方案详情
文
Purpose: Development of a single quantitative method for multi-class veterinary drugs in animal meat products.
Methods: 3 μL injections of extracted meat (chicken, beef, and pork) spiked withveterinary drugs were injected onto a C18 reverse phase column. Compounds of interest were separated and eluted using a standardized gradient elution profile. A high performance triple quadrupole mass spectrometer with a heated electrospray source(HESI) was used to analyze the compounds of interest in positive and negative ionization, and the data were collected, analyzed, and reported using customized software.
Results: The method provided excellent result s for most compounds at or below the required Maximum Residue Limits (MRLs)1 in the matrices studied. Several compounds could not be incorporated into the final method due to high polarity or poor chromatographic response under the proposed generic mobile phase conditions.
方案详情

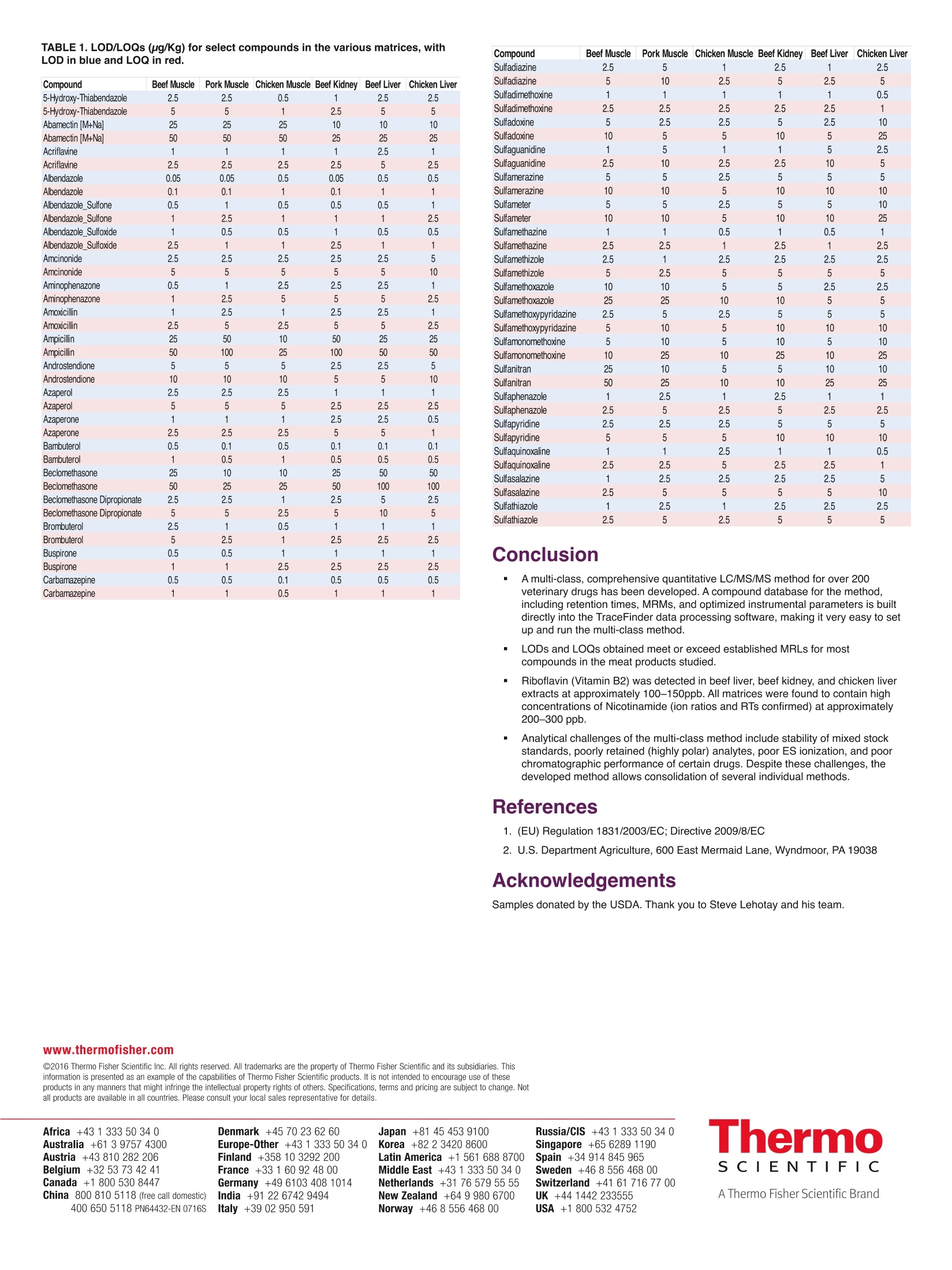
2 Determination of a Single Methodology for the Analysis and Quantitation of Multi-class Veterinary Drugs in Different Animal Matrices Used for Consumption Charles Yang, Ed George, Dipankar Ghosh, Mary Blackburn;Thermo Fisher Scientific, San Jose, CA Overview Purpose: Development of a single quantitative method for multi-class veterinary drugsin animal meat products. Methods: 3 pL injections of extracted meat (chicken, beef, and pork) spiked withveterinary drugs were injected onto a C18 reverse phase column. Compounds ofinterest were separated and eluted using a standardized gradient elution profile. A highperformance triple quadrupole mass spectrometer with a heated electrospray source(HESI) was used to analyze the compounds of interestin positive and negativeionization, and the data were collected, analyzed, and reported using customizedsoftware. Results: The method provided excellent result s for most compounds at or below therequired Maximum Residue Limits (MRLs) in the matrices studied. Several compoundscould not be incorporated into the final method due to high polarity or poorchromatographic response under the proposed generic mobile phase conditions. Introduction Veterinary drugs are pharmacologically active compounds that are used to treat andprevent diseases of animals in livestock. Their use can result in non-desirable drugresidues in the food products for consumption. Animals with high amounts of thesedrugs can cause harm in humans or can render the use of antibiotics in hospitalsuseless due to the counter effects of the drugs. Therefore, strict regulatory guidelinesexist for these compounds in meat products. Required MRLs typically are in the rangeof sub-ug/Kg up to 20,000 pg/Kg depending on the substance and specific regulation,with some compounds set at“zero"tolerance. The quantification of over 200 multi-class veterinary drugs from different meat productsusually involves a series of different extraction methods with either SPE or LLE. Eachrequires substantial time in both sample preparation and analytical run time withmultiple HPLC and mass spectrometer methods. A new robust method, utilizing a singlechromatography run and a triple quadrupole mass spectrometer is described in thisposter. It can be used to quantitate over 200 veterinary drugs by LC/MS in a variety ofmatrices. In addition, the instrumental method can be set up to perform targetedscreening of samples. Methods Sample Preparation Sample preparation of blank matrices used for matrix spiked calibration curves wereperformed by the U.S. Department of Agriculture (USDA)based upon the QueCHeRS(Quick, Easy, Cheap, Effective,Rugged, and Safe) method. Steps below: 1. 2.0 gram sample in 10 mL of acetonitrile/water (8:2). 2. Shake for 10 minutes using platform shaker. 3. Centrifuge at 3700 G for 5 minutes. 4. Extract was filtered using 0.45 micron PVDF syringe filter. Matrices studied: Beef muscle, chicken muscle, chicken liver, beef liver, beef kidney,pork muscle Liquid Chromatography (See Figure 1) Thermo ScientificTM Dionex TM UItiMate TM 3000 RSLC systemColumn: Thermo Scientific TM AccucoreTM aQ C18 Polar Endcapped(100×2.1mm, 2.6 pm) Column temperature: 30°C Mobile phase:A=H,O+0.1% FA, B=MeOH+0.1% FA Flowrate: 300 pL/min Inject volume: 3 pL FIGURE 1. LC Gradient ProgramUsed for Multi-classVeterinary Drugs. Mass Spectrometry Mass spectrometer: Thermo Scientific TM TSQ EnduraTM triple-stage quadrupole LC-MS Spray voltage: 3500V positive, 2500 negative Capillary temperature: 250°C Vaporizer temperature:300°C Sheath gas: 40arb Aux Gas: 5arb Sweep gas: 1arb Collision gas: 1.5 Q1 and Q3 (FWHM): 0.7 Data Analysis Thermo Scientific TM TraceFinderTM software with a specific veterinary drugs compounddatabase (283 analytes) was used for data processing. The compound databasecontains 3-5 SRM transitions percompound, including optimized CEs, lens voltages,and retention times. The database allows easy setup of the full method or selectedcompounds on the TSQ Endura triple-stage quadrupole LC-MS. Results Instrument Calibration All matrices were spiked with standard mixtures prepared from individual stocksolutions. Total of 11 mixes containing approximately 25 compounds per mix. Total of255 veterinary drugs, to final volume of 500 pL with internal standards added at 20 ppb.Calibration curve levels in each matrix: 0.05, 0.1,0.5,1,2.5,5, 10,25,50, 100, 200, 300 ppb (12 point curve) and matrix blank. (See Figure 2) Prepared in 40 %ACN:Water Seven replicates at each concentration level were analyzed to obtain% RSD at eachconcentration level FIGURE 2. Example calibration curve for Bambuterol with seven replicates ateach calibration level in the beef kidney matrix. Analytical Challenges Multi-Class compounds using a generic mobile phase and column resulted in someanalytes being poorly retained and/or poorly ionized. As a result, some compoundswere removed from the final quantitative method. Peak shapes weak and broad: For several acidic compounds, tetracyclines,perfloxacin Unknown stability and quality of standards: Majority of neat standards dissolved inACN:MeOHand/or DMSO At 40% acetonitrile, peak shapes are generally good; 3 ul is a good compromiseinjection volume. The submitted matrices were not completely free of incurred veterinary drugs. All ofthe matrices contained nicotinamide at high concentration (>100 ppb) and Riboflavin(Vitamin B2) in beef liver, beef kidney, and chicken liver at concentrationsapproximately 100-500 ppb. (See Figures 3 and 4) FIGURE 3. Nicotinamide in beef muscle compared to solvent spike. TheceSompound was detected in all matrices studied with passing ion ratios; estimatedconcentration at 150 ppb. FIGURE 4. Riboflavin (Vitamin B2) in beef kidney matrix compared to solventstandard. Ion ratios passed in all samples (beef kidney, beef liver, and chickenliver). LOD and LOQ The limit of detection (LOD) and limit of quantitation (LOQ) were determined byanalyzing a series of 7 replicates at each calibration level for each matrix. TraceFindersoftware then calculates the coefficient of variation and % RSD at each level. Thisprovides an estimate of the LOD and LOQ for each of the veterinary drugs using thefollowing criteria: LOD= <20% RSD for the calculated concentration LOQ=<15% RSD for the calculated concentration The ranges of LODs and LOQs were very similar in all the matrices studied. LODsranged from 0.05-100 pg/Kg and LOQs ranged from 0.1-200 pg/Kg. (See Table1) FIGURE 5. Digoxin at 10 ppb passing ion ratio qualitative confirmation. lonratios set to 25% (2 or 3 ions for most analytes). Thermo Tacehinder EFS IC Resl tme stetuis Usan charles yangi @alloa中委 APesesa Peak1im/301228) Peat2im/n5213121 0318_d_Ca77 Di..0918_dCal7-7 3i. RTImin)me521312000%-3350%3 000%-2817%5Z i0000- 0000- TABLE 1. LOD/LOQs (ug/Kg) for select compounds in the various matrices, withLOD in blue and LOQ in red. www.thermofisher.com 2016 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries. Thisinformation is presented as an example of the capabilities of Thermo Fisher Scientific products. It is not intended to encourage use of theseproducts in any manners that might infringe the intellectual property rights of others. Specifications, terms and pricing are subject to change. Notall products are available in all countries. Please consult your local sales representative for details. Conclusion ( A multi-class, comprehensive quantitativ e LC/MS/MS m ethod for over 200veterinary drugs has been developed. A compound database for the method, i ncluding r etention times, MRMs, and op t imized instrumental par a meters is builtdirectly into the TraceFinder data processing software, making it very easy to setup and run the m ulti-class method. ) ( LODs and LOQs obtained meet or exceed established MRLs for most compounds in the meat products st u died. ) Riboflavin (Vitamin B2) was detected in beef liver, beef kidney, and chicken liverextracts at approximately 100-150ppb. All matrices were found to contain highconcentrations of Nicotinamide (ion ratios and RTs confirmed) at approximately200-300 ppb. Analytical challenges of the multi-class method include stability of mixed stockstandards, poorly retained (highly polar) analytes, poor ES ionization, and poorchromatographic performance of certain drugs. Despite these challenges, thedeveloped method allows consolidation of several individual methods. References 1. (EU) Regulation 1831/2003/EC; Directive 2009/8/EC 2. U.S. Department Agriculture, 600 East Mermaid Lane, Wyndmoor, PA 19038 Acknowledgements Samples donated by the USDA. Thank you to Steve Lehotay and his team. Africa +43 1 333 50 34 0 Denmark +45 70 23 62 60 Japan +81 45 453 9100 Australia +61 39757 4300 Europe-Other +43 1 333 50 34 0Korea +82234208600 Austria +43 810 282 206 Finland +358 10 3292 200 Latin America +1 561 688 8700 Belgium +32 53 73 42 41 France +331 60 92 48 00 Middle East +43 1 333 50 34 0 Canada +1 800 530 8447 Germany +49 6103 408 1014 Netherlands +31 76 579 55 55 China 800 810 5118 (free call domestic) India +91 22 6742 9494 New Zealand +64 9 980 6700 Italy +39 02 950 591 Norway +46 8 556 468 00 Russia/CIS +43 1 333 50 340 Singapore +65 6289 1190 Spain +34 914 845 965 Sweden +46 8 556 468 00 Switzerland +41 61 716 77 00 UK +44 1442 233555 Veterinary drugs are pharmacologically active compounds that are used to treat andprevent diseases of animals in livestock. Their use can result in non-desirable drugresidues in the food products for consumption. Animals with high amounts of thesedrugs can cause harm in humans or can render the use of antibiotics in hospitalsuseless due to the counter effects of the drugs. Therefore, strict regulatory guidelinesexist for these compounds in meat products.
确定


还剩1页未读,是否继续阅读?
赛默飞色谱与质谱为您提供《兽用药中主要成分分析TSQ Endura检测方案(气相色谱仪)》,该方案主要用于兽用药中含量测定检测,参考标准--,《兽用药中主要成分分析TSQ Endura检测方案(气相色谱仪)》用到的仪器有赛默飞TRACE 1300系列 模块化气相色谱仪
推荐专场
相关方案
更多
该厂商其他方案
更多
















