方案详情
文
此应用方法可简单、快速、经济、有效的测定蜂蜜中7种新烟碱类杀虫剂。使用SPEX GG2010组织研磨处理实验样品,只需1min,即可得到均匀一致的样品。
方案详情

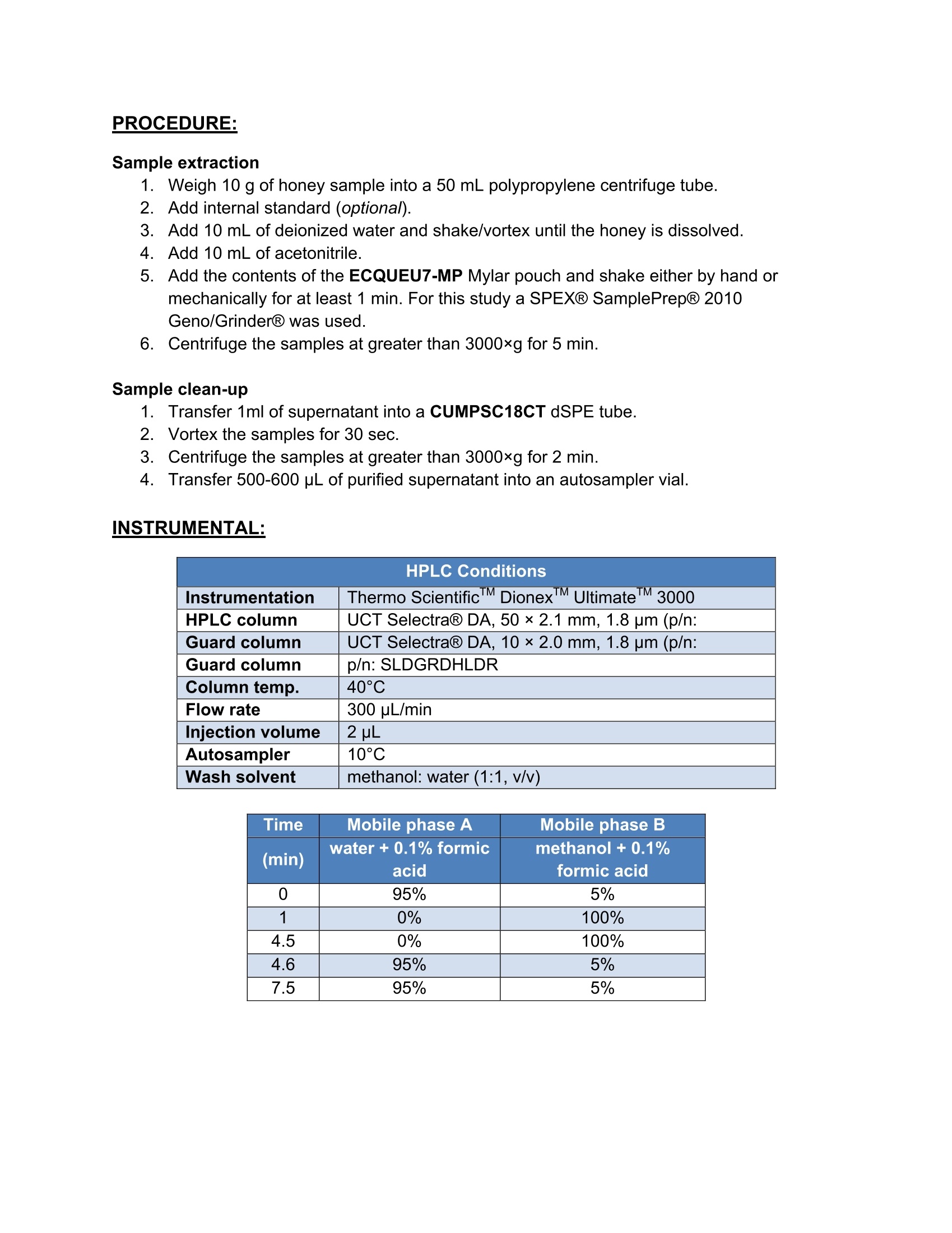
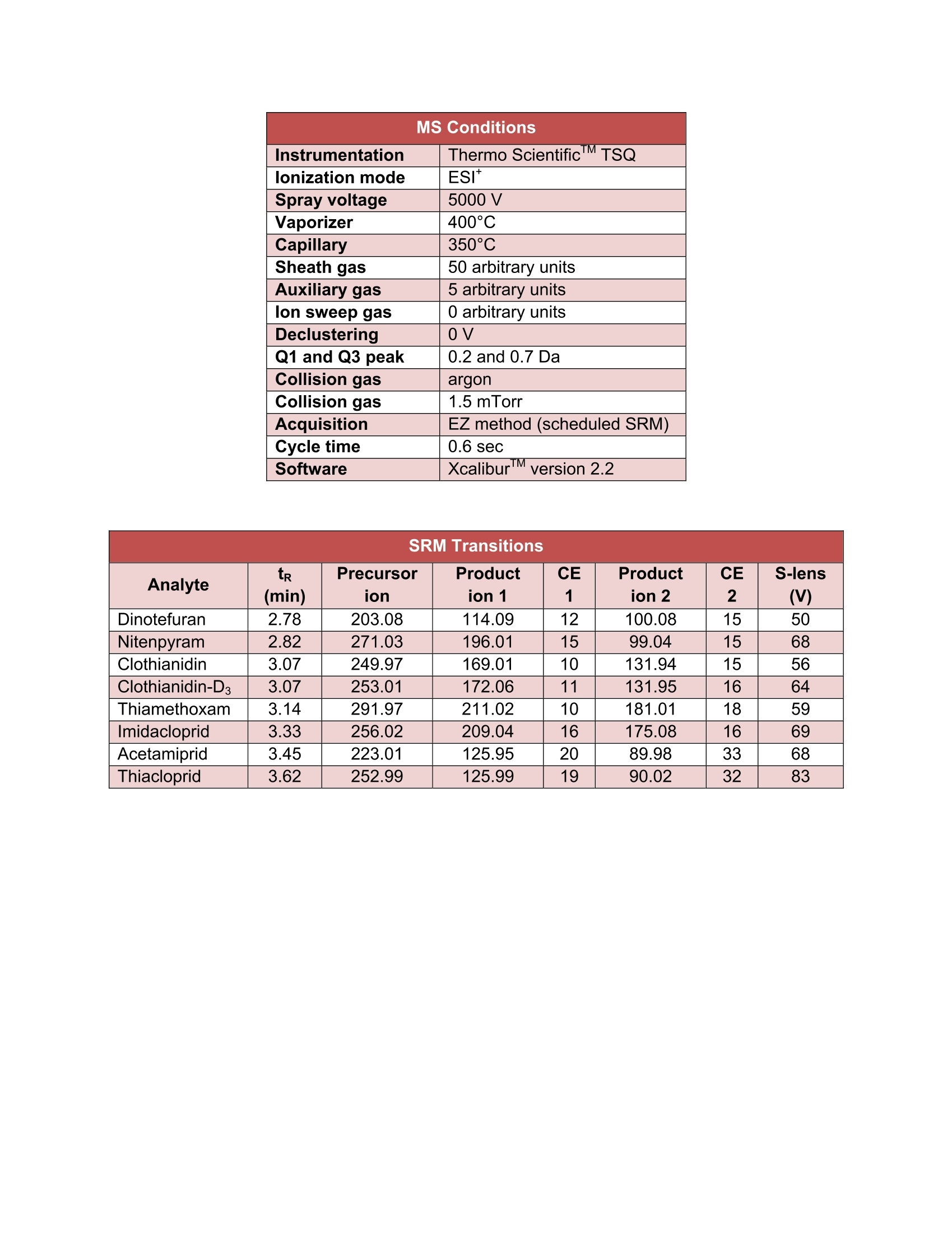
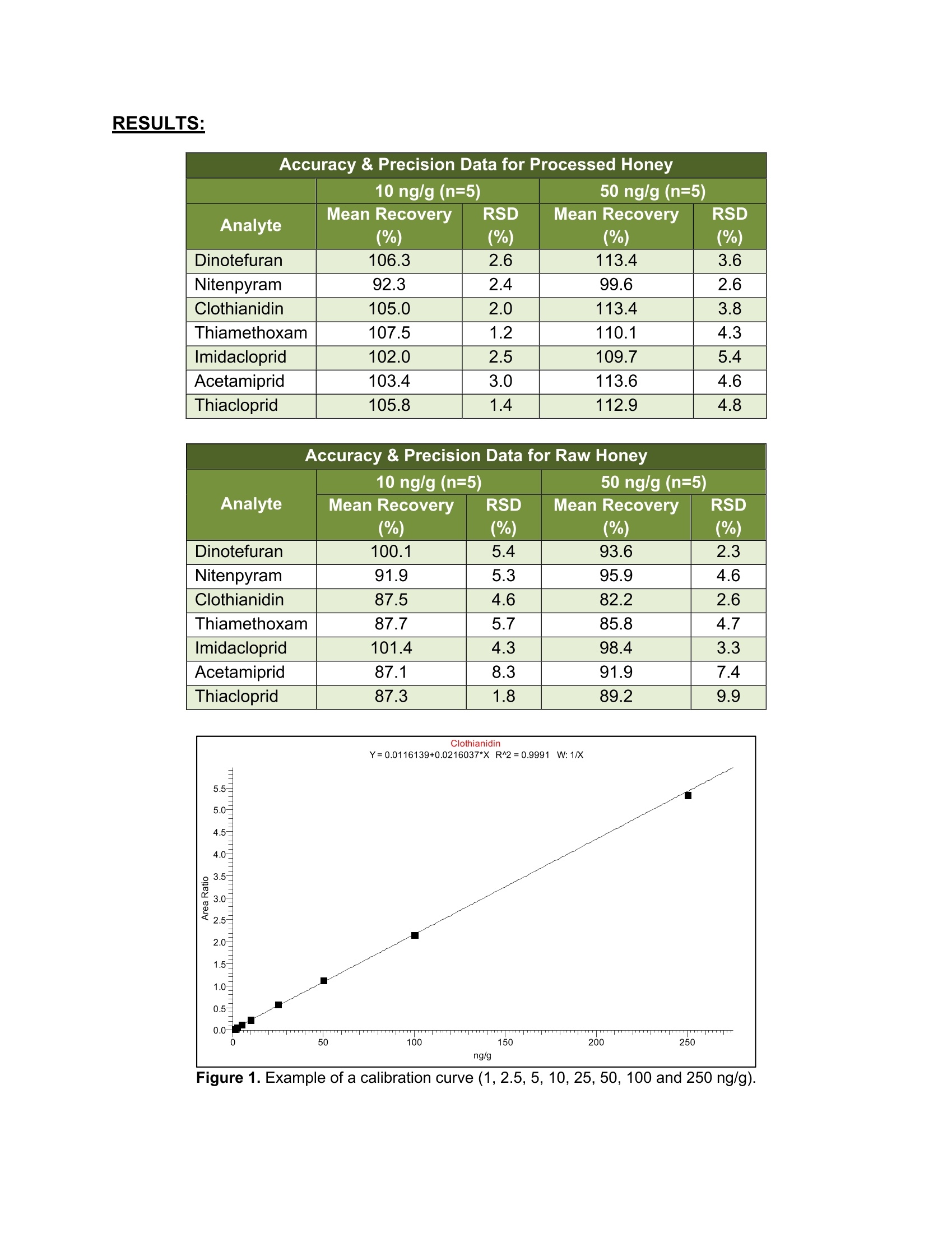
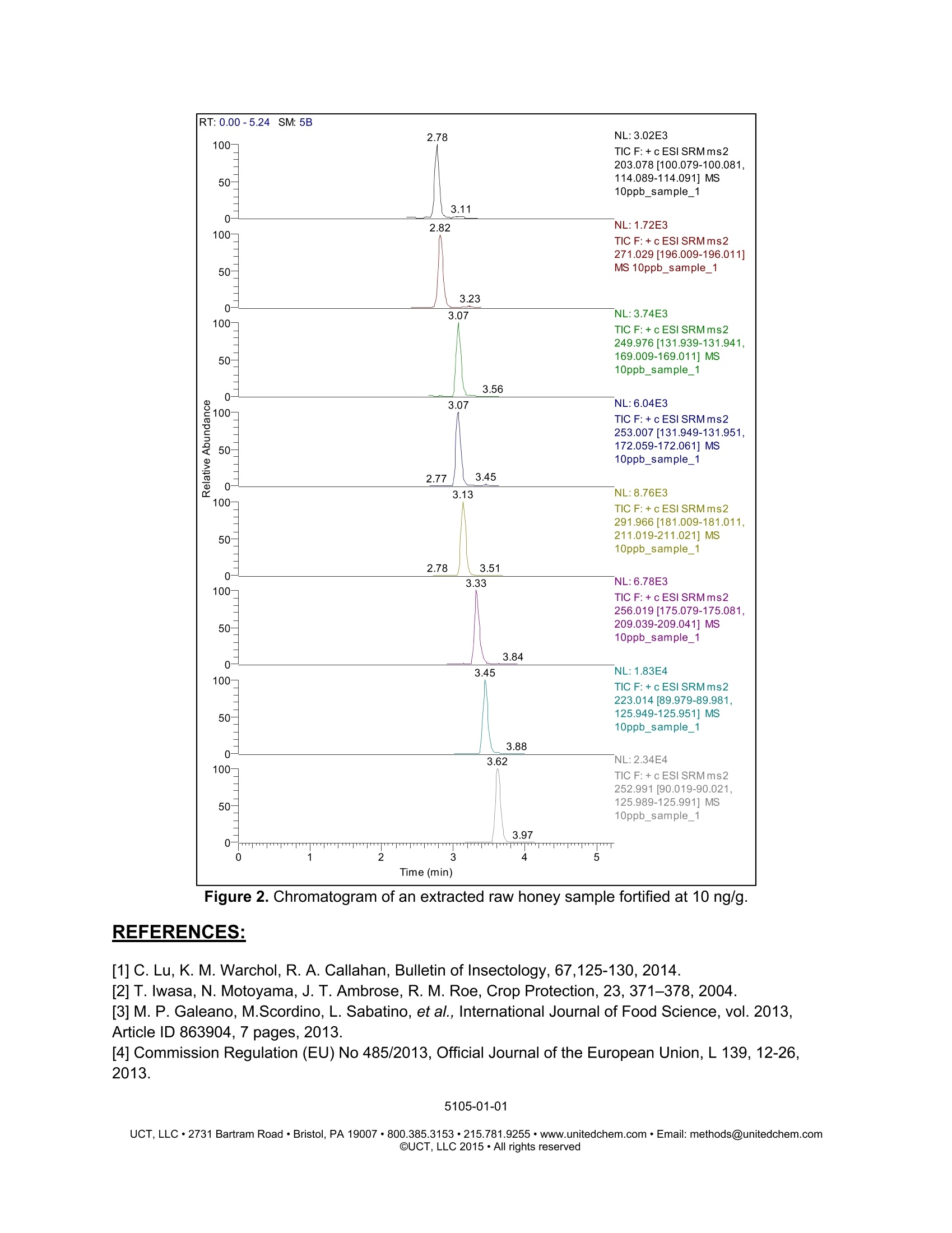
5105-01-01 Analysis of Neonicotinoids in Honey by QuEChERS andUHPLC-MS/MS UCT part numbers: ECQUEU7-MP-Mylar pouch containing 4 g MgSO4, 1 g NaCl, 0.5 gsodium citrate dibasic sesquihydrate and 1 g sodium citrate tribasicdehydrate CUMPSC18CT-150 mg MgSO4, 50 mg PSA and 50 mg endcapped C18;2 mL dSPE tube SLDA50ID21-18UM-SelectraQ DA, 50×2.1 mm, 1.8 um UHPLC columnSLDAGDC20-18UM-SelectraQ DA, 10×2.0 mm, 1.8 um guard cartridgeSLDGRDHLDR-Guard cartridge holder May 2015 INTRODUCTION: Neonicotinoids are a relatively new class of insecticide that were introduced as analternative to organophosphate, carbamate and pyrethroid insecticides. Their novelmode of action works by irreversibly binding to nicotinic acetylcholine receptors,resulting in paralysis and death of insects. Since their introduction in the 1990s theneonicotinoids have been used extensively in crop protection. However, they haverecently come under increasing scrutiny over their environmental and ecological impact,especially their role in bee deaths and colony collapse disorder (CCD). It has beenreported that neonicotinoid residues can accumulate in the pollen and nectar of treatedplants and poses a potential risk to honey bees2. In addition, neonicotinoid residuescan be transferred to products derived from bees, including the popular food sourcehoney3. Due to their potential negative impact, the European Union recently restrictedthe use of three neonicotinoids (clothianidin, thiamethoxam, and imidacloprid) for aperiod of 2 years4. This application note outlines a simple, fast and cost-effective method for thedetermination of 7 neonicotinoid pesticides in honey. Honey is dissolved in water andextracted using a citrate-buffered QuEChERS procedure. The sample extract thenundergoes cleanup by dispersive-SPE with PSA/C18 sorbent to remove unwantedwaxes, pigments and carbohydrates that are present. Analysis is performed byUHPLC/MS-MS using a SelectraQ DA UHPLC column. Recovery studies were carriedout by spiking raw and processed honey at two concentration levels (10 and 50 ng/g).Matrix-matched calibration curves, ranging from 1-250 ng/g, were used for quantitation.The mean recovery was found to be in the range of 82 to 113%, while repeatability wasless than 10%. PROCEDURE: Sample extraction 1. Weigh 10 g of honey sample into a 50 mL polypropylene centrifuge tube.2 .Add internal standard (optional). 3. .Add 10 mL of deionized water and shake/vortex until the honey is dissolved. Add 10 mL of acetonitrile. 45Add the contents of the ECQUEU7-MP Mylar pouch and shake either by hand ormechanically for at least 1 min. For this study a SPEXQ SamplePrepQ2010Geno/GrinderQ was used. 6.Centrifuge the samples at greater than 3000xg for 5 min. Sample clean-up 1Transfer 1ml of supernatant into a CUMPSC18CT dSPE tube.234 Vortex the samples for 30 sec. Centrifuge the samples at greater than 3000xg for 2 min. Transfer 500-600 pL of purified supernatant into an autosampler vial. INSTRUMENTAL: HPLC Conditions Instrumentation Thermo ScientificM DionexTMUltimate'TM3000 HPLC column UCT SelectraQ DA, 50×2.1 mm,1.8 um (p/n: Guard column UCT Selectra@ DA, 10×2.0 mm, 1.8 um (p/n: Guard column p/n: SLDGRDHLDR Column temp. 40°C Flow rate 300 uL/min Injection volume 2 uL Autosampler 10°C Wash solvent methanol: water (1:1, v/v) Time Mobile phase A Mobile phase B (min) water + 0.1% formic methanol+0.1% formic acid acid 0 95% 5% 1 0% 100% 4.5 0% 100% 4.6 95% 5% 7.5 95% 5% MS Conditions Instrumentation Thermo ScientificTM TSQ lonization mode ESI* Spray voltage 5000V Vaporizer 400°C Capillary 350°C Sheath gas Auxiliary gas 50 arbitrary units lon sweep gas 5 arbitrary units Declustering 0 arbitrary units 0v Q1 and Q3 peak 0.2 and0.7 Da Collision gas Collision gas argon 1.5 mTorr Acquisition Cycle time EZ method (scheduled SRM) Software 0.6 sec Xcalibur''V SRM Transitions Analyte t (min) Precursorion CE1 Product CE S-lens Production 1 Dinotefuran 2.78 203.08 114.09 12 ion 2 2 (V) Nitenpyram 2.82 271.03 196.01 100.08 15 50 Clothianidin 3.07 249.97 169.01 15 10 99.04 15 68 Clothianidin-D3 3.07 253.01 172.06 131.94 15 56 Thiamethoxam 3.14 291.97 211.02 131.95 16 64 Imidacloprid 3.33 256.02 209.04 10 181.01 18 59 Acetamiprid 3.45 223.01 125.95 16 20 175.08 16 69 89.98 33 68 RESULTS: Accuracy & Precision Data for Processed Honey 10 ng/g (n=5) 50 ng/g (n=5) Analyte Mean Recovery RSD Mean Recovery (%) (%) (%) (%) Dinotefuran 106.3 2.6 113.4 3.6 Nitenpyram 92.3 2.4 99.6 2.6 Clothianidin 105.0 2.0 113.4 3.8 Thiamethoxam 107.5 1.2 110.1 4.3 Imidacloprid 102.0 2.5 109.7 5.4 Acetamiprid 103.4 3.0 113.6 4.6 Thiacloprid 105.8 1.4 112.9 4.8 Accuracy & Precision Data for Raw Honey Analyte 10 ng/g (n=5) 50 ng/g(n=5) Mean Recovery RSD Mean Recovery RSD (%) (%) (%) (%) Dinotefuran 100.1 5.4 93.6 2.3 Nitenpyram 91.9 5.3 95.9 4.6 Clothianidin 87.5 4.6 82.2 2.6 Thiamethoxam 87.7 5.7 85.8 4.7 Imidacloprid 101.4 4.3 98.4 3.3 Acetamiprid 87.1 8.3 91.9 7.4 Thiacloprid 87.3 1.8 89.2 9.9 Figure 1. Example of a calibration curve (1, 2.5, 5, 10,25,50, 100 and 250 ng/g). RT: 0.00-5.24 SM: 5B 2.78 NL:3.02E3 TIC F:+c ESI SRM ms2203.078[100.079-100.081,114.089-114.091] MS 10ppb_sample_1 3.11 2.82NL: 1.72E3 TIC F:+c ESI SRM ms2271.029[196.009-196.011]MS10ppb_sample_1 3.23 3.07 NL:3.74E3 TIC F:+c ESI SRMms2 249.976 [131.939-131.941,169.009-169.011] MS 10ppb_sample_1 3.56 3.07 NL:6.04E3 TIC F: + c ESI SRMms2 253.007[131.949-131.951, 172.059-172.061] MS 10ppb_sample_1 2.77 3.45 3.13 NL:8.76E3 TIC F:+c ESI SRM ms2 291.966[181.009-181.011,211.019-211.021] MS 10ppb_sample_1 2.78 3.51 3.33 NL:6.78E3 TIC F:+c ESI SRM ms2 256.019[175.079-175.081, 209.039-209.041] MS Figure 2. Chromatogram of an extracted raw honey sample fortified at 10 ng/g. REFERENCES: [1]C. Lu, K. M. Warchol, R. A. Callahan, Bulletin of Insectology, 67,125-130,2014. [2] T. Iwasa, N. Motoyama, J. T. Ambrose, R. M. Roe, Crop Protection, 23, 371-378,2004. ( [3] M. P. Galeano, M.Scordino, L. Sabatino, et al., International Journal o f Food Science, vol. 2013,Article ID 863904, 7 pages,2013. ) [4] Commission Regulation (EU) No 485/2013, Official Journal of the European Union, L 139, 12-26,2013. ( UCT, L LC ·2731 Bartram Road· B ristol, PA 19007·800.385.3153·215.781.9255·www.unitedchem.com· Email: methods@unitedchem.com O UCT, LLC 2015·All rights reserved ) 新烟碱是一种相对较新的杀虫剂,作为有机磷、氨基甲酸酯和除虫菊精类杀虫剂的替代品而被引入。它们的新机能模式与烟碱型乙酰胆碱受体绑定,对其起着不可逆转的作用,导致昆虫发生瘫痪或死亡。自20世纪90年代引进以来,新烟碱在农作物保护中得到了广泛的应用。然而,由于对环境和生态的影响,特别是在蜜蜂死亡和蜂群崩溃失调病(CCD)中的作用,新烟碱受到越来越多的关注。据报道,新烟碱类残留物可以在花粉和被处理过的植物的花蜜中积累,并对蜂蜜产生潜在的风险。此外,新烟碱类残留物可以转移到来自蜜蜂的衍生品中,包括受欢迎的食物原料蜂蜜。由于其潜在的不良影响,欧盟实行临时禁令,2年内禁止使用三种新烟碱(可尼丁、塞速安和益达胺)农药。 此应用方法可简单、快速、经济、有效的测定蜂蜜中7种新烟碱类杀虫剂。使用SPEX GG2010组织研磨处理实验样品,只需1min,即可得到均匀一致的样品。 将蜂蜜溶解于水中,使用柠檬酸盐缓冲液按照QuEChERS方法提取。将样品提取液经过SPE的PSA/C18吸附柱,去除无用的基质、色素、糖类等物质。使用UHPLC-MS/MS分析样品。最终得到处理的样品和未处理的样品在两个浓度水平(10和50ng/g)下的回收率数据。1-250ng/g基质配制校准曲线,用于定量校准。平均回收率在82-113%之间,而相对标准偏差不到10%。 实验方法:开普敦大学部分药包剂编号:ECQUEU7-MP:聚酯膜包裹4g MgSO4、1g NaCl、0.5g柠檬酸氢二钠、1g无水柠檬酸三钠CUMPSC18CT:150mg MgSO4、50mg PSA、50mg C18柱芯,2mL dSPE 管SLDA50ID21-18UM:Selectra公司DA,50×2.1mm,1.8μm UHPLC柱SLDAGDC20-18UM:Selectra公司DA,10×2.0mm,1.8μm保护柱SLDGRDHLDR:保护柱架样品提取:1、称取10g蜂蜜样品,放入50mL聚丙烯离心管中2、加入内标(不同组别加入不同内标)3、加入10mL去离子水,摇匀至蜂蜜溶解4、加入10mL乙腈5、加入ECQUEU&-MP聚酯薄膜药包,使用SPEX公司Geno/Grinder2010破碎均质1min6、放入离心机中,离心力大于3000xg,离心5min样品纯化:1、将1mL离心上清液CUMPSC18CT dSPE管中2、圆周旋涡混匀样品30s3、放入离心机中,离心力大于3000xg,离心2min4、取500-600μL纯化后的上清液至自动进样器样品瓶中 仪器: 结果: 参考文献:[1] C. Lu, K. M. Warchol, R. A. Callahan, Bullentin of Insectology, 67, 125- 130, 2014.[2] T. Iwasa, N. Motoyama, J. T. Ambrose, R. M. Roe, Crop Protection, 23, 371-378, 2004.[3] M. P. Galeano, M.Scordino, L. Sabatino, et al., International Journal of Food Science, Vol. 2013, Article ID 863904, 7 Pages, 2013.[4] Commission Regulation(EU) No 485/2013, Official Jourmal of the European Union, L 139, 12-26, 2013.
确定

还剩3页未读,是否继续阅读?
产品配置单

培安有限公司为您提供《蜂蜜中新烟碱类杀虫剂检测方案(研磨机)》,该方案主要用于蜂蜜中农药残留检测,参考标准--,《蜂蜜中新烟碱类杀虫剂检测方案(研磨机)》用到的仪器有HG-600 Geno/Grinder (原SPEX 2010)高通量动植物组织研磨机
推荐专场
相关方案
更多
该厂商其他方案
更多