方案详情
文
今天的食品企业越来越关注食品的营养性和功能性,但维生素、益生菌不饱和脂肪酸等营养物质对氧气非常敏感,极其容易被氧化。因此企业需要改善包装的密封性,同时在包装时充氮气,减少食品包装中氧气的含量,以保持食品的营养成分和品质。传统探头无法测量狭小的顶层空间中的氧气含量,并且没有一种探头既可以测量气相也可以测量液相中的氧气含量。本文中利用荧光淬灭微型探头可以顺利测量食品包装袋狭小顶层空间中的氧气含量。
方案详情

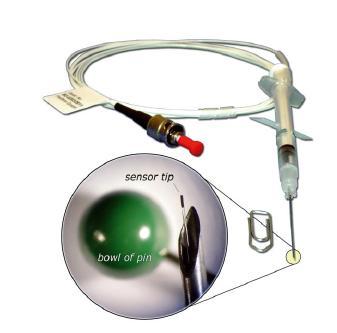
Oxygen concentration and volume of small headspacepackages. Y.WYSER,A.COLLET and C. PELLETIERNestle Research Center, Lausanne, Switzerland ABSTRACT Today's food business is strongly focused on nutrition and functional foods. The addition ofingredients such as vitamins, probiotics strains or poly-unsaturated fatty acids causes asignificant increase of product sensitivity against oxygen. In parallel to packaging barrier improvements, the use of modified atmosphere packages isone of the solutions to preserving the nutritional value and the overall quality of foods, throughreducing the amount of available oxygen inside the package after processing. Current technologies to monitor the quality of atmosphere modification are able to quantifyoxygen concentration, but fail to assess the total oxygen content of a package. Furthermore,these technologies require a fairly large headspace volume as a significant headspacesampling size is required for the measurement. The methodology proposed in this paper, based on fluorescent dye oxygen sensing, allowsprecise determination of the oxygen partial pressure in a package, independently ofheadspace volume and pressure. It further allows the determination of headspace volume, assuming either constant pressure(flexible packages) or constant volume (rigid packages) by mixing the headspace with aknown volume of gas of known oxygen partial pressure and applying a simple rule of mixture. The methodology therefore allows the determination of the main factor affecting the oxidationrate of foods, i.e. the partial pressure of oxygen in the headspace, as well as the total amountof oxygen available in the package through the determination of the volume, thus allowing theprediction of product quality loss in the absence of permeating oxygen, i.e. when using aperfect barrier.I. The limitation of the present technology lies in the testing of semi-rigid packages, for whichthe assumption of constant volume or pressure during the measurement cannot be made.While the determination of the oxygen partial pressure is still possible, the volume has to bedetermined separately, either through direct measurement, or through the determination ofthe initial pressure in the package. KEYWORDS: Headspace, oxygen,volume, methodology ( Co r responding author: y ves.wyser@rdls.nestle.com ) 1 INTRODUCTION Food industry is increasingly moving towards the production of food products with increasednutritional value,by using or adding components such as polyunsaturated fats and vitamins.Many of these components are known to be sensitive to oxidation and must be protectedagainst degradation; i.e. the level of oxygen encountered by these products must beminimized. There are several technologies that are used to limit the amount of oxygen coming intocontact with foodstuff, e.g. high barrier packaging materials and modified atmospherepackaging. Although perfect barrier packages exist, such as glass, tin-plate and aluminium, pressurearisen from cost, marketing and environmental considerations is very large and high barrierbut still oxygen permeable materials are often preferred. The latest generation of packaging material allow reaching oxygen permeation properties inthe range of 0.05 to 0.1 mL/m/day/atm. These low permeation values increase theimportance of a precise control of the composition of the head space during the MAP process,as exemplified below. If one considers a powdered product, packed under modified atmosphere (e.g.2% residualoxygen) and under a slight vacuum (e.g. 500 mbar) in a one litre packaging with 6 dm2packaging surface (i.e. a cubical packaging with a dimension of 10 cm in each direction) andconsidering an apparent density of the powder of approximately half its true density, i.e. 50 %of free volume between powder particles, the total amount of oxygen remaining in thepackaging after filling corresponds to approximately 5 mL. Taking a packaging material with apermeability of 0.05 mL/m²/day/atm, yields an oxygenuptake in the packaging of 6 10mL/pack/day in air or approximately 0.5 mL of oxygen withina 2 years shelf-life, i.e. a factor 10 below the residual oxygen at filling. A variation of 0.1 % inthe initial oxygen content of the headspace still amounts to 0.25 mL of oxygen,i.e. half thepermeating oxygen. Having this in mind, the importance of a precise control of gassing parameters as well asprecise quality control testing becomes crucial to guarantee the quality of an oxygen sensitiveproduct at the end of its shelf-life. 2 BACKGROUND Most oxygen concentration analysis equipments available today on the market function bypumping a given volume of the packaging headspace into the measuring cell, yielding anoxygen concentration given either in percentage or in ppm. Several types of sensing techniques are available, the most commonly used being zirconiumceramic, electrochemical sensing and paramagnetic sensing. The precision of themeasurement, thespecificity and the amount of headspace volume required for themeasurement depends on the sensor used and on the construction of the equipment. In therange of MAP oxygen concentrations, the precision of the measurement typically lies between0.01 and 0.1%(absolute) oxygen. The volume required to purge the sensor cell lies between2 mL and 20 mL. In terms of specificity, other headspace gasses are known to affect themeasurement by paramagnetic sensing, i.e. through modification of the paramagneticproperties of the analysed gas. This lack of accuracy can however be corrected either byusing adequate gas mixes for calibration or by calculation. A more important specificity issuearises in the case of zirconium cells. Indeed, the measurement in such equipment implies theheating of the headspace gas to 650 ℃, temperature at which oxidation of certain aromaticcompounds as well as carbon monoxide in the case of roast and ground coffee is very fast,and will consume part or all of the oxygen present in the gas prior to its detection. In manyinstances, measurements of 0% oxygen are observed when testing packed R&G coffee withsuch sensors. An additional drawback of these techniques is the fact that they require the removal of acertain amount of headspace gas from the packaging, which, for packages with smallheadspace volumes or rigid packages will create a significant bias, as the gas in the measurement cell will not be at ambient pressure. Indeed, all these equipment are measuringthe amount of oxygen present in the cell, and transform it into percentage of oxygen. If testinga rigid packaging containing 15 ml headspace, from which 5 ml are removed for themeasurement, the actual pressure in the cell will be approximately 3/4 of the internal pressureof the packaging,i.e. the measurement of oxygen concentration will be proportionally lowerthan the actual value. Additionally, as exemplified above, the total amount of oxygen available in the headspace isalso of paramount importance for quality preservation of oxygen sensitive products. Theequipments commercially available fail to measure this important parameter. The methodology presented in this paper has been developed to overcome these drawbacksby limiting or suppressing the need of headspace sample removal and enabling thedetermination of the actual headspace volume using simple calculations and models. Fluorescent dye oxygen sensing through dynamic quenching of luminescence One alternative capable of measuring oxygen are sensitive fluorescent tracers . Severalsystems exist on the market and are used for measurement of oxygen concentration inpermanent gases as well as in liquids. This technology is based on the quenching ofluminescence of a luminophore by molecular oxygen. By illumination with a light source theluminophore is excited and emits light until it encounters a molecule of oxygen. Theluminescence life time and intensity is mathematically related to the oxygen concentrationthrough the Stern-Volmer equation5.6. The luminophore can either be present on the tip of anoptical fibre, or be embedded in a sensor spot, which can be attached to the interior surfaceof a package. The parameter that is measured by this technology is the partial pressure ofoxygen, which, if the total pressure is known, can be translated in oxygen percentage. Itshould nevertheless be stressed out that, although oxygen percentage is the common way ofpresenting oxygen content in headspace, the actual parameter governing oxidation rate ofproducts is oxygen partial pressure, which is therefore a more sensible way of looking atheadspace composition. Optical fibres with diameters as small as 50 pm are available. They can be mounted into aneedle or a measurement cell of very small volume. This technology has been used in thisstudy,enabling the measurement of the oxygen content of very small headspace whilelimiting the amount of volume in the measurement system to reduce the bias induced by theair included therein, i.e. the dead volume of the system. Volume measurement Several methods exist for the measurement of headspace volume. The most straight forwardbeing the transfer of the headspace from the package to a graduated pipette filled with water. A set-up, consisting of a water bath and a graduated pipette ended with a funnel is used. Thepipette is filed to the upper end of its graduation and immersed in the water bath to a heightwhere the lower end of the graduation corresponds to the water level in the bath. The sampleis opened under the funnel, enabling the headspace gas to enter the pipette. The apparentvolume, directly read on the scale, must then be corrected to take the pressure induced bythe weight of the water column above the water level of the bath into account. Before the test, the dead volume of the pipette is at a pressure corresponding to theatmospheric pressure minus the pressure exerted by the water column when the pipette is full.After inserting the package headspace gas, the height of the water column is smaller, i.e. thepressure of the total volume of air in the pipette, corresponding to the headspace volumeadded to the dead volume, is higher. The actual volume, V, of the sample, in standardtemperature and pressure (STP) conditions, is therefore the total volume in the pipette,corrected for STP conditions minus the dead volume, also corrected for STP conditions. Thefollowing formula is used to calculateV: whhere Vnom isi st hteh en cnominal volume of the pipette (mL), the length of the full scale of thepipette (mm), Vaead the dead volume of the pipette (mL), T the standard temperature(273.15 K), T the testing temperature (K), P the standard pressure (1013 mbar), Pam theatmospheric pressure (mbar), V the apparent volume (mL), PH,o the water density at testingtemperature (g/mL) and g the gravitational constant (9.81 m/s). This method yields the volume of the headspace in given pressure and temperatureconditions, but it fails to give the volume taken by the headspace gas in the packaging. Another way to measure the volume of headspace is by using the perfect gas law and asimple rule of mixture. lf one considers a volume of gas 1 at a known partial pressure of oxygen and add to it aknown volume of gas 2 with another oxygen concentration, it is possible to calculate thevolume of the first gas by measuring the oxygen partial pressure obtained once the twogasses are mixed. The following equations describe the different systems: The amount of oxygen present in the two volumes is given by the law of perfect gas: Where n is the number of moles (mol) of oxygen present in volume V;(L), Po, the partialpressure of oxygen in volume i (mbar), T the testing temperature (K) and R the perfect gasconstant (83.14 L mbar/mol/K). The total amount of oxygen is simply the addition of the two contributions. After mixing, the new system is described by; As the total amount of oxygen is conserved and the temperature kept constant, the mixingprocess can be written as And 3 EXPERIMENTAL SETUP AND METHODS The equipment used in the frame of this study is a 50 um diameter optical fibre probe coatedwith fluorescent dye (measuring range from 0 to 500 mbar), fixed in a needle and connectedto a Microx oxygen analyser (Presens, Germany). In order to avoid fibre breakage upon contact with the product, the fibre tip was mounted onthe fixture of the needle used to pierce the package through a septum. This needle is itselfmounted on a syringe with a built-in valve, as depicted in Figure 1. The dead volume of the system was determined to be 47 and 69 pL, in the differentconfigurations used during the study. Figure 1: Experimental setup. Oxygen partial pressure measurement The package is pierced through a septum and left until stable reading of oxygen partialpressure is reached. The time to reach this equilibrium is rather large (5-10 minutes in theconfiguration used in this study) due the time required for the diffusive mixing of theheadspace gas to the gas contained in the dead volume of the system. Volume determination For volume measurement, the syringe is first filled with ambient air to a given volume and thevalve is closed. The package is pierced through a septum and left until stable reading ofoxygen partial pressure is reached. Once equilibrium is reached, the valve is opened and the gas inside the syringe is pushedinto the package. The piston of the syringe is then operated several times in order to allowgood mixing between the air of the syringe and the headspace gas. The piston is finally pulledback to its original position, and the equilibrium partial pressure is read. Sources of error In the case of packages for which the internal pressure equals the atmospheric pressure aswell as for rigid packages, the main source of error lies in the dead volume of the systemwhen applying the diffusive mixing. Indeed, the amount of oxygen present in the needle willcontribute to the measured oxygen partial pressure, and will increase the value measured.This effect will of course be highest for small volumes with low partial pressure. This error canhowever be corrected by using Equation [5], if the volume of the headspace is known. For volume measurement, the correction for the dead volume can directly be integrated in theformula, by rewriting equation [4] as follow: Yielding: Where Posyr is the partial pressure of the air in the syringe, V is the volume of air in thesyringe, Po, is the measured partial pressure (without correction for dead volume) andViead is the dead volume of the system. Measurement of different types of packaging. In the case of rigid packages, even if the internal pressure of the packages is different fromthe atmospheric pressure, i.e. the pressure in the syringe, the volume of the sample remainsconstant, and the volume of the mix is simply the addition of the two contributions. For flexible packages, the internal pressure of the packaging can always be considered as being equal tothe atmospheric packaging, as the material will deform to equilibrate external and internalpressure. Again the volume of the mix corresponds to the addition of the two contributions. In the case of semi-rigid packages at a pressure different than atmospheric pressure, e.g.rigid trays with a flexible membrane or vacuum packaging with flexible material, the situationis different as a change in pressure can induce a change in volume, which contrarily toflexible packages are not linearly related to each others. The volume of the mix can thereforenot be taken as the addition of the two contributions. There might thus be some cases wherethis approach to measuring headspace volume yields erroneous results. Samples The tests were performed on different types of samples, representing rigid, flexible and semi-rigid packages. Glass vials (for GC headspace analysis, crimped, with Teflon coated silicon septum)of different nominal volumes (2, 10 and 20 mL) were sealed under Nitrogenatmosphere (40-50 ppm residual oxygen, i.e. approximately 0.05 mbar oxygen partialpressure at 936 mbar atmospheric pressure). Soluble coffee stick packs (Nescafe Gold, 2 g) Plastic capsules for beverage systems (Nescafe Dolce-Gusto), containing roast andground coffee, milk or cocoa powder. Nominal volume 38mL 4 RESULTS Determination of oxygen partial pressure. The effective volume of the vials was determined by their weighing filled with water Table 1reports the effective volume of the vials. The oxygen partial pressure (Po2) in the vials wasdetermined using the method described above and the effective oxygen partial pressure wascalculated using Equation [4] with a dead volume of 69 uL. All results are given in Table 1. Table 1: Oxygen partial pressure measurement gassed at 0.05 mbaroxygen partial pressure. Sample Effective volumemL] Measured Po2[mbar1 Effective Po2[mbar Average [mbar1 Vial 2 ml 1 2.1 5.7 -0.3 0.1 Vial 2 ml 2 2.1 6.0 0.1 Vial 2 ml 3 2.1 6.2 0.2 Vial 2 ml 4 2.1 6.2 0.2 Vial 2 ml 5 2.1 6.1 0.1 Vial 10 ml 1 12.07 1.6 0.5 1 Vial 10 ml 2 12.07 1.8 0.7 Vial 10 ml 3 12.07 2.0 0.9 Vial 10 ml 4 12.07 2.0 0.9 Vial 10 ml 5 12.07 2.7 1.6 Vial 20 ml 1 21.53 1.4 0.8 0.9 Vial 20 ml 2 21.53 1.4 0.8 Vial 20 ml 3 21.53 1.6 1.0 Vial 20 ml 4 21.53 1.5 0.9 Vial 20 ml 5 21.53 1.7 1.1 As can be seen in Table 1, there is a slight bias between the corrected oxygen partialpressure measured in the vials and the partial pressure of the atmosphere in which they werepacked. In order to identify the cause of this difference, one of the 10 mL vials was measured afterdifferent periods of time. These measurements showed that the oxygen partial pressure in thevials rapidly rose due to oxygen permeation. A partial pressure increase rate of approximately12 mbar / day (or 0.5 mbar / hour) for an oxygen partial pressure gradient corresponding to 194 mbar (i.e. the oxygen partial pressure in air at a pressure of 930 mbar) was calculated.The sample having been taken out of the Nitrogen atmosphere early in the afternoon and themeasurements made a couple of hours later, the observed bias is most probably due tooxygen permeating through the septum. Unfortunately, the permeability of the 2 and 20 mLvials were not determined. These results clearly show that, if the sample headspace volume is known, the method iseffective for the measurement of the oxygen partial pressure of packages without headspacegas removal. Determination of headspace volume Glass vials The 10mL glass vials described above were also used to validate the approach of measuringthe headspace volume in a package, using Equation [7]. Table 2 reports the individual measurements made as well as the results of the application ofEquation [7]. The dead volume of the system used in these tests was 69 pL. Table 2: volume measurement of glass vials with various volumes of mixed air and variousheadspace compositions. Vsyr[mLT Po2Syr[mbar] Po2.1mbarl Po2,mix[mbar1 V。'sample[mL] Effective volumemL] Relativedifference 2.0 191.1 2.3 28.8 12.2 12.07 0.9% 4.0 187.9 53.8 86.5 12.3 12.07 2.2% 3.0 186.1 53.2 79.2 12.3 12.07 1.6% 2.0 196.1 144.5 151.7 12.3 12.07 1.6% Soluble coffee stick packs The headspace volume of the stick packs was determined both by the methodologydeveloped in this study and by using the graduated pipette method using equation 1, slightlymodified to have the volume at testing temperature and pressure.The latter tests resulted inan average headspace volume of 8.63±0.62 mL. As those packages are not gassed, the syringe was filled with pure Nitrogen instead of air, inorder to have a large partial pressure difference between the two gasses. The results arereported in Table 3. The dead volume of the system used in these test was 47 pL. Table 3: Headspace volumes of soluble coffee stick packs Vsyr[mL1 Po2syr [mbar] Po2.1 imbar1 Po2.mix [mbar] V.sample[mL1 AveragemL1 1.5 0.08 178.8 149.9 7.73 8.66±0.48 1.5 0.1 176.4 151.2 8.95 2.5 0.2 176.9 138.2 8.87 2.5 0.1 177.8 138.6 8.79 2.5 0.2 179.9 142.7 9.53 2.5 0.1 179.6 140.1 8.81 2.5 0.1 178.9 138.3 8.46 2.0 0.6 180.1 145.4 8.30 2.0 0.3 179.3 146.2 8.77 2.0 0.3 180.2 145.7 8.38 Plastic capsules The plastic capsules tested were all at least 2 months old and were filled under reducedoxygen atmosphere (<1 % residual O2). Most capsules had internal pressures slightly higherthan atmospheric at the time of testing (the membranes were swollen). In this case, the volume was determined by subtracting the volume of packed product(obtained from the weight of product and its true density) form the nominal volume of thecapsule,i.e. neglecting the volume increase due to the swell of the membrane. The results(averaged over 9 specimens each) are given in Table 4, together with the internal pressure inthe package). The volume of air in the syringe was kept constant at 2.5 mL and the deadvolume was 47 pL. Table 4. Volume of headspace in capsule containing different products VsamplemL1.] P intmbar1 Calculatedvolume[mL] Relative Difference R&G coffee 32.7 1120 31.2 4.9% Chocolate 26.0 950 26.9 -3.4% Milk powder 23.2 950 23.6 -1.5% 5 DISCUSSION The results presented in the previous section clearly show that the methodology presented inthis paper is a powerful tool to determining the headspace composition and volume ofsamples that are difficult to measure by conventional means. Although, as shown in Table 1, the effect of the dead volume can be very significant in thecase of small headspaces with low residual oxygen, the measured value can easily becorrected if the volume of the headspace is known. As for the volume determination, the results obtained on rigid packages (glass vials) andflexible ones (stick pack) show that the difference between classical volume measurementsand this methodology lies in the range of 2 % or bellow. Considering the precision of classicalmethods for volume determination, this difference is statistically not significant. In the experimental section of this paper, it was stated that the measurement of the volume onsemi-rigid packages might be biased by the non-linear relationship between pressure andvolume. This statement seems to be confirmed by the results given in Table 4, were a moresignificant difference appears between the two methods of determining headspace volume. Itcan however be observed that the difference increases with increasing internal pressure, thelatter causing swelling of the membrane and increasing the volume, which is not taken intoaccount in the calculation of the volume based on nominal volume and product density. Anapproximation of this volume increase can be made by calculating the volume of a sphericalcap using the diameter of the capsule and the height of the membrane. In the case of theR&G coffee capsules, the height amounts to 3.3 mm,yielding a volume of approximately3 mL, yielding a relative difference between measured and calculated volume of-4.4%. Themethod developed in this paper therefore underestimates the volume of semi-rigid packageswith over pressure. Methodology improvement The results shown in the previous sections have shown that the methodology presented inthis paper is effective to measure the oxygen content and the volume of headspace in smallpackages. The main drawback of this method is the time required to make a measurement, i.e. 5 to 10minutes for determination of the oxygen partial pressure and an additional 5 minutes for thedetermination of volume. In production conditions, this lead time is much too long and shouldbe reduced by at least a factor ten to be applicable in factories. One approach taken to overcome this issue was to, after filling the syringe to the specifiedvolume, move the piston of the syringe by an additional volume with closed valve, creating aslight under-pressure in the syringe. Once the sample is punctured, the valve is shortlyopened, causing the replacement of the dead volume by headspace gas and allowing themeasurement to be done in less than a minute. This of course implies the removal of a part ofthe headspace. However,preliminary tests have shown that a volume of 200 pL wassufficient to "clean" the 69 pL dead volume, i.e. a factor 10 to 100 below the headspacevolume removed by traditional methods. Although working in theory, the setup used during the study had dead volume parts that were not flushed (e.g. in the needle in which the opticalfibre is fixed), requiring again diffusive mixing, i.e. time. A modified setup avoiding this is inpreparation. 6 CONCLUSION This paper presents a methodology based on fluorescent oxygen sensing enabling thedetermination of the oxygen partial pressure in the headspace of small packages, as well asdetermining the volume of the headspace.This combined measurement allows the simpledetermination of the total amount of oxygen present in the package, partly responsible of theoxidative degradation of a packed product. The tests preformed during this study have demonstrated that, when applying adequatecorrections to take the effect of the dead volume into consideration, a very precise value ofoxygen partial pressure can be obtained, even for very small headspace volumes. Theyfurther showed that the volume determined by this methodology is at least as precise as lessconvenient traditional methods for flexible and rigid packages, whereas it might be slightlyless efficient for semi-rigid packages. It has finally been shown that the main drawback of the method is the lead-time to performone measurement. An approach has however been proposed to improve this limitation. 7 REFERENCES [1] Wolfbeis, O.S. Fiber Optic Chemical Sensors and Biosensors, CRC: Boca Raton, 1991. [2] Holst, G., Kuhl, M., and Klimant,I. A novel measuring system for oxygen micro-optodesbased on a phase modulation technique. Verga Scheggi, A. M. 2508-45,387-398.1995.SPIE- The International Society for Optical Engineering. [3] Wolfbeis, O. S.,Klimant, 1., Werner, T., Huber, C., Kosch, U., Krause, C., Neurauter, G.,and Durkop, A., Set of luminescence decay time based chemical sensors for clinicalapplications, Sensors and Actuators B: Chemical, vol. 51, no. 1-3, pp. 17-24, 1998. [4] Draaijer, A., van Veen, S., and Konig, H."Non-invasive: Novel method for oxygendetermination. Verpackungs-Rundschau, 3, 97-98.2002. [5]1Huber, C. and Krause, C., Instruction manual: Fibox 3 fiber-optic oxygen meterRegensburg: PreSens Precision Sensing GmbH, 2003. [6] Stern,O. and Volmer, M., "Ueber die Abklingzeit der Fluoreszenz," Phys.Z., vol. 20 pp.183-188, 1919.
确定






还剩7页未读,是否继续阅读?
科艺仪器有限公司为您提供《食品包装中氧气含量检测方案(溶解氧测定仪)》,该方案主要用于包装中氧气含量检测,参考标准--,《食品包装中氧气含量检测方案(溶解氧测定仪)》用到的仪器有微量氧气测量仪、氧气透过率测量仪
推荐专场
相关方案
更多