含氟有机化合物具有独特的性质,其在生命科学的各个领域,特别是在医药和作物保护领域得到越来越多的开发,市场潜力巨大。
例如,许多含氟化合物,如氟硅酸盐(SiF6)-2,应用于杀虫剂和防腐剂等,较低浓度时用于牙膏和漱口水的制备。或用于制造特氟隆(Teflon®)(聚四氟乙烯),一种抗酸耐腐蚀的含氟聚合物材料,也可以应用于汽车工业和一些特殊容器的生产,例如不粘的器具的涂层。此外,一些氟碳氢化合物作为添加成分应用于润滑油,可以让润滑油性能更加稳定。
此外,在过去的十五年中,含氟药物已经成为药物化学的一个重要组成部分,这一点可以从目前市场上可买到的广泛应用的药物中得到证明。许多氟化合物已经被开发和测试,用于提高代谢稳定性,影响人体内酸碱度的水平。
方案详情

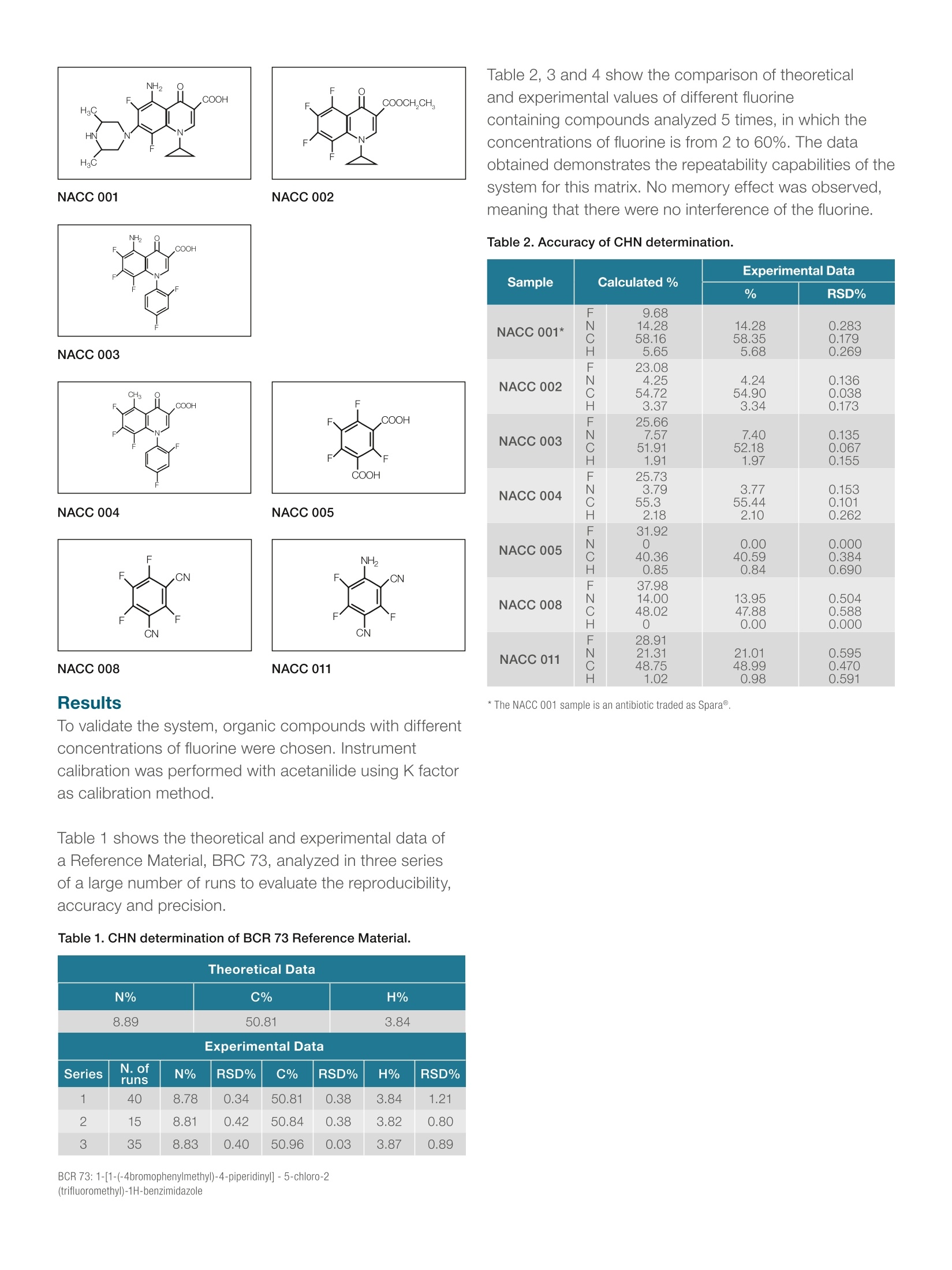
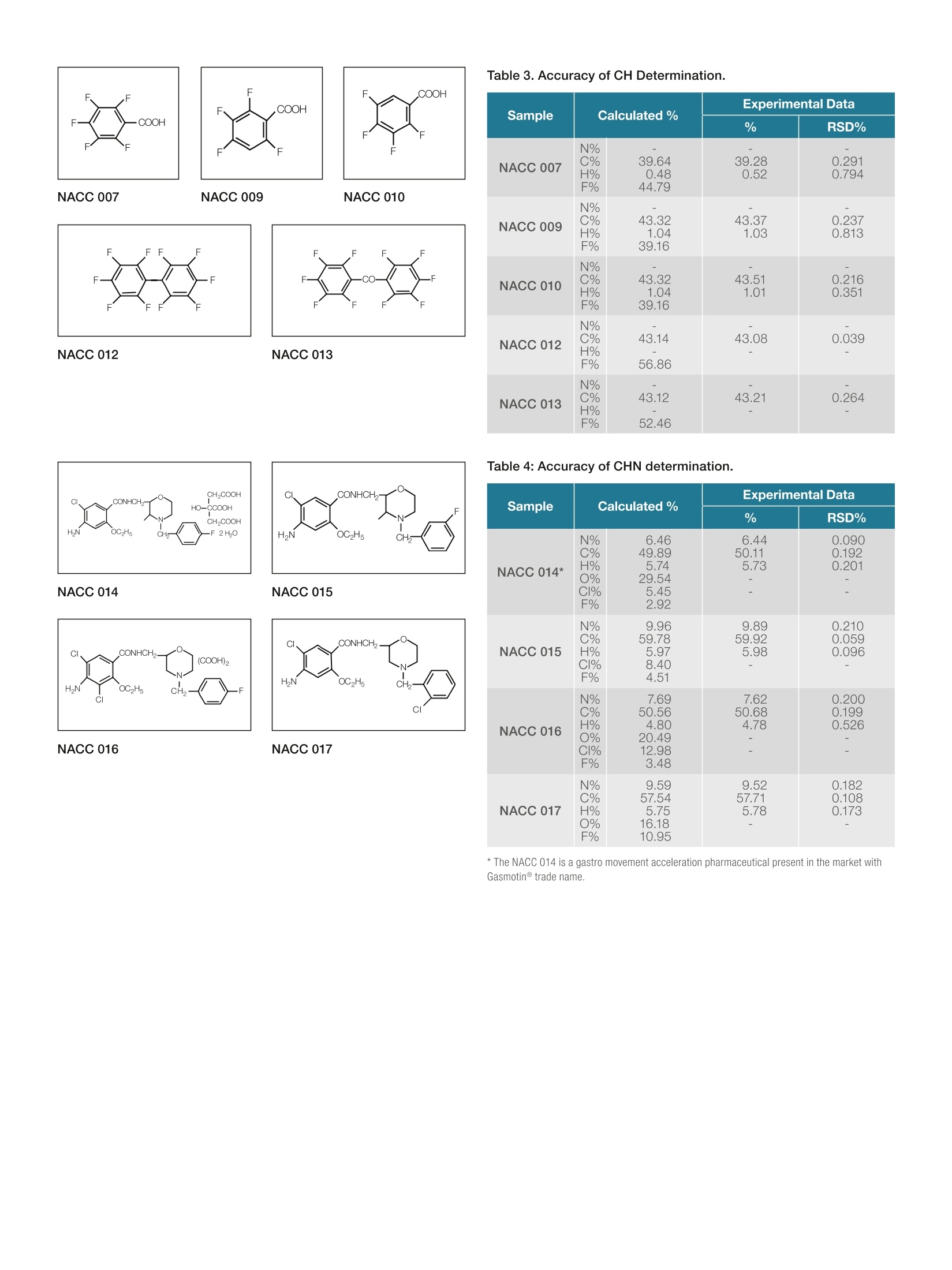
ThermoFisherSCIENTIFIC thermoscientific APPLICATION NOTE 42162 0t 心 CHN determination in Fluorine-Compoundswith the Thermo Scientific FlashSmartElemental Analyzer Authors Introduction Dr. Liliana Krotz, and Dr. Guido Giazzi. Thermo Fisher Scientific. Fluoro-organic compounds exhibit unique properties and their potential isincreasingly being exploited in various areas of life sciences, particularly inpharmaceutical and crop-protection fields. Milan, Italy Keywords CHN, Fluorine Compounds, IQ/OQ, Organic Synthesis, Pharmaceuticals Goal For example, many of the fluoro-compounds, the fluorosilicate (SiF)2, areused in industrial solutions such as insecticides and antiseptics, and atlow concentrations are used in toothpaste and mouthwash preparation.Applications have also been found using Teflon@(PTFE-polytetrafluoroethilene),a fluoro-polymer which is very resistant to acid attack and is used in the carindustry and in the production of special vessels such as non-stick lookingutensils. Furthermore, some per-fluorate hydrocarbons are used as verystable lubricant oils. This application note shows theaccuracy and robustness of theThermo Scientific FlashSmart EAused for fluorine applications. However, in the last fifteen years fluorine-containing drugs have become animportant tool in medicinal chemistry,as shown by its widespread use in thedrugs currently available on the market. Many fluorine compounds have beendeveloped and tested to enhance metabolic stability, which influences acidityand basicity levels in the human body. This application note was developed with the greatsupport of Dr Keiko Bando of DaiNippon, Osaka,Japan, who has supplied us with many of the samplesevaluated, and supported us with her wide experience influorine-compounds analysis. Fluorine compounds in OEA analysis Fluorine is very reactive. It reacts with catalysts, the tincontainer and with the silica present in the quartz reactor. Consequently, it affects the robustness of the analyticalsystem and the resistance time is proportional to thefluorine concentration in the molecule. Figure 1. Thermo Scientific FlashSmart Elemental Analyzer. The fluorine compounds, which were analyzed for thisapplication have a concentration range from 3% to55%.Although fluorine reaction affects the analytical system,the Thermo ScientificFlashSmartElemental Analyzerprovides a solution to overcome this challenge. A short layer of FluoAdso, constitutinga mixture ofdifferent oxides, was placed in the hot area of theoxidation reactor where the flash combustion occurs.The adsorber reacts with the fluorine-compounds, savingcatalysts and the quartz reactor wall. Method The FlashSmart Elemental Analyzer is based on thedynamic combustion of the sample. The materials to beanalyzed are weighed in tin containers and introducedinto the combustion reactor via the Thermo ScientificMAS Plus Autosampler with oxygen. After combustionof the sample, the gases produced are carried by ahelium flow to the combustion reactor filled with theFluoAdso, then through the oxidation reduction catalysts,a GC column. They are finally detected by a ThermalConductivity Detector (TCD). Figure 2. CHN configuration for Fluorine-Containing Compounds. A complete CHN report is automatically generated by theThermo ScientificEagerSmartData Handling Softwareand displayed at the end of the analysis. Analytical Conditions Oxidation/Reduction Furnace 950℃ GC Column Oven 75°℃ Helium Carrier Flow 140 ml/min Helium Reference Flow 100 ml/min Oxygen Flow 250 ml/min Oxygen Injection Time 5 sec Sample Delay Time 12 sec Total Run Time less than 8 min Standard Acetanilide Standard Weight 0.8-1.2 mg Sample Weight 0.8-1.2 mg Calibration Method K factor COOHCOOCH,CH, XO NACC 001 NACC 002 Table 2,3 and 4 show the comparison of theoreticaland experimental values of different fluorinecontaining compounds analyzed 5 times, in which theconcentrations of fluorine is from 2 to 60%.The dataobtained demonstrates the repeatability capabilities of thesystem for this matrix. No memory effect was observed,meaning that there were no interference of the fluorine. Table 2. Accuracy of CHN determination. NACC 003 NACC 004 NACC 005 NACC 008 NACC 011 Sample Calculated% Experimental Data % RSD% NACC 001* FNCH 9.6814.2858.16 5.65 14.2858.355.68 0.2830.1790.269 NACC 002 FNCH 23.08 4.25 4.24 0.1360.0380.173 54.903.34 54.72 3.37 NACC 003 F NCH 25.667.5751.911.91 7.4052.181.97 0.1350.0670.155 NACC 004 FN 25.73 3.79 3.7755.44 0.1530.1010.262 NACC 005 CH FNCH 55.3 2.18 31.92 0.0000.3840.690 O 40.362 0.85 NACC 008 FNCH 37.98 13.9547.880.00 0.5040.5880.000 14.0048.02O NACC 011 FNCH 28.9121.31 21.0148.990.98 0.5950.4700.591 48.751.02 Results *The NACC 001 sample is an antibiotic traded as Spara@. To validate the system, organic compounds with differentconcentrations of fluorine were chosen. Instrumentcalibration was performed with acetanilide using K factoras calibration method. Table 1 shows the theoretical and experimental data ofa Reference Material, BRC 73, analyzed in three seriesof a large number of runs to evaluate the reproducibility,accuracy and precision. Table 1. CHN determination of BCR 73 Reference Material. Theoretical Data N% C% H% 8.89 50.81 3.84 Experimental Data Series N.ofruns N% RSD% C% RSD% H% RSD% 1 40 8.78 0.34 50.81 0.38 3.84 1.21 2 15 8.81 0.42 50.84 0.38 3.82 0.80 3 35 8.83 0.40 50.96 0.03 3.87 0.89 ( BCR 7 3 : 1 - [ 1- (-4 b romo p heny l met h y l) - 4 - p i p eri di nyl ] -5-chl o ro-2(t ri f l u o r o methyl)-1H -b enzim i d a zole ) Table 3. Accuracy of CH Determination. NACC 007 NACC 009 NACC 010 FF NACC 012 NACC 013 Sample N%C%H%F% Experimental Data NACC 013 N% C%H% F% RSD% NACC 007 N%C%H% F% 39.640.48 44.79 39.280.52 0.2910.794 43.321.0439.16 43.371.03 0.2370.813 - 43.511.01 0.2160.351 43.08 - 0.039 43.21 0.264 Table 4: Accuracy of CHN determination. CH,COOH C CONHCH- HO-CCOOH CH,COOH HN OC2H5 -F2HO H2N OC2H5 NACC 014 NACC 015 CI CONHCH2- CI. CONHCH (COOH)2 H2N OC2H5 H2N OC2H5 CI CI NACC 016 NACC 017 Sample Calculated% Experimental Data % RSD% NACC 014* N%C%H%0%C1%F% 6.46 49.895.7429.545.452.92 6.4450.115.73- 0.0900.1920.201 一 NACC 015 N%C%H%CI%F% 9.9659.785.978.404.51 9.8959.925.98 0.2100.0590.096 NACC 016 N%C%H%0%CI%F% 7.6950.564.8020.4912.983.48 7.6250.684.78 0.2000.1990.526 NACC 017 N%C%H%0%F% 9.5957.545.7516.18 10.95 9.5257.715.78 0.1820.1080.173 * The NACC 014 is a gastro movement acceleration pharmaceutical present in the market withGasmotintrade name. Conclusion Among halogens, only fluorine concentrations affectsCHN determination as it causes tailing or splitting of thehydrogen peak. The use of a special FluoAdso, placedin the hot part of the combustion reactor eliminates thehigh activity of the fluorine compounds and it allowsthe quantitative determination of CHN with excellentaccuracy and precision. The lifetime of the reactor, when analyzing fluorinecompounds, is 150 - 200 samples, depending on thesample nature and the fluorine concentration in thechemical compounds. The use of the adsorber in CHNconfiguration completely eliminates any interference offluorine and other halogens atoms,chlorine, bromineand iodine do not influence CHN determination and nodedicated hanlding measures need to be taken. Dr Keiko Bando,Nichiei Sangyo Co.,Ltd, DainipponPharmaceutical, Osaka, Japan Find out more at thermofisher.com/OEA ( O2017 Th e rmo Fi s h e r S cientifi c I n c . All r igh ts r e served. T e flo n is a r eg is t e red t ra d emark o f E. I. du Po nt d e N emours a nd Co m pany.All ot h e r tra d em a rks are t h e p r o p e rty of T h er mo F isher S c ienti fi c. T h is i nf o rm at ion is p resen te d a s an example o f th e capabilities o f Ther mo Fish er Scie n tific pr o ducts. I t is not in ten d ed t o en courage u s e of t hese produc t s in a n y ma n ner th a t m i ght inf ri ng e the i n te llect u al p rope r ty r i gh t s o f o t h e rs. Spe c ific at ion s , ter m s an d pric i n g ar e s u bje c t t o ch ang e. Not all pro d u ct s a re availa b le in a ll c ount r ies. P l ease co n sult y o ur lo c a l s al e s r epre s e nt at i ve f or de t ails. A N 42162-E N 0517 ) 含氟有机化合物具有独特的性质,其在生命科学的各个领域,特别是在医药和作物保护领域得到越来越多的开发,市场潜力巨大。 例如,许多含氟化合物,如氟硅酸盐(SiF6)-2,应用于杀虫剂和防腐剂等,较低浓度时用于牙膏和漱口水的制备。或用于制造特氟隆(Teflon®)(聚四氟乙烯),一种抗酸耐腐蚀的含氟聚合物材料,也可以应用于汽车工业和一些特殊容器的生产,例如不粘的器具的涂层。此外,一些氟碳氢化合物作为添加成分应用于润滑油,可以让润滑油性能更加稳定。 此外,在过去的十五年中,含氟药物已经成为药物化学的一个重要组成部分,这一点可以从目前市场上可买到的广泛应用的药物中得到证明。许多氟化合物已经被开发和测试,用于提高代谢稳定性,影响人体内酸碱度的水平。
确定


还剩3页未读,是否继续阅读?
大昌华嘉科学仪器为您提供《含氟有机化合物中CHN元素检测方案(有机元素分析)》,该方案主要用于其他中含量分析检测,参考标准--,《含氟有机化合物中CHN元素检测方案(有机元素分析)》用到的仪器有有机元素分析仪(碳氢氧氮硫)、元素分析仪(CN/CNS)、全自动元素分析仪(CHN)、总氮分析仪(杜马斯燃烧法)、杜马斯蛋白质分析仪(定氮仪)
推荐专场
相关方案
更多
该厂商其他方案
更多