方案详情
文
将现代计算机技术和先进的微波控制技术应用于实验室的化学合成和萃取。主要由微波固液相合成仪主机、回流冷凝系统、多通道高精度温度传感器、电磁机械双通道搅拌系统、电脑智能控制系统、计算机程序控制系统、微波化学合成数据库组成。
方案详情

Bioresource Technology 123 (2012) 72-77 73H. Zhang et al./Bioresource Technology 123 (2012) 72-77 http://dx.doi.org/10.1016/j.biortech.2012.06.082 Contents lists available at SciVerse ScienceDirect Bioresource Technology ELSEVIER journalhomepage: www.elsevier.com/locate/biortech Microwave assisted esterification of acidified oil from waste cooking oil byCERP/PES catalytic membrane for biodiesel production Honglei Zhang, Jincheng Ding, Zengdian Zhao * School of Chemical Engineering, Shandong University ofTechnology, Zibo 255049, PR China HIGHLIG H T S We prepared CERP/PES hybridcatalytic membrane for biodieselproduction. We conducted esterification ofacidified oil under traditionalheating method. We conducted esterification ofacidified oil under microwaveirradiation. -Biodiesel production withmicrowave irradiation needs lesstime and catalyst. Esterification with microwaveirradiation offers a fast, easy andeffective route. A RTICLE IN F O Article history: Received 21 March 2012Received in revised form 23 June 2012Accepted 25 June 2012 Available online 4 July 2012 Keywords: BiodieselCation ion-exchange resinHybrid catalytic membraneEsterificationMicrowave irradiation GRAPHICALABSTRACT Compared with traditional heating esterification (THE), microwave assisted esterification (MAE) needsless reaction time, lower reaction temperature, less energy and lower methanol additive. Thus, manufac-turing biodiesel using microwave represents a fast, easy and effective route with advantages of a shortreaction time, a low methanol/acidified oil mass ratio, an ease of operation, reduced energy consumptionand all with lower production cost. A BSTRACT The traditional heating and microwave assisted method for biodiesel production using cation ion-exchange resin particles (CERP)/PES catalytic membrane were comparatively studied to achieve eco-nomic and effective method for utilization of free fatty acids (FFAs) from waste cooking oil(WCO). Theoptimal esterification conditions of the two methods were investigated and the experimental resultsshowed that microwave irradiation exhibited a remarkable enhanced effect for esterification comparedwith that of traditional heating method. The FFAs conversion of microwave assisted esterification reached97.4% under the optimal conditions of reaction temperature 60℃,methanol/acidified oil mass ratio 2.0:1,catalytic membrane (annealed at 120 ℃) loading 3 g, microwave power 360 W and reaction time 90 min.The study results showed that it is a fast, easy and green way to produce biodiesel applying microwaveirradiation. The enormous exhaust emissions of fossil fuels lead to seriousandgreat concerns about the energy and environment and ( * C orresponding author. Tel.: +86 533 2781225,+8613 3 95338337; fax: +86 533 2781664. E-mail a ddress: zhaozengdian@sdut.edu.cn (Z. Zh a o). ) biodiesel has gained much attention for it is believed to be renew-able and environmental friendly (Foo and Hameed, 2012; Hong,2011). Biodiesel, a mixture of mono-alkyl esters of long-chain fattyacids, is an alternative diesel fuel derived from vegetable and animal oils/fats by transesterification and esterification withshort-chain alcohols (e.g. methanol or ethanol). It has many advan-tagessuch asenvironmental1friendliness, biodegradability,renewability, low toxicity, high security of use and storage, adapt-ability to presently used engines and good blending ability withpetroleum-based diesel fuels (Wang et al., 2006; El Sherbinyet al., 2010). However, biodiesel are not currently competitive with fossilfuels due to its higher cost of raw material and production(Yan et al., 2011). One way to reduce the cost of biodiesel is toselect cheaper oil feeds like waste cooking oils (WCO) (Fenget al., 2010; Phan and Phan, 2008). WCO contains a lot of free fattyacids (FFAs) and cannot be directly used to prepare biodiesel byalkaline-catalyzed transesterification for soap formation (Shiet al., 2011; Liu et al., 2010). Therefore, the FFAs in waste cookingoils were often firstly esterified by homogeneous strong acid-cata-lysts, such as sulphuric and hydrochloric acid (Marchetti andErrazu, 2008; Zhang and jiang, 2008). But liquid acids are diffi-cult to be separated from the reaction medium and producewastewater and strong equipments corrosion (Shi et al., 2010;Zhu et al.,2010). Catalytic membranes, as one form of heterogeneous catalystswere developed to overcome the shortcoming of liquid acid cata-lysts for they have a lot of advantages, such as catalyst separation,mild reaction conditions, no corrosion to equipment and no pollu-tion to the environment (Ding et al., 2011). Polyethersulfone (PES)is a good candidate as polymeric material for the preparation ofcatalytic membrane supports since it possesses good mechanicaland thermal stability and good chemical resistance to aliphatichydrocarbons, alcohols and acids. Cation ion-exchange resins canoffer better selectivity towards the desired product(s) and betterreusability compared with homogeneous acid catalysts (Fenget al., 2011). In our previous work, cation ion-exchange resin par-ticles/PES hybrid catalytic membrane was prepared and used ascatalyst for the esterification of acidified oil with different alcoholsand the membrane showed good catalytic activity in biodiesel pro-duction (Ding et al., 2011; Zhang et al.,2012). Microwave irradiation is an unconventional energy sourcewhich has been widely used for a large number of chemical reac-tions including esterification. As polar molecules can selective ab-sorb microwave energy and non-polar molecules are inert to themicrowave dielectric loss, chemical reactions can be greatlyacceleratedl under microwave irradiation condition (Kumaret al., 2011).Microwave irradiation has a selective heating func-tion and requires less energy input for heating compared withconventional heating method. It is possible to perform reactionsquickly,efficiently and safely using scientific microwave appara-tus (Kumar et al., 2011). Some previous studies on microwave-as-sisted synthesis of biodiesel reported that microwave irradiationcan accelerate the reaction and reduce the reaction time (Hernan-do et al., 2007; Hsiao et al., 2011; Refaat et al., 2008; Su et al.,2010; Immacolata et al., 2012; Yuan et al.,2009). Azcan and Dan-isman (2008) reported a significant reduction in the reaction timeand an increase in product yield in microwave irradiated transe-sterification. However, the esterification of high FFAs oil withcatalytic membranes under microwave condition was littlereported. In the present work, we applied CERP/PES catalytic membranefor esterification of acidified oil with methanol for biodiesel pro-duction under traditional heating method and microwave irradia-tion. The main affecting parameters including reaction time,reaction temperature, catalytic membrane loading,methanol/acid-ified oil mass ratio and microwave power were analyzed to find outthe optimal reaction conditions. Furthermore, the compositions ofthe produced biodiesel were analyzed by Gas chromatography-mass Spectrometry. 2.1.Materials PES was purchased from Solvay Advanced Polymer Co. Ltd. Cat-ionion-exchange resin NKC-9 (average size of 0.83mm) in acidicform with total exchange capacity of ≥4.7 mmol/g, average porediameter of 56 nm and moisture content of <5% was purchasedfrom the Chemical Plant of Nankai University. NKC-9 was milledin frozen state using liquid nitrogen to obtain fragments (CERP)and the average particle size of the CERP particles analyzed by La-ser Scattering Particle Analyzer (BT-9300H, Dandong Baite Instru-ment CO. Ltd. Dandong, China) was 24.96 um. Acidified oil (FFAs)from waste cooking oil with an acid value of 152.3 mg KOH/gwas kindly supplied by Hubei Haolin Bioenergy Company and fil-tered to remove impurities. No water was detected by Karl Fischerwater tester with a sensitivity of 500 ppm (KF-1A, Shanghai Preci-sion and Scientific Instrument Co. Ltd., China). The main composi-tions of the feedstock oil analyzed by Gas chromatography-massSpectrometry (GC-MS: 6890N GC/5973 MS, Agilent Technologies)were dodecanoic acid, tetradecanoic acid,hexadecanoic acid andoctadecadienoic acid. The other chemicals were all AR grade andused without further purification. 2.2. Membrane preparation The PES and CERP were dried at 120°C for 1hand 50°C for24 h, respectively in a vacuum oven to remove a trace amount ofwater in them. Twenty grams PES was dissolved in 80 g NMP undercontinuous stirring for 12 h at room temperature to obtain PESsolution and then 20 g CERP was added into the above solutionand stirred till a homogeneous solution was obtained. The solutionwas cast onto glass plates and a copper wire was used to controlthe thickness of the membranes. The membranes were immersedinto secondary distilled water to phase inversion and washed for6 h to remove the solvents. Then the membranes were peeled offfrom the glass plates and dried in a vacuum oven at 50 C for24 h. The obtained membranes were annealed at 80, 120, 150and 200℃, respectively for 1 h in a heating oven.Finally, the mem-branes were cut into pieces for further use. 2.3. Traditional heating esterification (THE) Traditional heating esterification (THE) was performed in athree-necked batch reactor (100 ml) equipped with a reflux con-denser and mechanical stirrer at atmospheric pressure. The reactorwas heated by a heating jacket and a thermocouple was inserted inthe reactor to measure the reaction temperature. The catalyticmembranes were cut into small pieces (3 mmx3 mm) to achievebetter contact with reactants and to eliminate the influence of dif-fusion on esterification. Some amount of acidified oil was firstintroduced into the reactor and heated to the determined temper-ature. Then the desired amount of methanol and the catalyticmembranes were added into the reactor and the reaction beganat pre-determined conditions. The basic optimal reaction condi-tions discussed in our previous paper (Ding et al.,2011)were: acid-ified oil 20 g, methanol/acidified oil mass ratio 2.5:1 (methanol/acidified oil molar ratio 29:1), catalytic membrane loading 5 g,reaction temperature 65C and reaction time 8 h. The sampleswere taken out from the reaction system every one hour and trea-ted by decompression distillation with a rotational evaporator toremove unreacted methanol and produced water to determinateacid value and FFAs conversion. In repeated experiments, themembranes were reused without any treatment. 2.4. Microwave assisted esterification (MAE) As shown in Fig. 1, microwave assisted esterification (MAE) wascarried out with a microwave accelerated reaction system (BeijingXiangHu Science and Technology Development Co. Ltd.,XH-200A).The system is equipped with a reflux condenser, a magnetic stirrerand an infrared temperature measurement which allows continu-ous stirring and constant temperature control. A three-neckedbatch reactor (500 ml) was used for the microwave assisted reac-tion and a flask (250 ml) full of water was settled in the chamberto absorb microwave during each experiment. The basic reactionconditions were: acidified oil 20 g, reaction time 120 min, reactiontemperature 65℃, methanol/acidified oil mass ratio 2.0:1, cata-lytic membrane loading 3 g and microwave power 360 W. 2.5.Separation and purification of biodiesel After reaction completion, the reaction mixture was cooled toroom temperature and settled to separate the liquid phase (biodie-sel, methanol and unreacted acidified oil) and solid phase (catalyticmembrane). The liquid phase was poured into a separating funneland settled over night to separate methanol and the biodiesel. Thebiodiesel was further purified by decompression distillation with arotational evaporator under vacuum (10±1mmHg) at 50 °C to re-move excess methanol and other impurities.The biodiesel waswashed twice with secondary distilled water and treated by anhy-drous sodium sulfate to remove moisture. The product obtainedwas qualitatively analyzed by Gas chromatography-mass Spec-trometry (GC-MS: 6890N GC/5973 MS, Agilent Technologies).The temperatures of injector and detector were kept at 250C.The nitrogen was used as a carrier gas at a flow rate of 40 mL/min. Column was heated from 70 to 240℃ at heating rate of10C/min and maintained at 240°℃ for 10 min. 2.6. Determination of the acid value and FFAs conversion The acid value was determined by titration according to ASTMD1980-87(1998) (Standard Test Method for Acid Value of FattyAcids and Polymerized Fatty Acids). The brief steps were intro-duced as follows: about 0.20 g samples were added into 150 ml Fig. 1. Schematic diagram of the microwave accelerated reaction system. (a)Condenser pipe; (b) display panel; (c) control panel; (d) reaction chamber switch;(e) mains switch; (f) magnetic stirring knob;(g) infrared temperaturemeasurement. neutralized boiling ethanol and was fully dissolved; 0.5 ml phenol-phthalein was used as indicator; the sample was then titrated by0.1 mol/L KOH solution. The acid value was calculated using Eq.(1): where S is the acid value (mg KOH/g acidified oil), CkoH is the con-centration of KOH used for titration (mol/L), VKoH is the volume ofKOH employed for titration (ml), m is the weight of the sample ta-ken to be analyzed (g). FFAs conversion is defined as the ratio of acid value variationrate of acidified oil before and after reaction compares to acid valueof the initial oil. The conversion is calculated according to Eq.(2): where S; refers to initial acid value (mg KOH/g acidified oil) and Strefers to the acid value at pre-determined reaction time (mg KOH/g acidified oil). The reported values were the mean of at least fivemeasurements and the average estimated error was ±5%. 3. Results and discussion 3.1. Traditional heating esterification 3.1.1. Effect of membrane annealing temperature In order to improve the catalytic activity and reusability, themembranes were annealed at 80, 120, 150 and 200 ℃ for 1 h,respectively. Five grams of membranes annealed at different tem-peratures were used in the esterification. It is found that annealingtemperatures markedly affected the FFAs conversion. FFAs conver-sions increase from 77.86% to 90.87% and 94.98% with the anneal-ing temperature increasing from 50 to 80 and 120℃ becauseremoval of the absorbed water in the membranes could improve-SO3H group content in the drier membranes of unit quality. How-ever, FFAs conversion decreases greatly from 94.98% to 74.12%when the annealing temperature rose from 120 to 200℃ mainlybecause-SO3H groups in CERP reacted with -OH at higher temper-ature, such as 200℃ (Zhu et al., 2010). So the optimized annealingtemperature is 120 °C. 3.1.2. Effect ofstirring rate Stirring plays an important role in biodiesel production. Esteri-fication is a relatively slow process since this reaction can only oc-cur in the interfacial region between the liquids and FFAs andmethanol are not totally miscible. As a result, vigorous mixing isrequired to increase the area of contact between the two immisci-ble phases. Previously, researchers had reported that the stirringrate and mode were extraordinary important parameters for bio-diesel production (Meher et al., 2006; Veljkovic et al., 2006). Toinvestigate the effect of stirring rate in the reaction, esterificationwas conducted with different mechanical stirring rates of 120,240, 360, 480 and 600 rpm, respectively.The FFAs conversions atdifferent stirring rates are shown in Fig.2. It is observed that thereactions are incomplete when the stirring rates are 120, 240 and360 rpm and the FFAs conversions are only 71.15%, 80.24% and85.96%, respectively after 8 h. However, FFAs conversions at 480and 600 rpm are nearly the same of 94%. So the optimal stirringrate is 480 rpm which is in accordance with the result of Veljkovicet al. (2006). 3.1.3. Reusability of the catalytic membranes Besides the catalytic activity, reusability of the catalytic mem-branes is very important in saving produce cost. The catalyticmembranes annealed at different temperatures were employed Fig. 2. Effect of stirring rate on esterification. Reaction conditions: acidified oil 20 g,methanol/acidified oil mass ratio 2.5:1, reaction temperature 65℃, catalyticmembrane loading 5 g and reaction time 8 h. in the esterification for six runs and the results are shown in Fig. 3.It can be seen that all the membranes show good catalytic activityat the first run with FFAs conversion above 90%, higher than thatreported by Ozbay et al.(2008). The catalytic activity and catalyticstability of the membranes annealed at 120°C are higher than thatof others and the FFAs conversion reaches approximate 85.21% atthe sixth run. It implies that the membranes annealed at 120 ℃ ex-hibit the best catalytic activity and stability for producing biodie-sel. For the other four membranes, the catalytic activitydecreased with the runs and the FFAs conversion is only 75.34%for the membranes annealed at 200°C at the sixth run. The mem-branes become very fragile, easy broken into fine particles understirring and lost during the repeated experiments, which wouldlead to the decrease of the catalytic efficiency. In addition, the sat-uration of absorbing water produced from the esterification maybe another reason of the conversion decrease for all the mem-branes (Zhu et al., 2010). 3.2. Microwave assisted esterification 3.2.1. Effect of reaction temperature Effect of reaction temperature on FFAs conversion under micro-wave condition is shown in Fig. 4. It is found that FFAs conversionincreased with the increase of reaction temperature from 35 to Fig. 3. Catalytic performances of the membranes versus recycling times underoptimal conditions. Fig. 4. Effect of catalytic membrane loading and reaction time on esterification.Reaction conditions: acidified oil 20 g, reaction temperature 60℃, methanol/acidified oil mass ratio 2.0:1, microwave power 240 W and reaction time 90 min. 60℃ due to the promotion of the molecule motion and mass trans-fer rate with increasing temperature (Ding et al., 2011). FFAs con-version of 60C is almost the same with that of 65 C. However, theFFAs conversion decreases greatly from 87.5% to 72.6% when thetemperature further increases to 70°℃ because methanol willvaporize and form a large number of bubbles which may inhibitthe reaction when the reaction temperature exceeds the boilingpoint of methanol (El Sherbiny et al., 2010). In consideration ofproduction cost, the optimal reaction temperature is 60C. Thisimplies that microwave condition needs lower reaction tempera-ture as the optimal reaction temperature with traditional heatingmethod was 65 °C. 3.2.2. Effect ofreaction time To optimize reaction conditions, the effect of reaction time onthe esterification was investigated and the results are shown inFig. 4. FFAs conversion by CERP/PES catalytic membrane undermicrowave condition can be divided into three phases when reac-tion temperature is set as 60 C. At the first phase, the FFAs conver-sion over 85% can be obtained within 60 min; at the second one,esterification rate slows down from 60 min to 90 min with FFAsconversion of above 95%; at the last phase after 90 min, prolongingthe reaction time don't increase FFAs conversion but drive theesterification left as a result of side reaction. In view of reactiontime and production cost, the optimal reaction time is 90 min. 3.2.3. Effect of methanol/acidified oil mass ratio One of the most important variables affecting the esterificationis the molar ratio of methanol to FFA. The stoichiometric molar ra-tio for esterification requires one mole of methanol and one moleof FFA to yield one mole of free fatty methyl ester and one moleof water. However, esterification is an equilibrium reaction whichis often performed in excess methanol to drive the reaction to theright. Effect of methanol/acidified oil mass ratio on esterificationunder microwave condition is shown in Table 1.The FFAs conver-sion increases from 56.54% to 95.92% with the increase of metha-nol/acidified oil mass ratio rose from 0.5:1 to 2.0:1. However, theFFAs conversion decreased a lot when the mass ratio rise to3.5:1, which is mainly because excess methanol would adsorb onthe catalytic membrane surface and decrease the contact opportu-nities between acidified oil and the catalytic membranes and thusdecrease the catalytic activity or even deactivate the catalyst. Theresult was in good agreement with the swelling degree value re- Table 1Effect of methanol/acidified oil mass ratio on the esterification. Methanol/oil 0.5 1.0 1.5 2.0 2.5 3 3.5 mass ratio FFAs Conversion 56.54 64.87 76.63 95.92 93.84 80.33 78.42 (%) Reaction conditions: Acidified oil 20 g, reaction temperature 60℃, 120℃ annealedcatalytic membrane loading 3 g, microwave power 240 W and reaction time90 min. Table 2Effect of catalytic membrane loading on the esterification. Catalyst loading (g) 2 3 4 5 FFAs Conversion (%) 64.3 81.65 92.2 92.31 92.6 Reaction conditions: Acidified oil 20 g, reaction temperature 60℃, methanol/acidi-fied oil mass ratio 2.0:1, microwave power 240 W and reaction time 90 min. ported in our previous work (Zhang et al., 2012). So the optimalmethanol/acidified oil mass ratio is 2.0:1 in the reaction system. 3.2.4. Effect ofcatalytic membrane loading In order to optimize the catalytic membrane loading, differentexperiments were performed with different catalytic membraneloadings and the results are illustrated in Table 2. FFAs conversionis quite low when catalytic membrane loading is 1 and 2 g as thereare not enough active sites. This indicates that the reaction couldnot proceed efficiently with a small amount of CERP/PVA hybridcatalytic membranes as there were not enough active sites. TheFFAs conversion is almost the same of 92.5% with the catalyticmembrane loading of 3, 4 and 5 g and it seems meaningless forthe FFAs conversion to further increase catalytic membrane load-ing. Thus the optimal catalytic membrane loading is 3 g in the reac-tion system. This strongly demonstrates that the experimentsunder microwave condition need low catalyst loading. 3.2.5. Effect of microwave power Microwave power is another important parameter in analyzingthe reaction process so as to evaluate the production cost. The ef-fect of microwave power on FFAs conversion was investigated. It isfound that FFAs conversion increased a lot from 83.93% to 94.76%when microwave power increased from 120 W to 360W for theraised reaction temperature. However, it seems meaningless to in-crease the microwave power from 360 W to 480 W, with the FFAsconversion of 94.7%. It is also found that the FFAs conversion de-creased slightly (about 90.34%) when the power further increasedto 600 W, which is mainly because that with the increase of micro-wave power, heating rate increases greatly and methanol willvaporize promoting the side reaction and inhibiting the esterifica-tion. So the optimal microwave power is 360 W in this reactionsystem. 3.2.6. Effect of different alcohols Alcohols used in acid-catalyzed esterification mainly includedmethanol, ethanol, propanol and butanol. Methanol and ethanolare used most frequently in both laboratory research and the bio-diesel industry. The low cost of methanol makes it the first choicefor the esterification reaction. Ethanol, however, is derived fromagriculture products (renewable sources) and biologically lessobjectionable to the environment than methanol. Thus, ethanol isthe ideal candidate for the synthesis of fully biogenerated fuel.To study the esterification of methanol and ethanol with acidifiedoil under microwave condition, different experiments were per-formed and the results are shown in Fig. 5. It is observed thatmethanol has better FFAs conversion than ethanol. This is Fig.5. Effect of different alcohols on esterification.Reaction conditions: acidified oil20 g, reaction temperature boiling point of each alcohol, alcohol/acidified oil molarratio 23.5:1, microwave power 240 W and reaction time 90 min. associated with the fact that methanol has stronger microwaveabsorption capacity and it can easily react with acidified oil underthe microwave condition (Yuan et al., 2009). 3.3. Comparison of THE and MAE catalyzed by CERP/PES membranes The traditional heating esterification and microwave assistedreaction were performed under optimal reaction conditions,respectively and the results are shown in Fig. 6. As can be seenin Fig. 6, FFAs conversion of THE is about 98.4% under the opti-mized conditions for 8 h reaction time; however, FFAs conversionof MAE could reach 97.4% under the optimal conditions of reactiontemperature 60 ℃, methanol/acidified oil mass ratio 2.0:1, 120℃annealed catalytic membrane loading 3 g, microwave power360 W and reaction time 90 min. The reaction temperature,cata-lyst loading and methanol consumed in MAE are slightly lowerand the reaction time is much lower than that in THE, which couldlead to lower cost in biodiesel production. These results stronglydemonstrates that the application of microwave condition couldoffer a fast, economical and easy route to produce biodiesel andMAE catalyzed by CERP/PES catalytic membrane is an appropriateoption for biodiesel production. Fig. 6. Comparison of traditional heating method and microwave assisted esteri-fication catalyzed by CERP/PES membrane under optimal conditions, respectively. Table 3Compositions of the product. Peak Retention Constituents Fatty acid time(min) 15.73 Dodecanoic acid methyl ester C12:0° 18.73 Tetradecanoic acid methyl ester C14:0 20.69 Hexadecanoic acid methyl ester C16:0 22.84 9,12-Octadecadienoic acid(Z,Z) methyl ester C18:2 23.02 Octadecanoic acid methyl ester C18:0 The first number stands for the number of the carbon in the compound; thesecond number stands for the number of C=C double bond in the compound. 3.4.Qualitative analysis of the biodiesel The compositions of the produced FAME were investigated andare illustrated in Table 3. The reaction product mainly containedfive constituents: dodecanoic acid methyl ester, tetradecanoic acidmethyl ester, hexadecanoic acid methyl ester, 9, 12-octadecadie-noic acid (Z, Z) methyl ester and octadecanoic acid methyl ester.The two C18 esters of 9, 12-octadecadienoic acid (Z, Z) methyl es-ter, octadecanoic acid methyl ester should be produced from theoctadecadienoic acid in the acidified oil that are difficult to sepa-rate in the GC test. So the compositions in the acidified oil weretransformed to their corresponding fatty acid methyl ester (FAME)in the reaction product by esterification with the CERP/PES cata-lytic membrane as heterogeneous acid catalyst under microwavecondition. 4. Conclusion In the present investigation, biodiesel was prepared by esterifi-cation of acidified oil with methanol under microwave irradiationcondition. Compared with traditional heating method, microwaveassisted esterification needs less reaction time, lower reaction tem-perature, less energy and lower methanol additive. Thus, manufac-turing biodiesel using microwave represents a fast, easy andeffective route with advantages of a short reaction time, a lowmethanol/acidified oil mass ratio, an ease of operation,reduced en-ergy consumption and all with lower production cost. Furtherexperiments need to be done in the future to investigate the possi-bility of scale-up for industrial application. References Azcan, N., Danisman, A., 2008. Microwave assisted transesterification of rapeseedoil. Fuel 87, 1781-1788. Ding, J., He, B., Li, J.. 2011. Cation ion-exchange resin/polyethersulfone hybridcatalytic membrane for biodiesel production. J. Biobased Mater. Biol. 5(7), 85-91. ( E l Sherbiny, S.A., Refaat, A.A., El Sheltawy, S.T., 2010. Production of biodiesel u sing the m icrowave technique. J. Adv. R e s. 1, 3 09-314. ) Feng, Y., He, B., Cao, Y., Li, J., Liu, M., Feng, Y., Liang, X., 2010. Biodiesel productionusing cation-exchange resin as heterogeneous catalyst. Bioresour.Technol. 101,1518-1521. Feng, Y.,Zhang, A., Li, J., He, B., 2011. A continuous process for biodiesel productionin a fixed bed reactor packed with cation-exchange resin as heterogeneouscatalyst. Bioresour. Technol.102,3607-3609. Foo, K.Y., Hameed, B.H., 2012. Microwave-assisted preparation and adsorptionperformance of activated carbon from biodiesel industry solid reside: influenceof operational parameters. Bioresour. Technol. 103, 398-404. Hernando, J., Leton, P., Matia, M.P., Novella, J.L., Alvarez-Builla, J., 2007. Biodieseland FAME synthesis assisted by microwave: homogenous batch and flowprocesses. Fuel 86, 1641-1644. Hong,J., 2011. Uncertainty propagation in life cycle assessment of biodiesel versusdiesel: global warming and non-renewable energy. Bioresour. Technol. 113, 3-7. Hsiao,,M.C., Lin, C.C., Chang, Y.H., 2011. Microwave irradiation-assistedtransesterification of soybean oil to biodiesel catalyzed by nanopowdercalcium oxide. Fuel90, 1963-1967. Immacolata, M., Laura, G., Vittorio, V., Massimo, O., 2012. Microwave technology forthe biodiesel production: analytical assessments. Fuel 5, 108-112. Kumar, R., Kumar, G.Ravi., Chandrashekar, N., 2011. Microwave assisted alkali-catalyzed transesterification of Pongamia pinnata seed oil for biodieselproduction. Bioresour.Technol. 102,6617-6620. Liu, Y., Yan, Y., Hu, F., Yao, A., Wang, Z., Wei, F., 2010. Transesterification forbiodiesel production catalyzed by combined lipases: optimization and kinetics.AIChE J.56,1659-1665. Marchetti, J.M., Errazu, A.F., 2008. Esterification of free fatty acids using sulfuric acidas catalyst in the presence of triglycerides. Biomass Bioenerg. 32 (9), 892-895. Meher, L.C., Dharmagadda, V.S.S., Naik, S.N., 2006. Optimization of alkali-catalyzedtransesterification of Pongamia pinnata oil for production of biodiesel.Bioresour. Technol.97, 1392-1397. Ozbay, N., Nuray, O., Tapan, N.A., 2008. Esterification of free fatty acids in wastecooking oils (WCO): role of ion-exchange resins. Fuel 87, 1789-1798. Phan, A.N., Phan, T.M., 2008. Biodiesel production from waste cooking oils. Fuel 87,3490-3496. Refaat, A.A., El Sheltawy, S.T., Sadek, K.U., 2008. Optimum reaction time,performance and exhaust emissions of biodiesel produced by microwaveirradiation. Int. J. Environ. Sci. Technol. 5,315-322. Shi, W., He, B., Ding, J., Li, J., Yan, F., Liang, X., 2010. Preparation and characterizationof the organic-inorganic hybrid membrane for biodiesel production. Bioresour.Technol.101,1501-1505. Shi, W., He, B., Li, J., 2011. Esterification of acidic oil with methanol by SPES/PEScatalytic membrane. Bioresour. Technol. 102(9),5389-5393. Su, Z., Yuan, G.,Yu,J.,Meng, L., Dong, Y., Thomas,E., 2010. Rapid microwave-assistedtransesterification of yellow horn oil to biodiesel using a heteropolyacid solidcatalyst. Bioresour.Technol. 101,931-936. Veljkovic, V.B., Lakicevic, S.H., Stamenkovic, O.S., Todorovic, Z.B., Lazic, M.L., 2006.Biodiesel production from tobacco (Nicotiana tabacum L.) seed oil with a highcontent of free fatty acids. Fuel 85, 2671-2675. Wang,Y., Ou, S., Liu, P., Xue, F., Tang, S., 2006. Comparison of two different processesto synthesize biodiesel by waste cooking oil. J. Mol. Catal. A: Chem. 252, 107-112. Yan, J., Yan, Y., Liu, S., Hu, J., Wang, G., 2011. Preparation of cross-linked lipase-coated micro-crystals for biodiesel production from waste cooking oil.Bioresour. Technol. 102,4755-4758. Yuan, H., Yang, B., Zhu, G., 2009. Synthesis of biodiesel using microwave absorptioncatalysts. Energy Fuel 23, 548-552. Zhang, H., Ding, J., Qiu, Y., Zhao, Z., 2012. Kinetics of esterification of acidified oilhers11with different alcohols by a cation ion-exchange resin/polyethersulfone hybridcatalytic membrane. Bioresour. Technol. 112, 28-33. Zhang, J, Jiang, L., 2008. Acid-catalyzed esterification of Zanthoxylum bungeanumseed oil with high free fatty acids for biodiesel production. Bioresour. Technol.99(18),8995-8998. Zhu, M., He, B., Shi, W., Feng, Y., Ding, J., Li, J., Zeng, F., 2010. Preparation andcharacterization of PSSA/PVA catalytic membrane for biodiesel production. Fuel89, 2299-2304. 为改善膨润土对Cr(VI)的吸附性能,并提高其固液分离回收能力,研究构筑了一种壳聚糖修饰的复合磁性吸附材料——磁性柠檬酸膨润土(Magnetic Citric Acid Bentonite,MCAB),并利用 SEM、XRD、VSM、FT-IR 对改性前后的材料进行表征对比,结果表明壳聚糖中的‒NH2、‒OH 等功能基团及磁性 Fe3O4 微粒均负载到柠檬酸膨润土(Citric Acid Bentonite,CAB)上。 MCAB 对Cr(VI)的吸附性能研究结果显示:相对于CAB,复合后的 MCAB 吸附性能明显提高,并具有良好的磁分离能力;pH值对Cr(VI)的吸附影响较大, pH 值在 2.0~3.0;当吸附剂投加量为 1.6 g·L‒1、 pH 值为 3.0、Cr(VI)的初始浓度为 10 mg·L‒1 时,MCAB 对 Cr(VI)的去除率高达 99%;吸附等温方程和动力学方程的拟合结果分别符合 Langmuir 吸附等温模型和准二级动力学模型。由于 MCAB 上修饰的‒NH2 在酸性条件下易质子化,会增强对 Cr(VI)的静电吸引作用,因此 MCAB 对 Cr(VI)的吸附去除率得到了明显提高;同时,加磁赋予了 CAB 快速的固液分离能力。研究表明 MCAB 对模拟废水中的 Cr(VI)去除效果良好,有望应用于实际废水中 Cr(VI) 的去除。
确定
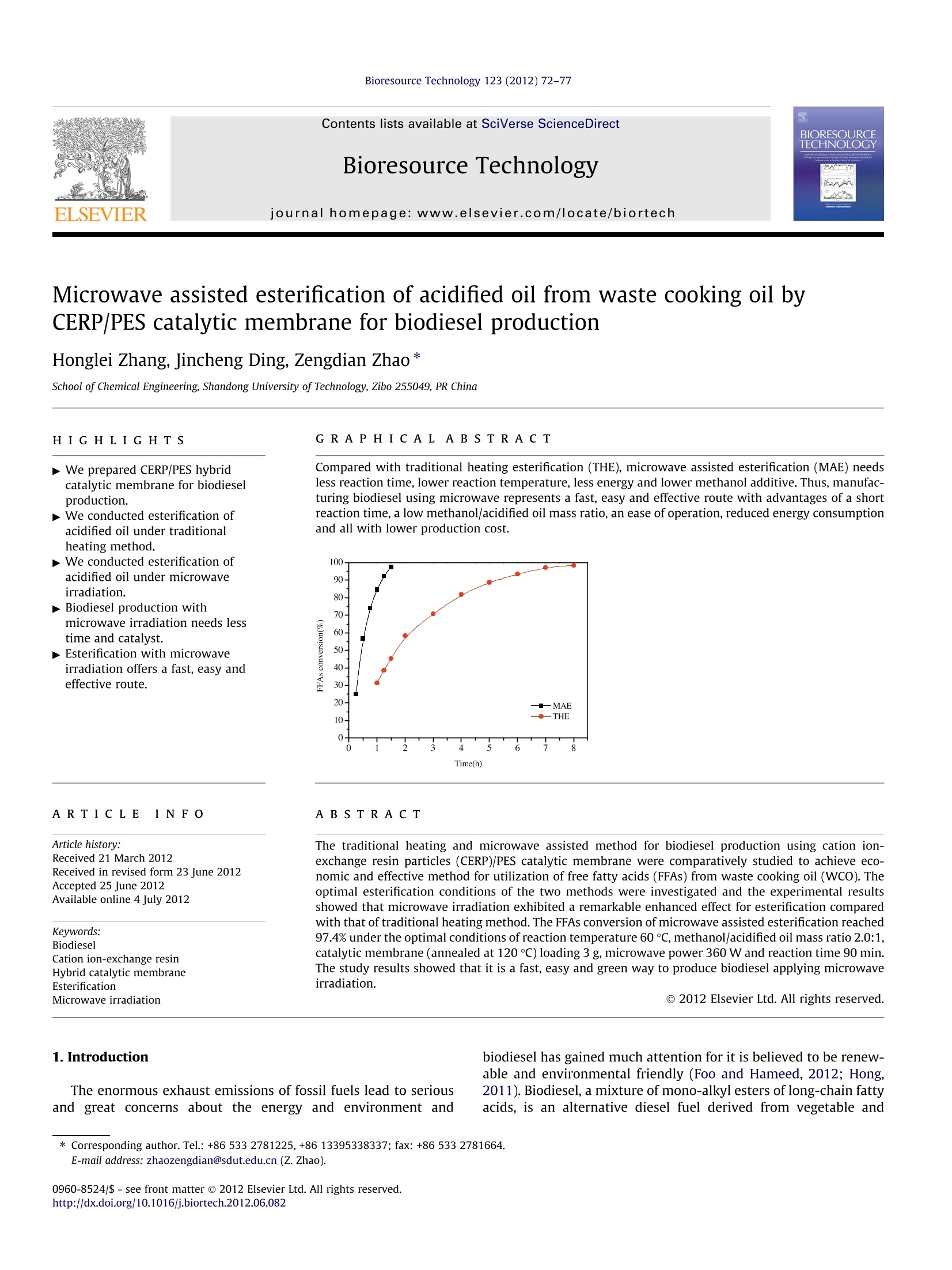

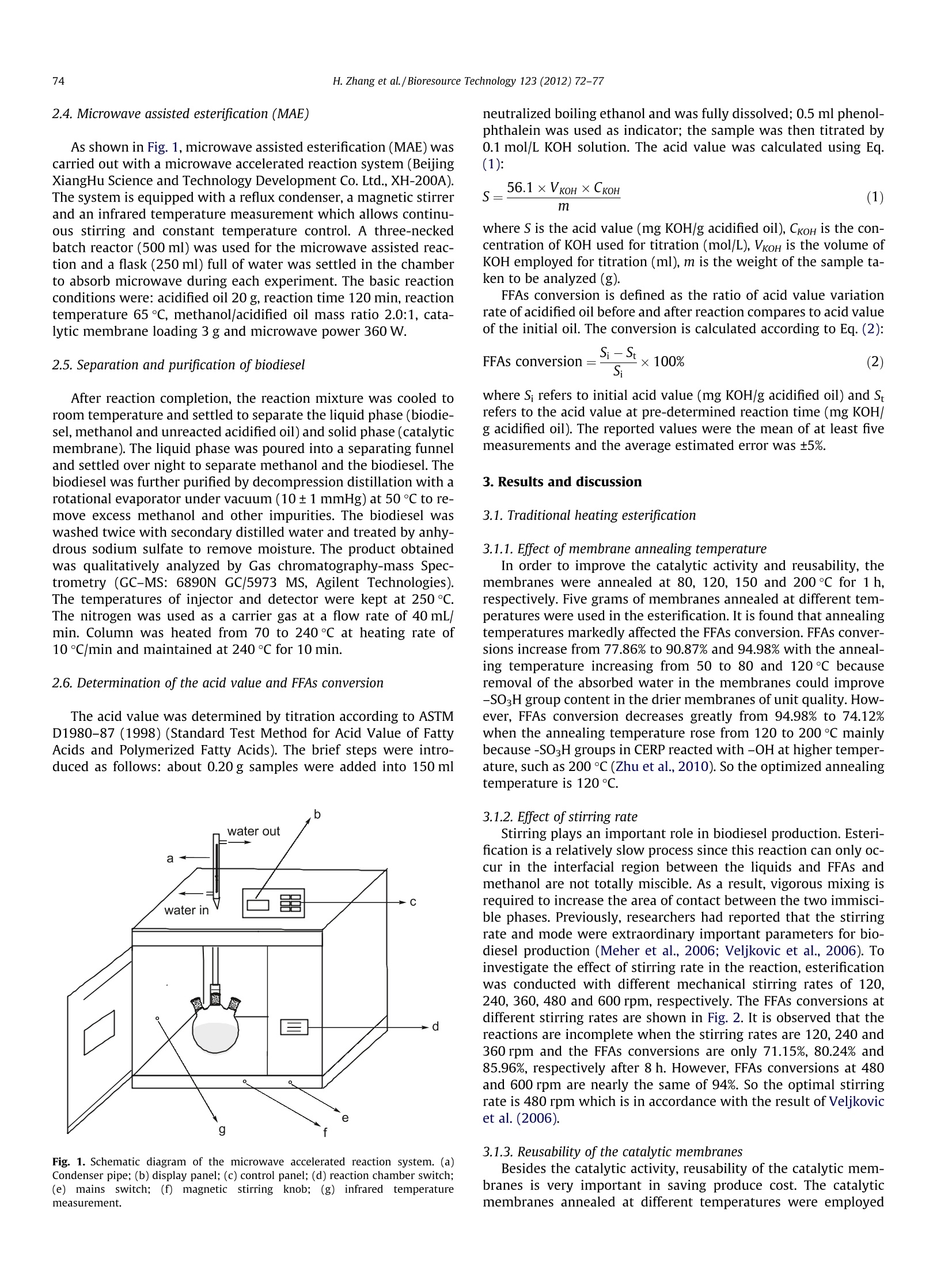
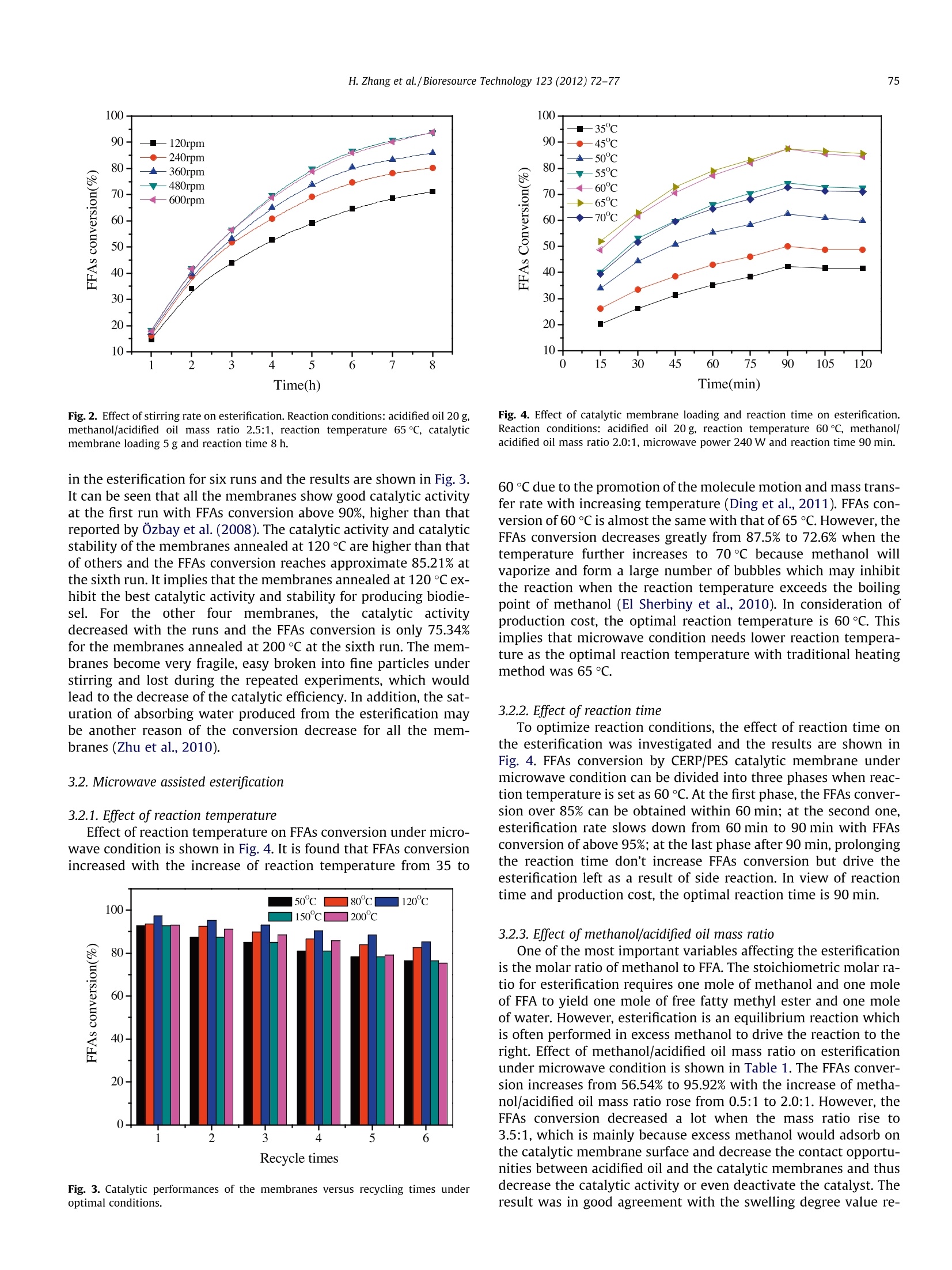
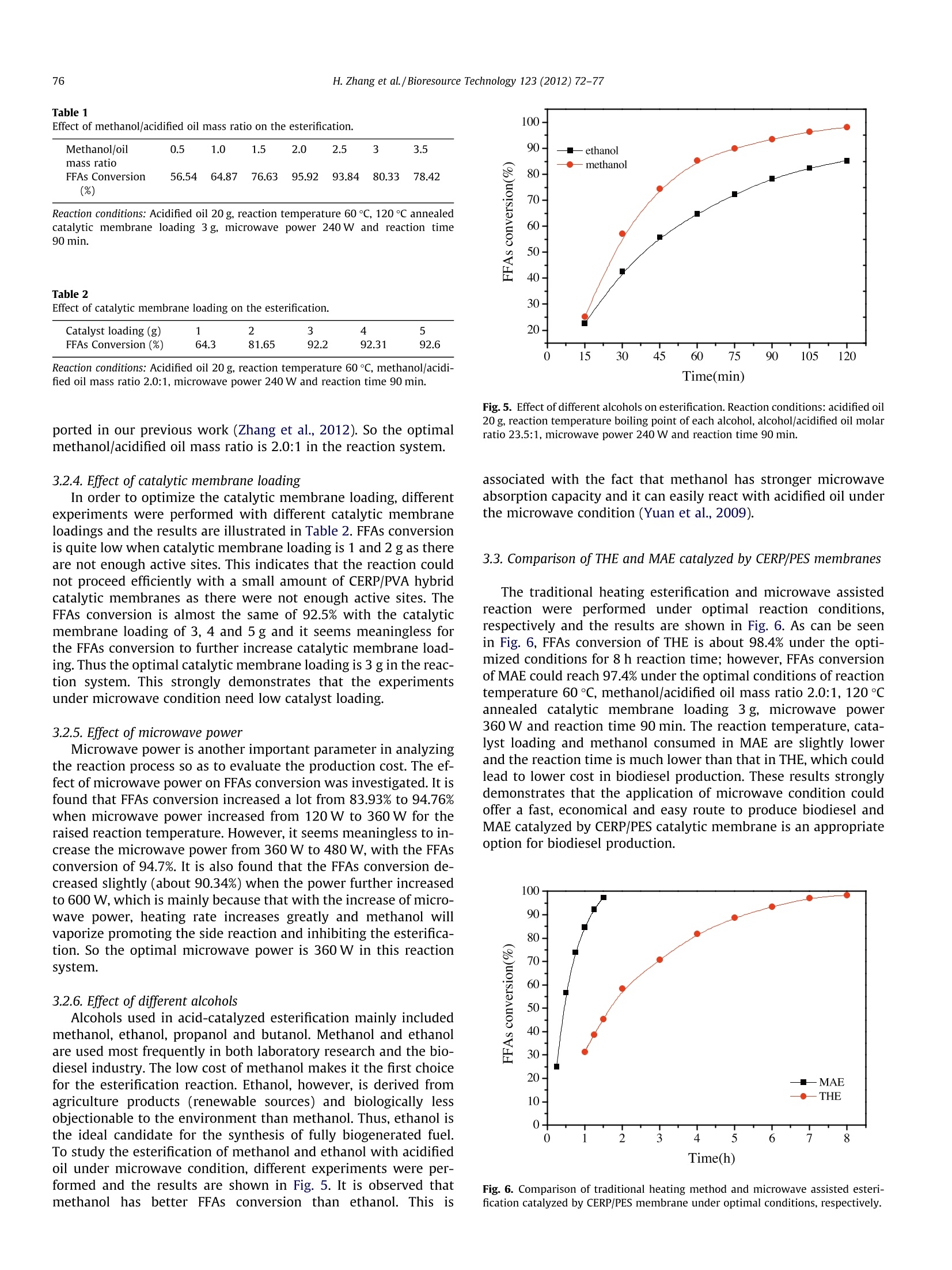

还剩4页未读,是否继续阅读?
北京祥鹄科技发展有限公司为您提供《烟梗纤维中形态及抄造性能的变化检测方案(微波合成仪)》,该方案主要用于烟草制品中形态及抄造性能的变化检测,参考标准--,《烟梗纤维中形态及抄造性能的变化检测方案(微波合成仪)》用到的仪器有电脑微波固液相合成萃取仪
推荐专场
相关方案
更多
















