ANOVAfollowed by Bonferroni post-test or a Studentt test was used to analyzedifference between mean relaxation to one dose of NaHS or SNP. P <0.05 was considered statistically significant for all tests. All graphsand statistical analyses were performed using GraphPad Prism 5.0(GraphPad Software,La Jolla,CA). The results are given as mean ±S.E.M. Results Release of H2S from NaHS, Na2S, and GYY4137. Therelease of free HS from the donors was examined by use of amicrosensor in closed containers containing PSS. The H2Smicrosensor responded with changes in current to micromolarconcentrations of Na2S, and the output current of the probescorrelated linearly with the concentrations of Na2S (Fig. 1A).The amount of HS released from 300 uM of NaHS in PSS atpH 7.4 was 43 uM (14%); during the 30 minutes of measure-ments the concentration slowly decreased to 33 uM (11%)(Fig. 1B). The corresponding curve for 300 uM Na2S in PSS atpH 7.4 had a similar appearance, with a fast release of HSreaching a maximum of 50 uM (17%) after 2 minutes, and aslow decrease resulting in a final concentration of 39 uM (13%)after 30 minutes (Fig. 1C). Addition of 300 uM GYY4137 toPSS at pH 7.4 failed to change the electrode current, whereas300 uM GYY4137 added to PSS at pH 3.0 gave a slower releaseof H2S than NaHS and Na2S, reaching a maximum after 6 minutes, after which a stable level of HS at 3 uM (1%) wasobtained that remained stable through the next 25 minutes(see Fig. 1D). Effect of Exogenous HS. To test the response of mes-enteric arteries to different HS donors, CRCs for NaHS,Na2S, and GYY4137 were obtained in arteries contracted toNE. NE induced stable contractions; however, in some casesthey were oscillatory around a stable mean tension as pre-viously described for rat mesenteric small arteries (see Fig. 2,A and B) (Peng et al., 2001). NaHS and Na2S inducedcontraction starting from 10 uM (Fig.2A), and only at higherconcentrations (100 uM) was the contraction followed byrelaxation; at 3 mM the arteries relaxed 100% (see Fig. 2, Aand C). NaHS induced comparable relaxations in NE- andU46619 (9,11-dideoxy-11a, 9o-epoxymethano-prostaglandinF2)-contracted mesenteric arteries (n =6, results not shown).GYY4137 induced relaxations starting at a concentration of30 uM,and maximum relaxation was reached at 300 uM; noconstriction was observed with GYY4137 (see Fig. 2, B and C).Based on the added amounts, the compounds caused equipo-tent relaxations;NaHS gave EC50 values of 188±33 uM(n=13),Na2S of 187 ±52uM(n=5),and GYY4137 of 107±21uM(n = 6). Impurities in NaHS have been suggested as anexplanation for low sensitivity, but our results show thatNaHS and Na2S induced equipotent and also reproduciblerelaxations in mesenteric arteries. Based on the estimatedconcentration of free H2S showing that a maximum of,respectively, 14% of NaHS, 17% of Na2S, and 1%ofGYY4137 (at pH=3.0) is present on the H2S form, the curvesdepicted in Fig. 2D are obtained. Based on the measured freeHS,GYY4137 caused more relaxation in response to releasedfree HS with EC50 values of 1.3± 0.2 uM(n =6), whereasNaHS and Na2S were equipotent, yielding EC50 values of 21±4 uM for NaHS (n=13) and 34± 12 uM for Na2S(n=5) in ratmesenteric small arteries. To correlate the actual HS concentrations to the relaxa-tions,simultaneous measurements of tension and H2S con-centrations were obtained for NaHS and GYY4137. NaHS(1-1000 uM) induced concentration-dependent increases inthe current of the HS microsensors and simultaneouslyrelaxed the mesenteric artery (Fig.3B). At 300 uM NaHS,the HS concentration measured with the intraluminal andextraluminal H2S-sensitive microsensors was, respectively,15 ± 1.6 uM and 6.5±0.5 uM, and the artery relaxed 61 ±1.9 uM (n = 3). GYY4137 failed to induce any increase incurrent of the H2S microsensors; at 300 uM GYY4137, the H2Sconcentration measured with the intraluminal and extra-luminal H2S-sensitive microsensors was, respectively 0.7 ±0.8 uM and 0.04± 0.1 uM (Fig. 3C). GYY4137 did not yielddetectable H2S at pH 7.4 in closed containers or in themyograph; therefore, NaHS was used for the further investi-gation ofthe mechanisms underlying H2S-induced relaxationin rat mesenteric arteries. Effect on Smooth Muscle Calcium Levels of NaHS.Simultaneous measurements of tension and intracellularcalcium levels were conducted. Norepinephrine induced stableincreases in intracellular calcium and contraction (Fig.4A).Increasing the concentration of NaHS resulted in slightincreases in calcium, while the artery relaxed at concentra-tions from 100 pM to 1 mM. At concentrations equal to orabove 1 mM, NaHS simultaneously reduced the intracellularcalcium concentration and tension (Fig. 4,Aand B). In arteries Fig.3. Simultaneous measurements ofHS and relaxation in rat superiormesenteric artery. (A) A typical calibration curve of the H2S sensor madeby addition of Na2S to PSS at pH below 4.0. Insert is the linear regressionshowing the relationship between [H2S] and electrode current, R²=0.99.(B) A typical trace in superior mesenteric arteries contracted with 1 uMNE after addition of NaHS showing the relationship between tension and[H2S] measured in the lumen and in the organ bath. (C) A typical trace insuperior mesenteric arteries contracted with 1 uM NE after addition ofGYY4137 showing the relationship between tension and [HS] measuredin the lumen and in the organ bath. contracted with high extracellular potassium, the fall incalcium was abolished, and the maximum relaxation wasreduced compared with the NaHS relaxation in NE-contractedpreparations (Fig. 4,B and C). The lowering of calcium in NE-contracted arteries showsthat HS-induced vasodilation involves mechanisms depen-dent on changes in intracellular calcium levels. The calcium-dependent mechanisms likely involve the K channels because c3to log NaHS (M) c3to log NaHS (M) Fig. 4. Simultaneous intracellular calcium levels and tension in responseto exogenous HS. (A) Original trace illustrating the change in contractionand calcium ratios induced by NaHS in rat small mesenteric arteriescontracted by NE (3uM). (B) Contraction and calcium ratio in mesentericarteries contracted with NE (3 uM) (C) Contraction and calcium ratio inmesenteric arteries contracted with 60 mM K(K60PSS).Results are mean ±S.E.M. of arterial segments from five to six animals. *P < 0.05 comparedwith the initial level of, respectively, contraction or calcium level. relaxation was attenuated in potassium-contracted arteries.Another part of the relaxation is independent of changesin calcium, as we still observed some relaxation in thepotassium-contracted arteries where no change in calciumwas observed. Effect of K Channels Blockers on NaHS Relaxation.To investigate the involvement of K channels in H2S-inducedrelaxation, CRCs for NaHS were performed on arteries in-cubated with blockers of different K channels. None of theblockers changed contraction levels to NE (SupplementalTable 1). Glibenclamide (10 uM) failed to inhibit NaHS-induced relaxation (Fig. 5A), but it significantly reducedrelaxations induced by pinacidil, an opener of KATp channels(Supplemental Fig1). This suggests that K channels otherthan KATp Pc.hannels are involved in NaHS relaxation. In-hibition with TEA (1 mM and 3 mM), which has beensuggested to inhibit BKCa (Nelson and Brayden, 1993) andKv7 channels expressed in Chinese hamster ovary cells withIC50 values of 3-5 mM (Hadley et al., 2000), yielded asignificant reduction of NaHS-induced relaxation (Fig. 5B);the higher concentration ofTEA (3 mM) had a larger effect onNaHS relaxation than 1 mM. TEA (1 mM) failed to reduceSNP relaxation (n = 4, results not shown) or GYY4137-induced relaxation (n =5-6,results not shown). Iberiotoxin,a selective blocker of BKca, failed to inhibit NaHS relaxation(Fig.5C) and the same was observed when inhibiting voltage-dependent K channels by 4-aminopyridine (Fig. 5D). XE991 (10 uM), an inhibitor of Ky7 channels, reduced therelaxations induced by retigabine, an opener of Ky7 channels(n=5P=0.004, results not shown). XE991 failed to changethe CRCs for NaHS, but it significantly reduced the relaxationto 300 uM NaHS (Fig. 5E). Another blocker of Ky7 channels,linopirdine, inhibited the CRCs for NaHS, giving further supportfor the involvement of Ky7 channels in NaHS relaxation (Fig.5F). Effect of NaHS on Phosphorylation of MLC andMYPT-1. To further investigate the role of calcium levelsor calcium desensitization in mesenteric arteries, we de-termined the phosphorylation levels of MLC and of MYPT-1(Fig. 6). A reduced amount of pMLC/MLC was observed inarteries exposed to NaHS (1 mM) compared with controlarteries contracted with NE (3uM) (Fig.6B), correspondingto our calcium measurements, where we see a decline inintracellular calcium levels in response to 1 mM NaHS (Fig.4B). To investigate the role of calcium desensitization,MYPT-1 phosphorylation was determined. In arteries treatedwith the Rho kinase inhibitor Y27632 (4-[(1R)-1-aminoethyl]-N-pyridin-4-ylcyclohexane-1-carboxamide) (1 uM), which servedas a control for our experiment, we observed inhibition ofMYPT-1 phosphorylation (Fig. 6C), but no difference was observed inpMYPT-1/MYPT-1 in arteries exposed to NaHS (1 mM) com-pared with control arteries contracted with NE (3uM). In bothin NE- and K-contracted arteries, 1 mM NaHS relaxed thearteries compared with control, but Y27632 (1 uM) failed tochange the contraction even though it reduced phosphorylationofMYPT-1(Fig.6D). A higher concentration of Y27632 (10uM)inhibited contraction without further change in MYPT phos-phorylation (n =3,results not shown). Investigation of Protein Kinase Inhibitors and Rotenoneon NaHS Relaxation. Following previously published re-sults, we investigated a series of pathways potentially involvedin NaHS relaxation. The effect of the Cl-/HCOs-exchangerinhibitor DIDS was found to inhibit the CRC not only for NaHS,but also for SNPand ACh (n=6, results not shown), suggestingthat the effect is either unspecific or dependent on the presenceof NO. However, the NaHS relaxations were unaltered in thepresenceof 100 uM L-NOARG, a NO synthase inhibitor (n =6,results not shown). B 120- 一-Control 100- -O-Glibenclamide D coco F 0- -6 -5 -4 -3 log [NaHS] (M) Log [NaHS] M Fig. 5. Effect of K channels blockers in NE (3 mM)-contracted mesenteric arteries on the responses to cumulative concentrations of NaHS (A) Effect ofglibenclamide(10 mM)(n=6-28).(B)Effect ofTEA (1 mM and3 mM)(n=7-28).(C)Effect of iberiotoxin (100nM)(n=7-9).(D)Effect of 4-aminopyridine(0.5mM)(n=6-7). (E) Effect of XE991(10 mM)#P,0.05 by t test (n=9-28). (F) Effect of linopirdine (10 mM)(n=9-28). All data are presented as mean6 S.E.M., *P, 0.05 by two-way ANOVA or Bonferroni posttest. To investigate the role of the cyclic nucleotide pathways inH2S relaxation, the cGMP and cAMP pathways were blocked.ODQ(3×10-6M), an inhibitor of guanylyl cyclase, markedlyreduced relaxations induced by the NO donor SNP but failedto change NaHS relaxation (Fig. 7, A and B). RP-8-cPTP-cGMPS (20 uM), an inhibitor of PKG, also inhibited SNP-induced relaxation but failed to change NaHS relaxation(Fig. 7, A and B). RP-8-cPTP-cGMPS and ODQ were alsofound to inhibit relaxation by polysulfides (K2Sn) (Supple-mental Fig. 2). KT5720 (200 nM), an inhibitor of protein kinase A, inhibited forskolin-induced relaxation but failed toreduce NaHS relaxation (Fig. 7, C and D). NaHS has been proposed to be converted by sulfide quinoneoxoreductase, leading to sulfation of the mitochondrial com-plexes. Therefore, rotenone and antimycin A were added,which are inhibitors of, respectively, complex I and complexIII. In vessels constricted with high extracellular potassium,NaHS 3 ×10-4 M induced 57%± 3% relaxation (n = 12),which was inhibited in the presence of rotenone (1uM) andantimycin A (1 uM) to 7.8%±3.8% and 0.4%± 0.4%, A B 200- Fig. 6. Phosphorylation of MYPT and MLC in rat mesenteric arteries. The immunoblottings were performed on 3 uM NE and 60 mM K(K60PSS)-contracted arterial segments in the absence and the presence of NaHS and Y27632, an inhibitor of Rho kinase. Immunoblots were analyzed bydensitometry.(A) Representative immunoblots for MLC, phosphorylated MLC,MYPT-1, and phosphorylated MYPT-1. (B) Immunoblots for MLC andpMLC. The columns are average, and the bars are S.E.M.; n=12-14.*P<0.05 compared with control (NA-contracted arteries). (C) Immunoblots forMYPT and pMYPT. The columns are average, and the bars are S.E.M.; n= 12-14 animals. *P <0.05 compared with control (NA-contracted arteries). (D)Contraction level in arteries. The columns are average, and the bars are S.E.M.; n=12-14 animals.*P <0.05 compared with control (NA-contractedarteries). respectively; these relaxations were not inhibited by thesuperoxide mimetic Tempol (300 uM) or the mitochondria-specific superoxide mimetic MITO-TEMPO (10 uM) (Fig. 8A).In NE-contracted arteries, rotenone and antimycin A alsoinhibited relaxation from NaHS (Fig. 8B), but the superoxidemimetics failed to affect relaxation. SNP (10-4 M), an NOdonor,relaxed the NE-contracted arteries to the same level inthe absence or presence of the inhibitors (Fig. 8C). Discussion The main findings of our study are that low micromolarconcentrations of free HaS lead to relaxations involving thelowering of smooth muscle [Ca ]. This is supported by theobservation that high extracellular K and inhibition of Ky7channels caused inhibition of NaHS-induced vasodilation,suggesting that low micromolar concentrations of free H2Slead to relaxations involving K channels followed by loweringof [Ca ]i and of MLC phosphorylation in NE-contractedpreparations.Moreover, in preparations activated with highextracellular K, NaHS induced relaxations independent of changes in [Ca+]i and MLC phosphorylation, probably viadirect inhibition of the mitochondrial electron transportleading to force suppression. The latter observation wassupported by the observation that NaHS relaxations weresensitive to inhibitors of mitochondrial complex I and III. Evaluation of HS Concentration and Relaxation.The HS concentration measured with microelectrodes inour study reflects the free [H2S],which is the amount addedto the bath where the electrode is placed minus clearance bydegradation and diffusion.The addition of 300 uM NaHS andNa2S salts to a closed container resulted in sustained freeHS concentrations of 14%-17%, but taking pH 7.4 intoaccount the measured levels correspond to the expectedlevels of free H2S. In the myograph bath, the free H2Sconcentrations were also lower compared with those in aclosed container. In pressurized rat mesenteric arteries,10-300 uM of added NaHS induced relaxation (Whiteet al., 2013); in our study, 100-1000 uM NaHS inducedrelaxations. Although there are differences in pressureversus isometric mounted small arteries (Buus et al., 1994;Wesselman et al., 1997), our electrode measurements B --Control RP-8-cPTP-cGMPS 120- 0DQ 100- -2 -8 -7 -6 -5 -2 Log [SNP](M) p-Control 80-co60-c40-o200--10 -9-3D-e- Control120-100c80oo60c40o20 -O-KT5720 0 -6 -5 -4 -3 Log [Forskolin] (M) Fig. 7. Effect of guanylyl cyclase andprotein kinase A and G on NaHS re-laxation. (A) Effect of ODQ (3 uM) andRP-8-cPTP-cGMPS(20 uM) inNE(3uM)-contracted mesenteric arterieson the responses to cumulative concen-trations of NaHS (two-way ANOVA, notstatistically significant, n = 3-7). (B)Effect of ODQ (3 uM) and RP-8-cPTP-cGMPS (20uM) in NE (3 uM)-contractedmesenteric arteries on the responses tocumulative concentrations of SNP (two-way ANOVA, *P < 0.05, n =3-7). (C)Effect of KT5720 (200 nM) in NE (3uM)-contracted mesenteric arteries on theresponses to cumulative concentrationsof NaHS (two-way ANOVA, P=N.S.,n=5-7). (D) Effect of KT5720 (200 nM) inNE (3 uM)-contracted mesenteric ar-teries on the responses to cumulativeconcentrations offorskolin (two-wayANOVA, *P<0.05, n=3). Data arepresented as mean ± S.E.M. underline that it is pivotal to measure the HS concentrationin the experimental setup used. Our simultaneous measure-ments of the H2S concentration and relaxation showed adirect relationship of free HS concentration to relaxationwhen Na2S was added. Plasma concentrations of H S have been reported to varyfrom 0.1 to 100 uM in patients (Goslar et al., 2011). Takinginto account that a maximum of 14%-17% oftheadded NaHSor NaS was observed to be in the free HS form, thissignificantly lowers the concentration of free HS needed toinduce vasodilation, giving us an estimated EC50 of 34 and21 uM. Furthermore, the simultaneousmeasurementsshowed that 15 uM of H2S at the luminal side caused 61%relaxation of the superior mesenteric artery. These findingssuggest that the concentration range of free HS generated bythe addition of NaHS salt in our study appears relevant forregulation of vascular tone. The slow-releasing H2S donor GYY4137 at 0.1 mM relaxesaorta and ophthalmic arteries by 50% (Li et al., 2008;Salomone et al., 2014), and GYY4137 increases the plasmalevels of total sulfides after intravenous and intraperitonealinjection (Li et al., 2008). However, in vitro GYY4137 onlyincreased the free H2S concentrations measured by the use ofa microsensor in acidic conditions (Li et al., 2008). Ourfindings support the previous observations; at pH 7.4 theHS levels were below the detection limit of the electrode,although GYY4137 causes relaxation equipotent to that in-duced by the NaHS and Na2S salts in mesenteric arteries.Together with the observation that L-cysteine is required tomeasure the release of H2S (Martelli et al., 2013), our findingssuggest that the GYY4137-induced release of free H2S mayalso depend on the presence of tissue thiols or it only releasesother sulfide species (e.g., HS and S-). Moreover, it shouldbe explored whether a sulfide-independent mechanism maycontribute to GYY4137 relaxations. Involvement of K Channels and Calcium Lowering inH2S-Induced Vasodilation. Opening of K channels leads toreduced [Ca]i and vasodilation. NaHS and Na2S were foundto activate calcium sparks and reduce global [Ca ]i (Liang et al., 2012;Jackson-Weaver et al., 2013). In the present study,simultaneous measurements of [Ca ] in NE-contractedpreparations revealed that NaHS lowered [Ca ]iand inducedrelaxation and, as expected based on the calcium measure-ments, phosphorylation of MLC decreased compared withcontrols. However, in preparations contracted with highextracellular potassium, NaHS induced less relaxation with-out any corresponding changes in [Ca ]i in rat mesentericarteries. These findings suggest K channels are involved inNaHS-induced relaxation. KATp channels (Zhao and Wang, 2002;Tang et al., 2005;Webb et al., 2008), Ky7 channels (Schleifenbaum et al., 2010;Martelli et al., 2013; Hedegaard et al., 2014), and BKca(Liang et al., 2012;Jackson-Weaver et al., 2013) have beensuggested to be involved in H2S vasodilatation. Glibenclamide,4-aminopyridine (a blocker of voltage-gated K channels),and iberiotoxin (a blocker of BKca channels) failed to affectNaHS relaxation in our study. However, we found that TEA(1-3 mM) reduced NaHS relaxation; this concentration rangewas previously found to inhibit Ky7 channels (Hadley et al.,2000). In agreement with these findings XE991 and linopirdine,blockers ofKy7 channels, inhibited NaHS relaxation,suggest-ing that Ky7 channels are involved in NaHS relaxation.However, the reductions in NaHS relaxation by XE991,linopirdine, and TEA were markedly less than observed inKPSS versus NE-contracted preparations, suggesting thatother K channel subtypes may also contribute to NaHSrelaxation. Involvement of Calcium-Independent Mechanismsin H2S-Induced Vasodilation. Vascular tone is dependenton the relative activities of MLC kinase and MLCP, whereactivation of MLC kinase or inhibition of MLCP can increasephosphorylation of MLC and thereby initiate vascular smoothmuscle contraction (Somlyo and Somlyo, 2003). Inhibition ofMLCP, but not Rho-kinase,reduced the HS-induced relaxa-tion of mouse gastrointestinal smooth muscle (Dhaese andLefebvre, 2009); in the pig bladder, H2S lowered tensionwithout changing the calcium concentration (Fernandeset al., 2013). In our study, H2S initially evoked vasodilation Fig.8. Effect of rotenone and antimycin A on NaHS and SNP relaxation.(A) Effect of rotenone, antimycin A, Tempol, and MITO-TEMPO in KPSS-contracted mesenteric arteries on the response to 300 uM NaHS (one-wayANOVA, *P <0.05 by Bonferroni post-test, n=5). (B) Effect of rotenone,antimycin A, Tempol, and MITO-TEMPO in NE (3 uM)-contractedmesenteric arteries on the response to 300 pM NaHS (one-way ANOVA,*P<0.05 by Bonferroni post-test, n=5). (C) Effect of rotenone, antimycinA, Tempol, and MITO-TEMPO in NE (3 uM)-contracted mesentericarteries on the response to 100 uM SNP(one-way ANOVA, *P <0.05 byBonferroni post-test, n=5-11). Data are presented as mean ± S.E.M. while the intracellular calcium levels increased or weremaintained at the same level. These results suggest thatbesides a lowering of [Ca ]i by high NaHS concentrations,calcium desensitization is involved in NaHS relaxation of ratmesenteric small arteries. However, the phosphorylated MYPT-1/MYPT-1 ratio did not change after exposure to NaHSin the mesenteric arteries, suggesting that other mechanismsthan MLCP are involved in the calcium desensitization of thevascular smooth muscle cell contractile apparatus. In large arteries,inhibition of phosphodiesterase type 5 wasfound to be involved in NaHS relaxation (Bucci et al.,2012),and NaHS was also recently suggested to contain polysulfidesand by oxidation of protein kinase G to cause relaxation inmouse mesenteric arteries (Stubbert et al., 2014). However, inour study ODQ and RP-8-cPTP-cGMPS, an inhibitor of proteinkinase G,inhibited SNP relaxation and relaxation induced bythe polysulfide K2Sn while NaHS relaxation was unaltered,suggesting that polysulfides and NaHS cause relaxationthrough different pathways. Nor did we observe any effecton NaHS relaxation when protein kinase A was inhibited byKT5720. These findings do not exclude an interaction of H2Swith phosphodiesterases or protein kinase A and G, but theysuggest that other mechanisms are involved in NaHS-induceduncoupling of calcium from the contractile apparatus inmesenteric small arteries. In perfused trout gills, it has been suggested that sulfideinduces vasoconstriction by a mechanism involving mitochon-drial complexes I, II, and IV, and by enhancing the formationof radical oxygen species (ROS) (Skovgaard and Olson, 2012).The interaction of H2S with the mitochondria is complex, aslower concentrations may stimulate the mitochondrial elec-tron transfer through a mechanism involving metabolism bysulfide quinone oxoreductase (Szabo et al., 2014), whereashigher concentrations of HS may inhibit mitochondrialelectron transfer by interaction with cytochrome C (Goubernet al., 2007; Modis et al., 2013). In preparations contractedwith high extracellular potassium to exclude the contributionof K channels, the mitochondrial complex I and III inhibitorsrotenone and antimycin A both abolished NaHS relaxation inrat mesenteric arteries, but rotenone and antimycin A did notchange the relaxation induced by exogenously added NO andhence the bioavailability. Moreover, NaHS relaxations werenot inhibited in the presence of the superoxide mimeticTempol or the mitochondrial superoxide scavenger MITO-TEMPO. Although ROS may lead to relaxation in mesentericarteries, these findings do not suggest that ROS contribute toNaHS relaxation. Inhibition of cytochrome C by NaHS wouldlead to decreased ATP synthesis (Modis et al.,2013). There-fore, one may speculate that the lower ATP levels resultingfrom NaHS inhibition of the mitochondria lead to reducedactin-MLC cross-bridge turnover or to an increased smoothmuscle AMP to ATP ratio followed by activation of AMPkinase and vascular relaxation (Rubin et al., 2005). ThatNaHS relaxation was also reduced by rotenone and antimycinA in NE-contracted preparations suggests that both K chan-nels and a mitochondrial pathway contribute to NaHS re- laxation in mesenteric small arteries. Our findings suggest that low micromolar concentrations offree H2S lead to relaxations involving K channels followed bylowering of smooth muscle calcium. Moreover, in preparationsactivated with high extracellular potassium, NaHS inducedrelaxations independent of the changes in [Ca]i and MLCphosphorylation, probably by direct inhibition of the mito-chondrial electron transport,leading to force suppression. Theeffect of H2S on both Ky7 channels and the mitochondrialpathways may contribute to a cardioprotective effect of lowconcentrations of HS. Acknowledgments The authors thank Henriette Gram Johansen and Heidi Knudsenfor technical assistance. Authorship Contributions Participated in research design: Hedegaard, Gouliaev, Skovgaard,Simonsen. Conducted experiments: Gouliaev, Winther, Arcanjo, Aalling,Renalton,Skovgaard, Simonsen. Contributed new reagents or analytic tools: Wood, Whiteman. Performed data analysis: Hedegaard, Gouliaev, Winther, Arcanjo,Aalling, Renalton, Skovgaard, Simonsen. Wrote or contributed to the writing of the manuscript: Hedegaard,Gouliaev, Skovgaard, Simonsen. References ( Ali MY, Ping CY, Mok YY, Ling L, W hiteman M, Bhatia M, and Moore PK (2006) R egulation o f vascular nitric oxide in vitro a n d in vivo; a new role for en d ogenoushydrogen sulphide? B r J P harmacol 1 4 9:625-634. ) Bucci M, Papapetropoulos A, Vellecco V, Zhou Z, Zaid A, Giannogonas P, CantalupoA, Dhayade S, Karalis KP, and Wang R, et al. (2012) cGMP-dependent proteinkinase contributes to hydrogen sulfide-stimulated vasorelaxation. PLoS One 7:e53319. Buus NH, VanBavel E, and Mulvany MJ (1994) Differences in sensitivity of ratmesenteric small arteries to agonists when studied as ring preparations or ascannulated preparations. Br J Pharmacol 112:579-587. Cheang WS, Wong WT, Shen B, Lau CW, Tian XY, Tsang SY, Yao X, Chen ZY,and Huang Y (2010) 4-aminopyridine-sensitive K* channels contributes to NaHS-induced membrane hyperpolarization and relaxation in the rat coronary artery.Vascul Pharmacol 53:94-98. Dhaese I and Lefebvre RA (2009) Myosin light chain phosphatase activation is in-volved in the hydrogen sulfide-induced relaxation in mouse gastric fundus. Eur JPharmacol 606:180-186. Dombkowski RA, Russell MJ, Schulman AA, Doellman MM, and Olson KR (2005)Vertebrate phylogeny of hydrogen sulfide vasoactivity. Am JPhysiol Regul IntegrComp Physiol 288:R243-R252. Eaton SL, Roche SL, Llavero Hurtado M, Oldknow KJ, Farquharson C, GillingwaterTH, and Wishart TM (2013) Total protein analysis as a reliable loading control forquantitative fluorescent Western blotting. PLoS One 8:e72457. d'Emmanuele di Villa Bianca R, Sorrentino R, Coletta C, Mitidieri E, Rossi A,Vellecco V, Pinto A, Cirino G, and Sorrentino R (2011) Hydrogen sulfide-induceddual vascular effect involves arachidonic acid cascade in rat mesenteric arterialbed.J Pharmacol Exp Ther 337:59-64. Fernandes VS, Ribeiro ASF, Barahona MV, Orensanz LM, Martínez-Sáenz A, RecioP, Martínez AC, Bustamante S, CarballidoJ, and Garcia-Sacristan A, et al. (2013)Hydrogen sulfide mediated inhibitory neurotransmission to the pig bladder neck:role of KATp channels, sensory nerves and calcium signaling.J Urol 190:746-756.Goslar T, Mars T, and Podbregar M (2011) Total plasma sulfide as a marker of shock severity in nonsurgical adult patients. Shock 36:350-355. Goubern M, Andriamihaja M, Nubel T, Blachier F, and Bouillaud F (2007) Sulfide,the first inorganic substrate for human cells. FASEB J 21:1699-1706. Hadley JK, Noda M, Selyanko AA, Wood IC, Abogadie FC, and Brown DA (2000)Differential tetraethylammonium sensitivity of KCNQ1-4 potassium channels.Br J Pharmacol 129:413-415. ( Hedegaard ER, Nielsen BD, Kun A, Hughes AD, Krpigaard C,Mo g ensen S, MatchkovVV, F robert O , a nd Simonsen U (2 0 14) KV 7 c h annels are inv o lved in h ypoxia- ) induced vasodilatation of porcine coronary arteries. Br J Pharmacol 171:69-82. Ishii I, Akahoshi N,Yamada H, Nakano S, Izumi T, and Suematsu M (2010) Cys-tathionine gamma-lyase-deficient mice require dietary cysteine to protect againstacute lethal myopathy and oxidative injury. J Biol Chem 285:26358-26368. Jackson-Weaver O, Osmond JM, Riddle MA, Naik JS, Gonzalez Bosc LV, Walker BR,and Kanagy NL (2013) Hydrogen sulfide dilates rat mesenteric arteries by acti-vating endothelial large-conductance Ca-activated K’ channels and smoothmuscle Ca’ sparks. Am J Physiol Heart Circ Physiol 304:H1446-H1454. Jackson-Weaver O, Paredes DA, Gonzalez Bosc LV, Walker BR, and Kanagy NL (2011) Intermittent hypoxia in rats increases myogenic tone through loss of hy-drogen sulfide activation of large-conductance Ca+-activated potassium channels. Circ Res 108:1439-1447. Kimura H (2014) Production and physiological effects of hydrogen sulfide. AntioxidRedox Signal 20:783-793. Kiss L, Deitch EA, and Szabo C (2008) Hydrogen sulfide decreases adenosinetriphosphate levels in aortic rings and leads to vasorelaxation via metabolicinhibition. Life Sci 83:589-594. Kubo S, Doe I, Kurokawa Y, Nishikawa H, and Kawabata A (2007) Direct inhibitionof endothelial nitric oxide synthase by hydrogen sulfide: contribution to dualmodulation of vascular tension. Toxicology 232:138-146. Lee SW, Cheng Y, Moore PK, and Bian JS (2007) Hydrogen sulphide regulates in-tracellular pH in vascular smooth muscle cells. Biochem Biophys Res Commun 358: 1142-1147. Li L, Whiteman M, Guan YY, Neo KL, Cheng Y, Lee SW, Zhao Y, Baskar R, Tan CH,and Moore PK (2008) Characterization of a novel,water-soluble hydrogen sulfide-releasing molecule (GYY4137): new insights into the biology of hydrogen sulfide.Circulation 117:2351-2360. Li Y, Zang Y, Fu S, Zhang H, Gao L, and Li J (2012) H2S relaxes vas deferens smoothmuscle by modulating the large conductance Ca+-activated K*(BKca) channelsvia a redox mechanism.J Sex Med 9:2806-2813. ( Liang GH, X i Q , L effler CW, a nd Jaggar JH ( 2 012) Hydrogen sulfide activatesCa sparks t o induce c e rebral arteriole dilatation. J Physiol 590:2709-2720. ) ( Martelli A, Testai L , Bresch i MC, Lawson K , McKay NG, Mi c eli F, T a glialatela M,and CalderoneV(2013) Vasorelaxation by hydr o gen sulphide invol v es activation of Kv7 potassium channels. Pharmacol Res 70:27-34. ) ( Modis K, C oletta C, Erdelyi K, Papapetropoulos A, and Szabo C (2013) I ntra-mitochondrial hydrogen sulfide production by 3-mercaptopyruvate sul f urtransfer-ase m aintains mitochondrial e l ectron flow and s u pports cellular b i oenergetics. FASEB J 27:601-611. ) ( Mulvany MJ an d Halpern W (1977) Contractile properties of small arterial resistancevessels in spontaneously hypertensive and normotensive rats. Circ Res 41:19-26. ) Nelson MT and Brayden JE (1993)Regulation of arterial tone by calcium-dependent ( K+ channels and ATP-sensitive K* channels.Cardiouasc Drugs Ther 7 (Suppl 3): 605-610. ) ( Olson K R (2011) The t h erapeutic p o tential of hydrogen sulfide: separating hype from hope. Am J Physiol Regul Integr Comp Physiol 301:R297-R312. ) ( Olson KR, DeLeon ER, and Liu F (2014) Controversies and conundrums in hydrogensulfide biology. Nitri c Oxide 41: 1 1-26. ) Papapetropoulos A, Whiteman M, and Cirino G (2015) Pharmacological tools forhydrogen sulphide research: a brief, introductory guide for beginners. Br J Phar-macol 172:1633-1637. Peng H, Matchkov V, Ivarsen A, Aalkjaer C, and Nilsson H (2001) Hypothesis for theinitiation of vasomotion. Circ Res 88:810-815. Rodriguez-Rodriguez R, Stankevicius E, Herrera MD, Ostergaard L, Andersen MR,Ruiz-Gutierrez V, and Simonsen U (2008) Oleanolic acid induces relaxation andcalcium-independent release of endothelium-derived nitric oxide. Br J Pharmacol155:535-546. Rubin LJ, Magliola L, Feng X, Jones AW, and Hale CC (2005) Metabolic activation ofAMP kinase in vascular smooth muscle. J Appl Physiol (1985) 98:296-306. Salomone S, Foresti R, Villari A, Giurdanella G, Drago F, and Bucolo C (2014)Regulation of vascular tone in rabbit ophthalmic artery: cross talk of endogenousand exogenous gas mediators. Biochem Pharmacol 92:661-668. Schleifenbaum J, Kohn C, Voblova N, Dubrovska G, Zavarirskaya O, Gloe T, CreanCS, Luft FC, Huang Y, and Schubert R, et al. (2010) Systemic peripheral arteryrelaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens 28:1875-1882 Simonsen U, Wadsworth RM, Buus NH, and Mulvany MJ (1999) In vitro simulta-neous measurements of relaxation and nitric oxide concentration in rat superiormesenteric artery. J Physiol 516:271-282. Skovgaard N and Olson KR (2012) Hydrogen sulfide mediates hypoxic vasocon-striction through a production of mitochondrial ROS in trout gills. Am J PhysiolRegul Integr Comp Physiol 303:R487-R494. Somlyo AP and Somlyo AV(2003) Ca+ sensitivity of smooth muscle and nonmusclemyosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Reu83:1325-1358. Stubbert D, Prysyazhna O, Rudyk O, Scotcher J, Burgoyne JR, and Eaton P (2014)Protein kinase G Ia oxidation paradoxically underlies blood pressure lowering bythe reductant hydrogen sulfide. Hypertension 64:1344-1351. Sun Y,Tang CS, Du JB, and Jin HF (2011) Hydrogen sulfide and vascular relaxation.Chin Med J (Engl) 124:3816-3819. Szab6 C (2007) Hydrogen sulphide and its therapeutic potential. Nat Reu Drug Discou.6:917-935. Szabo C, Ransy C, Modis K, Andriamihaja M, Murghes B, Coletta C, Olah G, YanagiK, and Bouillaud F (2014) Regulation of mitochondrial bioenergetic function byhydrogen sulfide. Part I. Biochemical and physiological mechanisms. Br J Phar-macol 171:2099-2122. Tang G, Wu L, Liang W, and Wang R (2005) Direct stimulation of KATp channels byexogenous and endogenous hydrogen sulfide in vascular smooth muscle cells. MolPharmacol 68:1757-1764. ( Webb GD, Lim LH, Oh V M , Yeo SB, Cheong YP, Ali MY, El Oakley R, Lee CN, WongPS, and C aleb MG, et al. (2008) C ontractile and vasorelaxant e ffects of hydrogensulfide and its biosynthesis in the human internal mammary artery. J Pharmacol Exp Ther 324:876-882. ) Wesselman JP,Schubert R, VanBavel ED, Nilsson H, and Mulvany MJ (1997) KCa-channel blockade prevents sustained pressure-induced depolarization in rat mes-enteric small arteries. Am J Physiol 272:H2241-H2249. White BJO, Smith PA, and Dunn WR (2013) Hydrogen sulphide-mediated vaso-dilatation involves the release of neurotransmitters from sensory nerves inpressurized mesenteric small arteries isolated from rats. Br J Pharmacol 168:785-793. ( Yang G, Wu L, J iang B, Yang W , Qi J, Cao K , M eng Q, Mustafa AK, Mu W,and Zhang S, e t a l . (2008) H 2S as a physiologic vasorelaxant: h ypertension in micewith deletion of cystathionine g amma-lyase. Science 322:587-590. ) Zhao W and Wang R (2002) H(2)S-induced vasorelaxation and underlying cellularand molecular mechanisms. Am J Physiol Heart Circ Physiol 283:H474-H480. Zhong G, Chen F, Cheng Y, Tang C, and Du J (2003) The role of hydrogen sulfidegeneration in the pathogenesis of hypertension in rats induced by inhibition ofnitric oxide synthase. J Hypertens 21:1879-1885. Address correspondence to: Dr. Elise R. Hedegaard, Assistant Professor,Department of Biomedicine, Pulmonary and Cardiovascular Pharmacology,Aarhus University, Wilhelm Meyers Alle 4, 8000 Aarhus C, Denmark. E-mail:erh@farm.au.dk Unisense微电极可应用与老鼠肠道体内的硫化氢含量,其测试原理是体系中的硫化氢通过外部压力进入到电极前端中传感器顶端膜后进入到碱性电解质液中并转化为相应的电信号。硫化氢电极对于温度较为敏感,本实验中的硫化氢是在37度(生理环境温度)下、PH小于4的体系环境中进行校正的。硫化氢的测试是在含有PSS(PH=7.4)的闭管中进行测试。
确定



还剩9页未读,是否继续阅读?
上海谓载科技有限公司为您提供《老鼠肠道中硫化氢检测方案(其他电化学仪)》,该方案主要用于绒毛膜组织中硫化氢检测,参考标准--,《老鼠肠道中硫化氢检测方案(其他电化学仪)》用到的仪器有丹麦unisense硫化氢测量仪
相关方案
更多