方案详情
文
在哺乳动物和昆虫世界里,信息素强烈影响其社会行为,如攻击性和配偶识别。果蝇的信息素以表皮烃类形式存在,在求偶中发挥着重要作用。GC/MS是目前研究果蝇表皮烃类的主要分析工具。虽然其重现性和灵敏度很高,但需要将果蝇放在毁灭性的有机溶剂中,因而无法再对其进一步的行为进行研究。我们提出了一种用实时直接分析(DART)MS分析活体动物烃类和其它表面分子的技术。用一种钢制小探针从清醒状态的果蝇腹部取样进行表面烃类分析。对探针进行DART质谱分析,检测以前鉴定过的许多不饱和烃类化合物质子化分子离子的质荷比(m/z)。与用GC/MS研究的结果一致,雄性和雌性的化学成分有很大差异。 我们还观察到了雄性表达轮廓图的空间差异。首先从一只处子状态的雌性果蝇取样,然后在其成功交配后45分钟和90分钟再取样,结果显示交配后顺vaccenyl醋酸酯、tricosene和pentacosene 的质谱信号强度增加。本方法适用于行为学研究时对个体动物的化学轮廓进行近瞬时分析,扩展了信息素介导的行为学模型。
已有研究表明,许多挥发性化学信号强烈影响着哺乳动物和昆虫的复杂社会行为,包括配偶选择、亲缘识别、攻击与聚集等。在昆虫和节肢动物中,这类信号,许多是表皮烃类化合物,除影响求偶、群体识别和攻击外,还可能标志其在社会网络中的角色。对果蝇的研究文献表明,烃类化合物起着催欲剂或抑制剂的作用。特别是,许多研究都将重点放在z-11-octadecenyl 醋酸酯[顺-vaccenyl 醋酸酯 (cVA)]上,认为其既是配偶识别的介导剂,又是攻击因子。通过提供从感觉输入到行为输出的信息,可以解析信息素受体和上游中性通路,为描绘复杂社会行为通路提供的方法。
表征昆虫烃类化合物所用的主要方法一直是GC/MS联用法。GC/MS分析除个别异构体不能分离外,可以定量测定烃类化合物。虽然这种方法重现性和灵敏度很高,但却有三个局限。首先,提取时要把动物放置到己烷或氯仿中,这种条件是毁灭性的,因此已无法在对其下一步的行为进行研究。第二,所用的溶剂和检测条件对表面化合物的类别是有选择性的,其它行为相关的表皮信息将无法用现有方法检测。第三,GC/MS分析时间较长,一般需要几十分钟到1小时以上。
针对这些局限,我们提出了一种分析清醒状态果蝇表皮烃类化合物和其它表面分子的方法。常压质谱是最近发展起来的技术,以最少的样品制备进行质荷比(m/z)测定。常压质谱的一种模式就是实时直接分析(DART),采用激发态氦原子使化合物直接从样品表面解吸并离子化,不需要化学提取或高真空条件。用DART MS研究果蝇烃类化合物,较过去的GC/MS方法有了较大改善,在平行进行行为学研究的同时,实现了动物化学轮廓图的快速分析。本方法可以追踪同一动物在其社会交往前后化学轮廓图的变化,控制表皮烃类表达的个体变化,还可以从所观察的个体动物中发现与行为差异相关的化学信号。采用DART MS技术,可以以高重现性对活体果蝇表皮进行化学轮廓分析、检测雄性和雌性轮廓图的差异、检测雄性烃类表达的空间特异性,并监测同一个体社会交往见后烃类化合物的变化。
方案详情

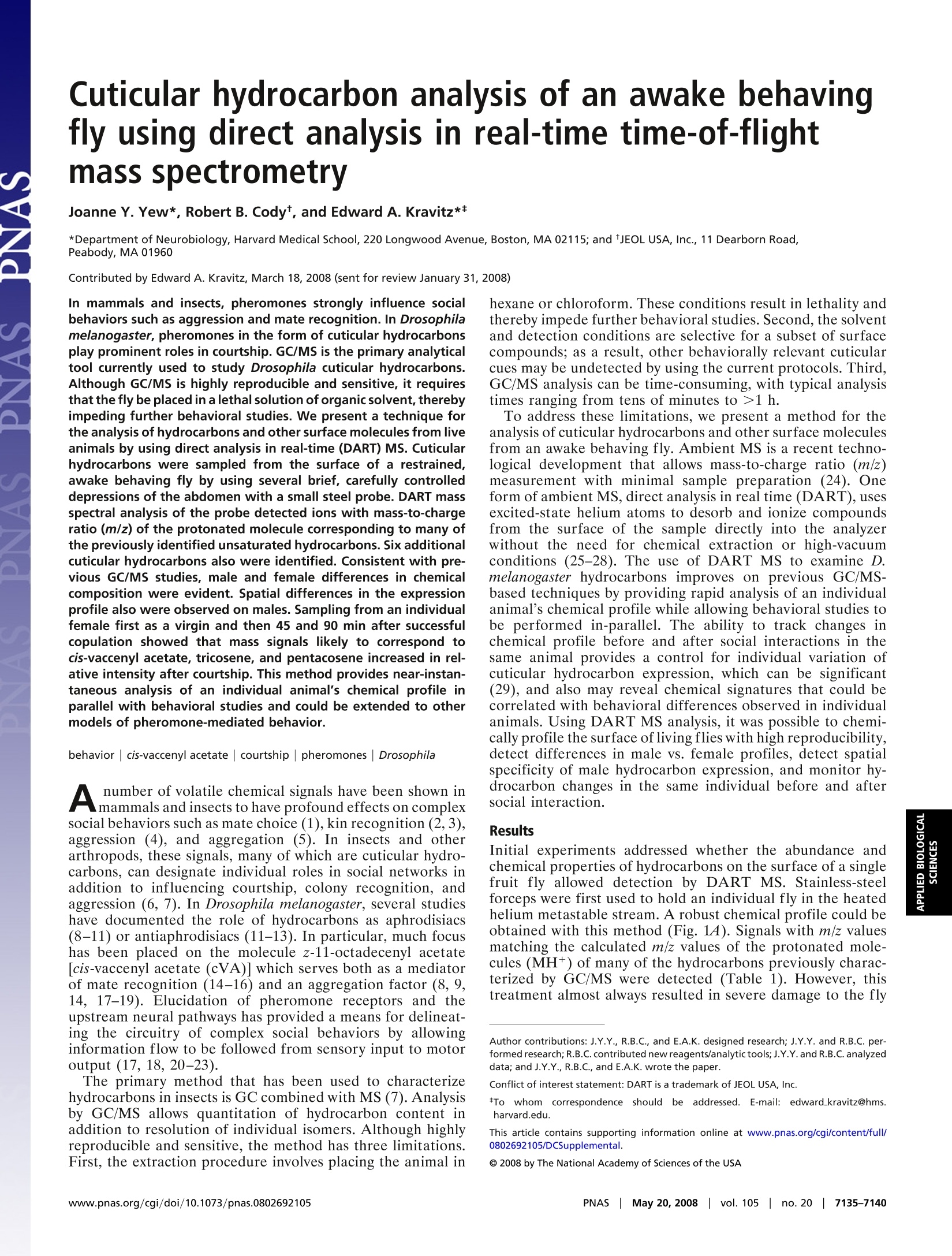
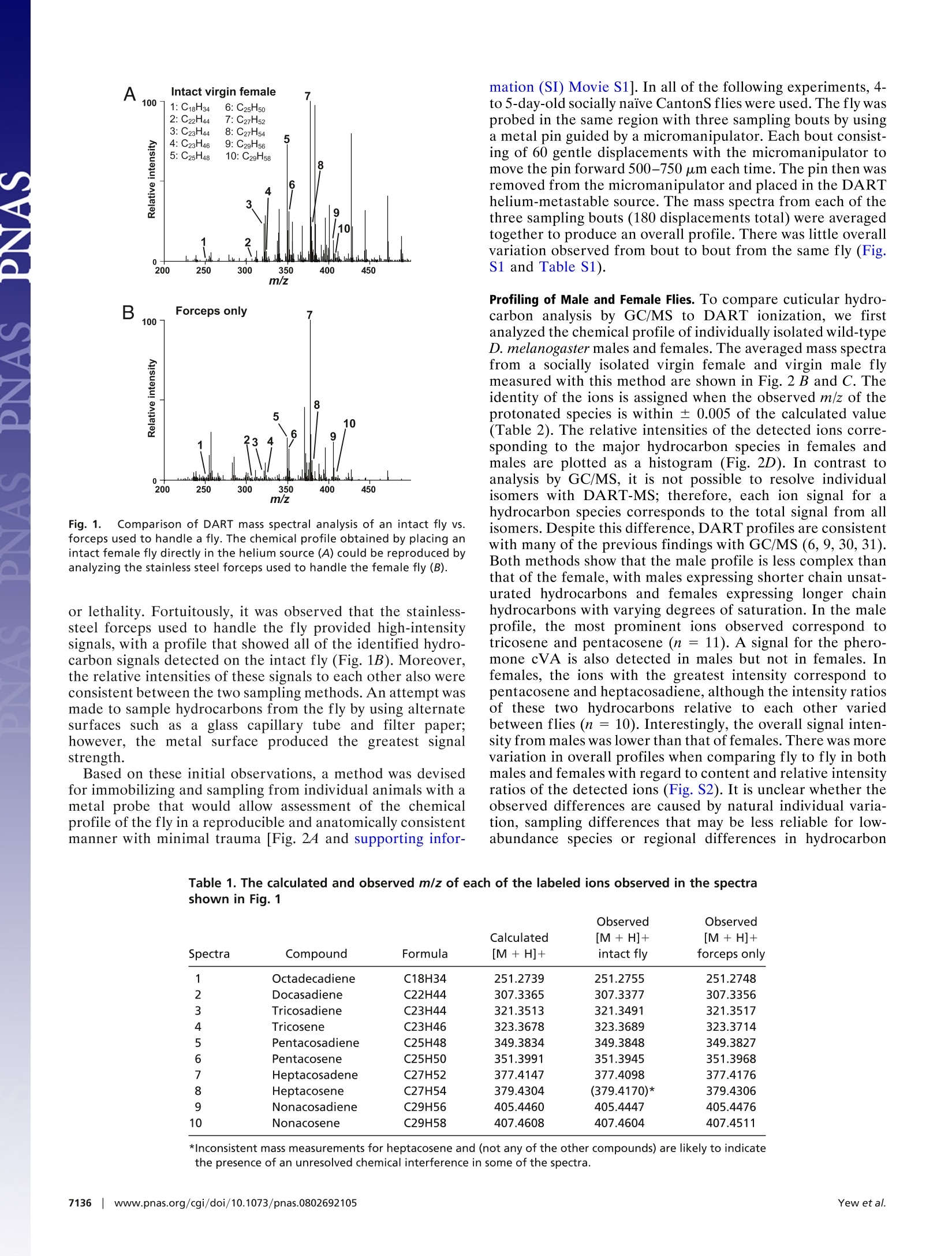
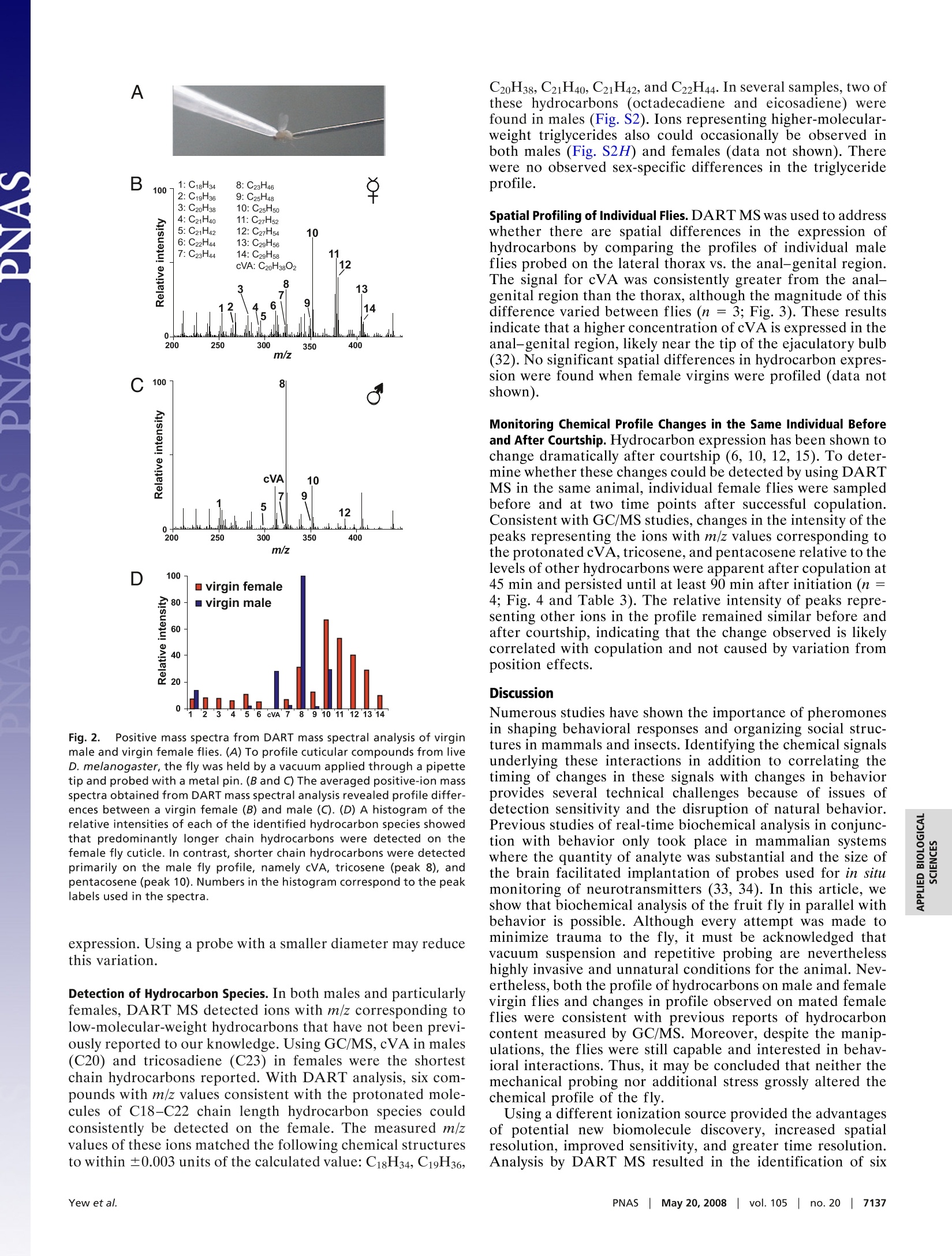
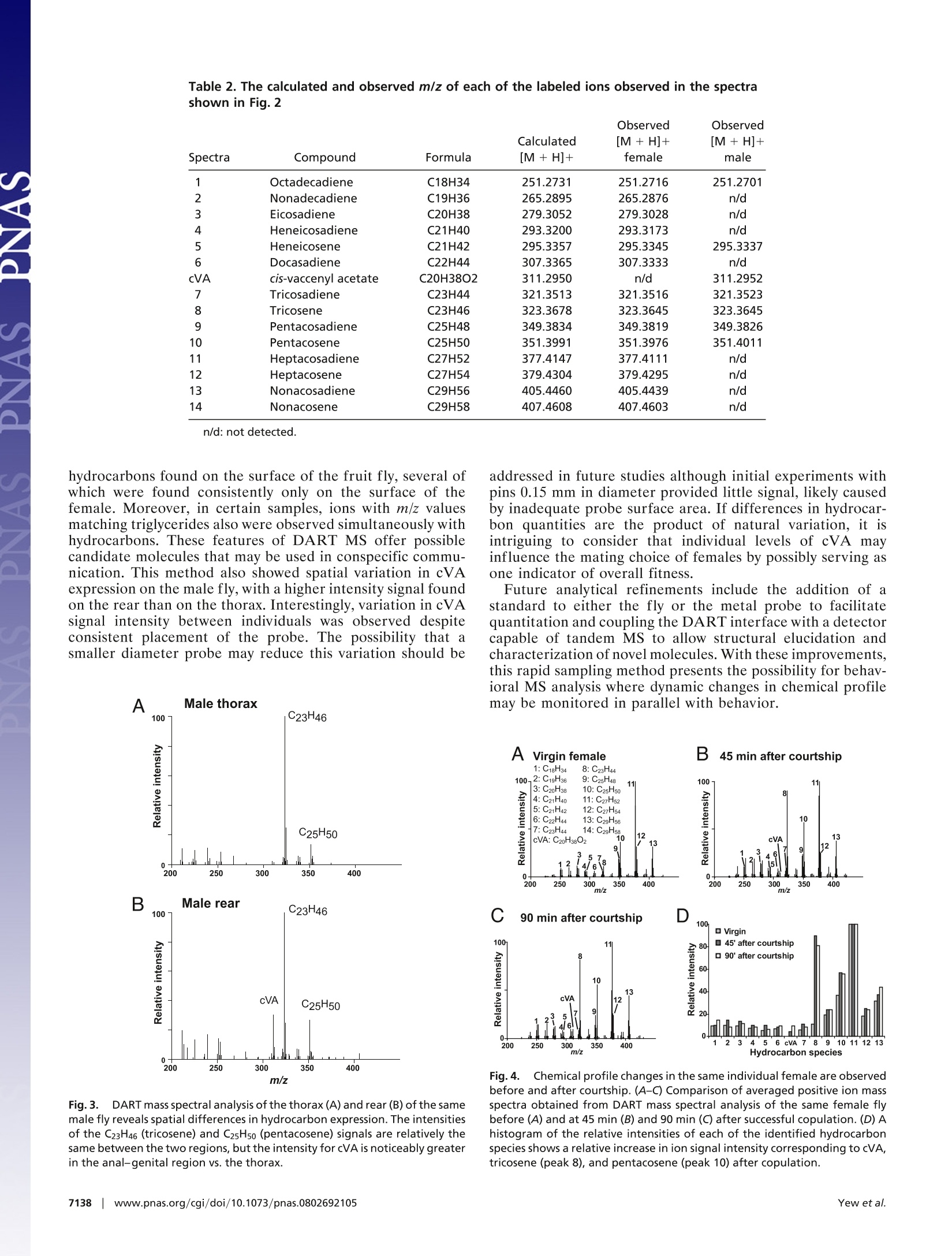
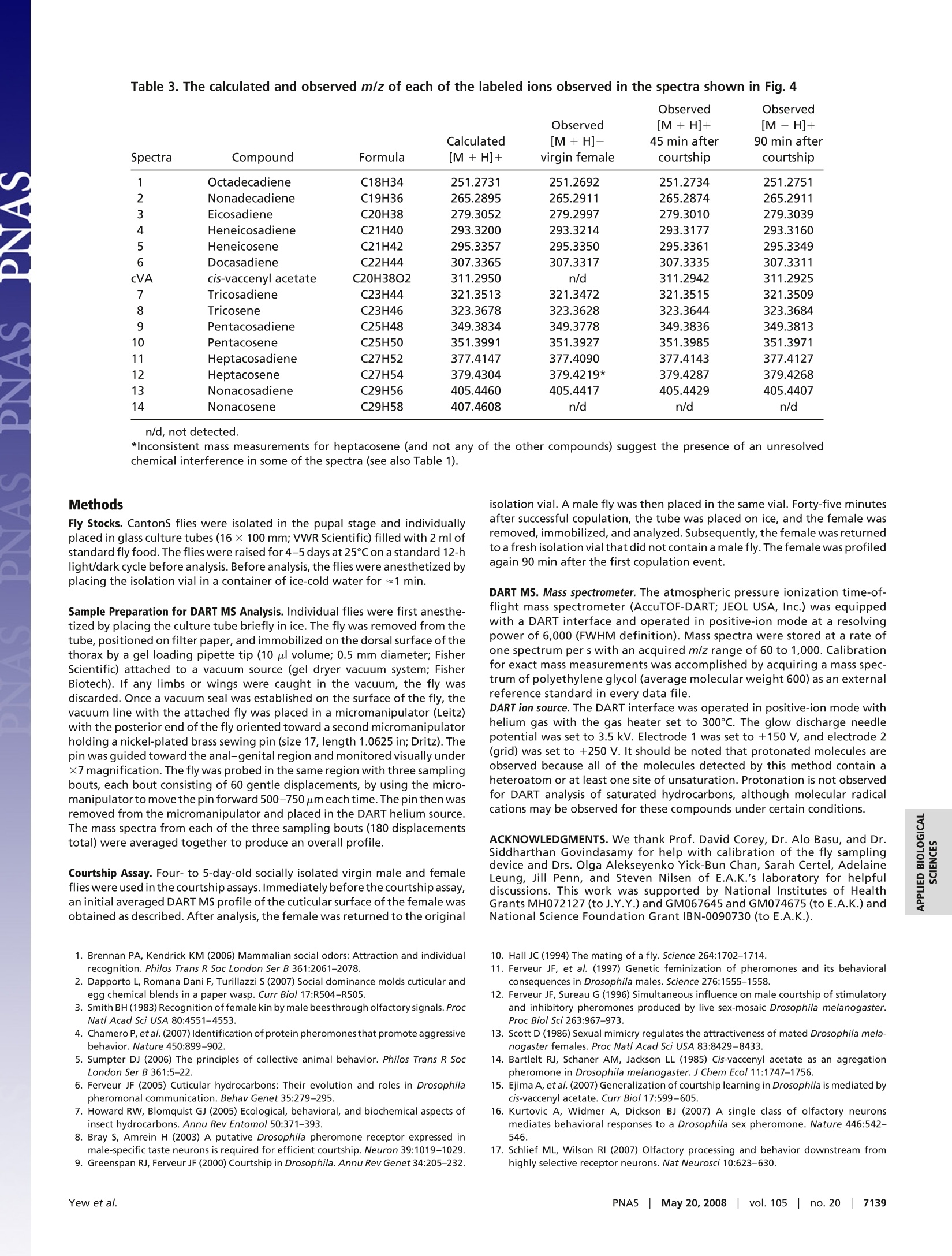
Table 3. The calculated and observed m/z of each of the labeled ions observed in the spectra shown in Fig. 4 Cuticular hydrocarbon analysis of an awake behavingfly using direct analysis in real-time time-of-flightmass spectrometry Joanne Y. Yew*, Robert B. Codyt, and Edward A. Kravitz** *Department of Neurobiology, Harvard Medical School, 220 Longwood Avenue, Boston, MA 02115; and tJEOL USA, Inc., 11 Dearborn Road,Peabody, MA 01960 In mammals and insects, pheromones strongly influence socialbehaviors such as aggression and mate recognition. In Drosophilamelanogaster, pheromones in the form of cuticular hydrocarbonsplay prominent roles in courtship. GC/MS is the primary analyticaltool currently used to study Drosophila cuticular hydrocarbons.Although GC/MS is highly reproducible and sensitive, it requiresthat the fly be placed in a lethal solution of organic solvent, therebyimpeding further behavioral studies. We present a technique forthe analysis of hydrocarbons and other surface molecules from liveanimals by using direct analysis in real-time (DART) MS. Cuticularhydrocarbons were sampled from the surface of a restrained,awake behaving fly by using several brief, carefully controlleddepressions of the abdomen with a small steel probe. DART massspectral analysis of the probe detected ions with mass-to-chargeratio (m/z) of the protonated molecule corresponding to many ofthe previously identified unsaturated hydrocarbons. Six additionalcuticular hydrocarbons also were identified. Consistent with pre-vious GC/MS studies, male and female differences in chemicalcomposition were evident. Spatial differences in the expressionprofile also were observed on males. Sampling from an individualfemale first as a virgin and then 45 and 90 min after successfulcopulation showed that mass signals likely to correspond tocis-vaccenyl acetate, tricosene, and pentacosene increased in rel-ative intensity after courtship. This method provides near-instan-taneous analysis of an individual animal's chemical profile inparallel with behavioral studies and could be extended to othermodels of pheromone-mediated behavior. behavior|cis-vaccenyl acetate| courtship|pheromones| Drosophila number of volatile chemical signals have been shown inAmammals and insects to have profound effects on complexsocial behaviors such as mate choice (1), kin recognition (2,3),aggression (4), and aggregation (5). In insects and otherarthropods, these signals, many of which are cuticular hydro-carbons, can designate individual roles in social networks inaddition to influencing courtship, colony recognition, andaggression (6,7). In Drosophila melanogaster, several studieshave documented the role of hydrocarbons as aphrodisiacs(8-11) or antiaphrodisiacs (11-13). In particular, much focushas been placed on the molecule z-11-octadecenyl acetate[cis-vaccenyl acetate (cVA)] which serves both as a mediatorof mate recognition (14-16) and an aggregation factor (8, 9,14, 17-19). Elucidation of pheromone receptors and theupstream neural pathways has provided a means for delineat-ing the circuitry of complex social behaviors by allowinginformation flow to be followed from sensory input to motoroutput (17, 18, 20-23). The primary method that has been used to characterizehydrocarbons in insects is GC combined with MS (7). Analysisby GC/MS allows quantitation of hydrocarbon content inaddition to resolution of individual isomers. Although highlyreproducible and sensitive, the method has three limitations.First, the extraction procedure involves placing the animal in hexane or chloroform. These conditions result in lethality andthereby impede further behavioral studies. Second, the solventand detection conditions are selective for a subset of surfacecompounds; as a result, other behaviorally relevant cuticularcues may be undetected by using the current protocols. Third,GC/MS analysis can be time-consuming, with typical analysistimes ranging from tens of minutes to >1 h. To address these limitations, we present a method for theanalysis of cuticular hydrocarbons and other surface moleculesfrom an awake behaving fly. Ambient MS is a recent techno-logical development that allows mass-to-charge ratio (m/z)measurement with minimal sample preparation (24). Oneform of ambient MS, direct analysis in real time (DART), usesexcited-state helium atoms to desorb and ionize compoundsfrom the surface of the sample directly into the analyzerwithout the need for chemical extraction or high-vacuumconditions (25-28). The use of DART MS to examine D.melanogaster hydrocarbons improves on previous GC/MS-based techniques by providing rapid analysis of an individualanimal's chemical profile while allowing behavioral studies tobe performed in-parallel. The ability to track changes inchemical profile before and after social interactions in thesame animal provides a control for individual variation ofcuticular hydrocarbon expression, which can be significant(29), and also may reveal chemical signatures that could becorrelated with behavioral differences observed in individualanimals. Using DART MS analysis, it was possible to chemi-cally profile the surface of living flies with high reproducibility,detect differences in male vs. female profiles, detect spatialspecificity of male hydrocarbon expression, and monitor hy-drocarbon changes in the same individual before and aftersocial interaction. Results Initial experiments addressed whether the abundance andchemical properties of hydrocarbons on the surface of a singlefruit fly allowed detection by DART MS. Stainless-steelforceps were first used to hold an individual fly in the heatedhelium metastable stream. A robust chemical profile could beobtained with this method (Fig. 14). Signals with m/z valuesmatching the calculated m/z values of the protonated mole-cules (MH+) of many of the hydrocarbons previously charac-terized by GC/MS were detected (Table 1). However, thistreatment almost always resulted in severe damage to the fly ( Author c o ntributions: J . Y.Y., R.B.C., and E .A.K. d esigned r e search; J . Y.Y. and R . B.C. p e r- formed research;R.B.C. contributed new reagents/analytic tools;J.Y.Y. and R.B.C. analyzed data; and J.Y.Y. , R.B.C., and E.A.K. wrote the paper. ) ( Conflict of interest statement: DART i s a trademark of JEOL USA, Inc. ) ( *To w hom c or respondence s h ould b e ad d ressed. E- m ail: edward_kravitz@hms. h arvard.edu. ) ( This a rticle contains supporting information o nli n e at w ww.pn a s . org/cgi/conte n t/full/ 0802692105/DCSup plem enta l . ) ( O 2008 by The National Academy of Sciences of the USA ) Fig. 1. Comparison of DART mass spectral analysis of an intact fly vs.forceps used to handle a fly. The chemical profile obtained by placing anintact female fly directly in the helium source (A) could be reproduced byanalyzing the stainless steel forceps used to handle the female fly (B). or lethality. Fortuitously, it was observed that the stainless-steel forceps used to handle the fly provided high-intensitysignals, with a profile that showed all of the identified hydro-carbon signals detected on the intact fly (Fig. 1B). Moreover,the relative intensities of these signals to each other also wereconsistent between the two sampling methods. An attempt wasmade to sample hydrocarbons from the fly by using alternatesurfaces such as a glass capillary tube and filter paper;however, the metal surface produced the greatest signalstrength. Based on these initial observations, a method was devisedfor immobilizing and sampling from individual animals with ametal probe that would allow assessment of the chemicalprofile of the fly in a reproducible and anatomically consistentmanner with minimal trauma [Fig. 2A and supporting infor- mation (SI) Movie S1]. In all of the following experiments, 4-to 5-day-old socially naive CantonS flies were used. The fly wasprobed in the same region with three sampling bouts by usinga metal pin guided by a micromanipulator. Each bout consist-ing of 60 gentle displacements with the micromanipulator tomove the pin forward 500-750 um each time. The pin then wasremoved from the micromanipulator and placed in the DARThelium-metastable source. The mass spectra from each of thethree sampling bouts (180 displacements total) were averagedtogether to produce an overall profile. There was little overallvariation observed from bout to bout from the same fly (Fig.S1 and Table S1). Profiling of Male and Female Flies. To compare cuticular hydro-carbon analysis by GC/MS to DART ionization, we firstanalyzed the chemical profile of individually isolated wild-typeD. melanogaster males and females. The averaged mass spectrafrom a socially isolated virgin female and virgin male flymeasured with this method are shown in Fig. 2 B and C. Theidentity of the ions is assigned when the observed m/z of theprotonated species is within ± 0.005 of the calculated value(Table 2). The relative intensities of the detected ions corre-sponding to the major hydrocarbon species in females andmales are plotted as a histogram (Fig. 2D). In contrast toanalysis by GC/MS, it is not possible to resolve individualisomers with DART-MS; therefore, each ion signal for ahydrocarbon species corresponds to the total signal from allisomers. Despite this difference, DART profiles are consistentwith many of the previous findings with GC/MS (6, 9, 30,31).Both methods show that the male profile is less complex thanthat of the female, with males expressing shorter chain unsat-urated hydrocarbons and females expressing longer chainhydrocarbons with varying degrees of saturation. In the maleprofile, the most prominent ions observed correspond totricosene and pentacosene (n = 11). A signal for the phero-mone cVA is also detected in males but not in females. Infemales, the ions with the greatest intensity correspond topentacosene and heptacosadiene, although the intensity ratiosof these two hydrocarbons relative to each other variedbetween flies (n =10). Interestingly, the overall signal inten-sity from males was lower than that of females. There was morevariation in overall profiles when comparing fly to fly in bothmales and females with regard to content and relative intensityratios of the detected ions (Fig. S2). It is unclear whether theobserved differences are caused by natural individual varia-tion, sampling differences that may be less reliable for low-abundance species or regional differences in hydrocarbon Table 1. The calculated and observed m/z of each of the labeled ions observed in the spectrashown in Fig. 1 Observed Observed Calculated [M+H]+ [M+H]+ Spectra Compound Formula [M +H]+ intact fly forceps only 1 Octadecadiene C18H34 251.2739 251.2755 251.2748 2 Docasadiene C22H44 307.3365 307.3377 307.3356 3 Tricosadiene C23H44 321.3513 321.3491 321.3517 4 Tricosene C23H46 323.3678 323.3689 323.3714 5 Pentacosadiene C25H48 349.3834 349.3848 349.3827 6 Pentacosene C25H50 351.3991 351.3945 351.3968 7 Heptacosadene C27H52 377.4147 377.4098 377.4176 8 Heptacosene C27H54 379.4304 (379.4170)* 379.4306 9 Nonacosadiene C29H56 405.4460 405.4447 405.4476 10 Nonacosene C29H58 407.4608 407.4604 407.4511 *Inconsistent mass measurements for heptacosene and (not any of the other compounds) are likely to indicatethe presence of an unresolved chemical interference in some of the spectra. C D Fig.2. Positive mass spectra from DART mass spectral analysis of virginmale and virgin female flies. (A) To profile cuticular compounds from liveD. melanogaster, the fly was held by a vacuum applied through a pipettetip and probed with a metal pin. (B and C) The averaged positive-ion massspectra obtained from DART mass spectral analysis revealed profile differ-ences between a virgin female (B) and male (C). (D) A histogram of therelative intensities of each of the identified hydrocarbon species showedthat predominantly longer chain hydrocarbons were detected on thefemale fly cuticle. In contrast, shorter chain hydrocarbons were detectedprimarily on the male fly profile, namely cVA, tricosene (peak 8), andpentacosene (peak 10). Numbers in the histogram correspond to the peaklabels used in the spectra. expression. Using a probe with a smaller diameter may reducethis variation. Detection of Hydrocarbon Species. In both males and particularlyfemales, DART MS detected ions with m/z corresponding tolow-molecular-weight hydrocarbons that have not been previ-ously reported to our knowledge. Using GC/MS, cVA in males(C20) and tricosadiene (C23) in females were the shortestchain hydrocarbons reported. With DART analysis, six com-pounds with m/z values consistent with the protonated mole-cules of C18-C22 chain length hydrocarbon species couldconsistently be detected on the female. The measured m/zvalues of these ions matched the following chemical structuresto within ±0.003 units of the calculated value: C18H34, C19H36, Spatial Profiling of Individual Flies. DART MS was used to addresswhether there are spatial differences in the expression ofhydrocarbons by comparing the profiles of individual maleflies probed on the lateral thorax vs. the anal-genital region.The signal for cVA was consistently greater from the anal-genital region than the thorax, although the magnitude of thisdifference varied between flies (n = 3; Fig. 3). These resultsindicate that a higher concentration ofcVA is expressed in theanal-genital region, likely near the tip of the ejaculatory bulb(32). No significant spatial differences in hydrocarbon expres-sion were found when female virgins were profiled (data notshown). Monitoring Chemical Profile Changes in the Same Individual Beforeand After Courtship. Hydrocarbon expression has been shown tochange dramatically after courtship (6, 10, 12,15). To deter-mine whether these changes could be detected by using DARTMS in the same animal, individual female flies were sampledbefore and at two time points after successful copulation.Consistent with GC/MS studies, changes in the intensity of thepeaks representing the ions with m/z values corresponding tothe protonatedcVA,tricosene, and pentacosene relativfei yto thelevels of other hydrocarbons were apparent after copulation at45 min and persisted until at least 90 min after initiation (n =4; Fig. 4 and Table 3). The relative intensity of peaks repre-senting other ions in the profile remained similar before andafter courtship, indicating that the change observed is likelycorrelated with copulation and not caused by variation fromposition effects. Discussion Numerous studies have shown the importance of pheromonesin shaping behavioral responses and organizing social struc-tures in mammals and insects. Identifying the chemical signalsunderlying these interactions in addition to correlating thetiming of changes in these signals with changes in behaviorprovides several technical challenges because of issues ofdetection sensitivity and the disruption of natural behavior.Previous studies of real-time biochemical analysis in conjunc-tion with behavior only took place in mammalian systemswhere the quantity of analyte was substantial and the size ofthe brain facilitated implantation of probes used for in situmonitoring of neurotransmitters (33, 34). In this article, weshow that biochemical analysis of the fruit fly in parallel withbehavior is possible. Although every attempt was made tominimize trauma to the fly, it must be acknowledged thatvacuum suspension and repetitive probing are neverthelesshighly invasive and unnatural conditions for the animal. Nev-ertheless,both the profile of hydrocarbons on male and femalevirgin flies and changes in profile observed on mated femaleflies were consistent with previous reports of hydrocarboncontent measured by GC/MS. Moreover, despite the manip-ulations, the flies were still capable and interested in behav-ioral interactions. Thus, it may be concluded that neither themechanical probing nor additional stress grossly altered thechemical profile of the fly. Using a different ionization source provided the advantagesof potential new biomolecule discovery, increased spatialresolution, improved sensitivity, and greater time resolution.Analysis by DART MS resulted in the identification of six Table 2. The calculated and observed m/z of each of the labeled ions observed in the spectrashown in Fig.2 Observed Calculated [M +H]+ [M + H]+ Spectra Compound Formula [M +H]+ female male 1 Octadecadiene C18H34 251.2731 251.2716 251.2701 2 Nonadecadiene C19H36 265.2895 265.2876 n/d 3 Eicosadiene C20H38 279.3052 279.3028 n/d 4 Heneicosadiene C21H40 293.3200 293.3173 n/d 5 Heneicosene C21H42 295.3357 295.3345 295.3337 6 Docasadiene C22H44 307.3365 307.3333 n/d cVA cis-vaccenyl acetate C20H38O2 311.2950 n/d 311.2952 7 Tricosadiene C23H44 321.3513 321.3516 321.3523 8 Tricosene C23H46 323.3678 323.3645 323.3645 9 Pentacosadiene C25H48 349.3834 349.3819 349.3826 10 Pentacosene C25H50 351.3991 351.3976 351.4011 11 Heptacosadiene C27H52 377.4147 377.4111 n/d 12 Heptacosene C27H54 379.4304 379.4295 n/d 13 Nonacosadiene C29H56 405.4460 405.4439 n/d 14 Nonacosene C29H58 407.4608 407.4603 n/d hydrocarbons found on the surface of the fruit fly, several ofwhich were found consistently only on the surface of thefemale. Moreover, in certain samples, ions with m/z valuesmatching triglycerides also were observed simultaneously withhydrocarbons. These features of DART MS offer possiblecandidate molecules that may be used in conspecific commu-nication. This method also showed spatial variation in cVAexpression on the male fly, with a higher intensity signal foundon the rear than on the thorax. Interestingly, variation in cVAsignal intensity between individuals was observed despiteconsistent placement of the probe. The possibility that asmaller diameter probe may reduce this variation should be B Male rear Fig. 3.DART mass spectral analysis of the thorax (A) and rear(B) of the samemale fly reveals spatial differences in hydrocarbon expression. The intensitiesof the C23H46(tricosene) and C25H50 (pentacosene) signals are relatively thesame between the two regions, but the intensity for cVA is noticeably greaterin the anal-genital region vs. the thorax. addressed in future studies although initial experiments withpins 0.15 mm in diameter provided little signal, likely causedby inadequate probe surface area. If differences in hydrocar-bon quantities are the product of natural variation, it isintriguing to consider that individual levels of cVA mayinfluence the mating choice of females by possibly serving asone indicator of overall fitness. Future analytical refinements include the addition of astandard to either the fly or the metal probe to facilitatequantitation and coupling the DART interface with a detectorcapable of tandem MS to allow structural elucidation andcharacterization of novel molecules. With these improvements,1LS,this rapid sampling method presents the possibility for behav-ioral MS analysis where dynamic changes in chemical profilemay be monitored in parallel with behavior. Fig.4. Chemical profile changes in the same individual female are observedbefore and after courtship. (A-C) Comparison of averaged positive ion massspectra obtained from DART mass spectral analysis of the same female flybefore (A) and at 45 min (B) and 90 min (C) after successful copulation. (D) Ahistogram of the relative intensities of each of the identified hydrocarbonspecies shows a relative increase in ion signal intensity corresponding to cVA,tricosene (peak 8), and pentacosene (peak 10) after copulation. Observed [M + H]+ [M+ H]+ Calculated [M + H]+ 45 min after 90 min after Spectra Compound Formula [M +H]+ virgin female courtship courtship 1 Octadecadiene C18H34 251.2731 251.2692 251.2734 251.2751 2 Nonadecadiene C19H36 265.2895 265.2911 265.2874 265.2911 3 Eicosadiene C20H38 279.3052 279.2997 279.3010 279.3039 4 Heneicosadiene C21H40 293.3200 293.3214 293.3177 293.3160 5 Heneicosene C21H42 295.3357 295.3350 295.3361 295.3349 6 Docasadiene C22H44 307.3365 307.3317 307.3335 307.3311 cVA cis-vaccenyl acetate C20H38O2 311.2950 n/d 311.2942 311.2925 7 Tricosadiene C23H44 321.3513 321.3472 321.3515 321.3509 8 Tricosene C23H46 323.3678 323.3628 323.3644 323.3684 9 Pentacosadiene C25H48 349.3834 349.3778 349.3836 349.3813 10 Pentacosene C25H50 351.3991 351.3927 351.3985 351.3971 11 Heptacosadiene C27H52 377.4147 377.4090 377.4143 377.4127 12 Heptacosene C27H54 379.4304 379.4219* 379.4287 379.4268 13 Nonacosadiene C29H56 405.4460 405.4417 405.4429 405.4407 14 Nonacosene C29H58 407.4608 n/d n/d n/d Methods Fly Stocks. CantonS flies were isolated in the pupal stage and individuallyplaced in glass culture tubes (16 × 100 mm; VVR Scientific) filled with 2 ml ofstandard fly food. The flies were raised for 4-5 days at 25Con a standard 12-hlight/dark cycle before analysis. Before analysis, the flies were anesthetized byplacing the isolation vial in a container of ice-cold water for~1 min. Sample Preparation for DART MS Analysis. Individual flies were first anesthe-tized by placing the culture tube briefly in ice. The fly was removed from thetube, positioned on filter paper, and immobilized on the dorsal surface of thethorax by a gel loading pipette tip (10 ul volume; 0.5 mm diameter; FisherScientific) attached to a vacuum source (gel dryer vacuum system; FisherBiotech). If any limbs or wings were caught in the vacuum, the fly wasdiscarded. Once a vacuum seal was established on the surface of the fly, thevacuum line with the attached fly was placed in a micromanipulator (Leitz)with the posterior end of the fly oriented toward a second micromanipulatorholding a nickel-plated brass sewing pin (size 17, length 1.0625 in;Dritz). Thepin was guided toward the anal-genital region and monitored visually under×7 magnification. The fly was probed in the same region with three samplingbouts, each bout consisting of 60 gentle displacements, by using the micro-manipulator to move the pin forward 500-750 um each time. The pin then wasremoved from the micromanipulator and placed in the DART helium source.The mass spectra from each of the three sampling bouts (180 displacementstotal) were averaged together to produce an overall profile. Courtship Assay. Four- to 5-day-old socially isolated virgin male and femaleflies were used in the courtship assays. Immediately before thecourtship assay,an initial averaged DART MS profile of the cuticular surface of the female wasobtained as described. After analysis, the female was returned to the original 1. Brennan PA, Kendrick KM (2006) Mammalian social odors:Attraction and individualrecognition. Philos Trans R Soc London Ser B 361:2061-2078. 2. Dapporto L, Romana Dani F, Turillazzi S (2007) Social dominance molds cuticular andegg chemical blends in a paper wasp. Curr Bio/ 17:R504-R505. 3. Smith BH(1983)Recognition of female kin by male bees through olfactory signals.ProcNatl Acad Sci USA 80:4551-4553. 4. Chamero P,etal. (2007)Identification of protein pheromones that promote aggressivebehavior. Nature 450:899-902. 5. Sumpter DJ (2006) The principles of collective animal behavior. Philos Trans R SocLondon Ser B 361:5-22. 6. Ferveur JF (2005) Cuticular hydrocarbons: Their evolution and roles in Drosophilapheromonal communication. Behav Genet 35:279-295. 7. Howard RW, Blomquist GJ (2005) Ecological, behavioral, and biochemical aspects ofinsect hydrocarbons. Annu Rev Entomo/ 50:371-393. 8. Bray S, Amrein H (2003) A putative Drosophila pheromone receptor expressed inmale-specific taste neurons is required for efficient courtship. Neuron 39:1019-1029. 9. Greenspan RJ, Ferveur JF (2000) Courtship in Drosophila. Annu Rev Genet 34:205-232. isolation vial. A male fly was then placed in the same vial. Forty-five minutesafter successful copulation, the tube was placed on ice, and the female wasremoved, immobilized, and analyzed. Subsequently, the female was returnedto afresh isolationvial that did not contain a male fly. The female was profiledagain 90 min after the first copulation event. DART MS. Mass spectrometer. The atmospheric pressure ionization time-of-flight mass spectrometer (AccuTOF-DART; JEOL USA, Inc.) was equippedwith a DART interface and operated in positive-ion mode at a resolvingpower of 6,000 (FWHM definition). Mass spectra were stored at a rate ofone spectrum per s with an acquired m/z range of 60 to 1,000. Calibrationfor exact mass measurements was accomplished by acquiring a mass spec-trum of polyethylene glycol (average molecular weight 600) as an externalreference standard in every data file. DART ion source. The DART interface was operated in positive-ion mode withhelium gas with the gas heater set to 300°C. The glow discharge needlepotential was set to 3.5 kV. Electrode 1 was set to +150 V, and electrode 2(grid) was set to +250 V. It should be noted that protonated molecules areobserved because all of the molecules detected by this method contain aheteroatom or at least one site of unsaturation. Protonation is not observedfor DART analysis of saturated hydrocarbons, although molecular radicalcations may be observed for these compounds under certain conditions. ACKNOWLEDGMENTS. We thank Prof. David Corey, Dr. Alo Basu, and Dr.Siddharthan Govindasamy for help with calibration of the fly samplingdevice and Drs. Olga Alekseyenko Yick-Bun Chan, Sarah Certel, AdelaineLeung, Jill Penn, and Steven Nilsen of E.A.K.'s laboratory for helpfuldiscussions. This work was supported by National Institutes of HealthGrants MH072127 (to J.Y.Y.) and GM067645 and GM074675 (to E.A.K.)andNational Science Foundation Grant IBN-0090730 (to E.A.K.). 10. Hall JC (1994) The mating of a fly. Science 264:1702-1714. 11. Ferveur JF, et al. (1997) Genetic feminization of pheromones and its behavioralconsequences in Drosophila males. Science 276:1555-1558. 12. Ferveur JF, Sureau G (1996) Simultaneous influence on male courtship of stimulatoryand inhibitory pheromones produced by live sex-mosaic Drosophila melanogaster.Proc Biol Sci 263:967-973. 13. Scott D (1986) Sexual mimicry regulates the attractiveness of mated Drosophila mela-nogaster females. Proc Natl Acad Sci USA 83:8429-8433. 14. Bartlelt RJ, Schaner AM, Jackson LL (1985) Cis-vaccenyl acetate as an agregation pheromone in Drosophila melanogaster. J Chem Eco/ 11:1747-1756. 15. Ejima A, et al.(2007) Generalization of courtship learning in Drosophila is mediated bycis-vaccenyl acetate. Curr Bio/ 17:599-605. 16. Kurtovic A, Widmer A, Dickson BJ (2007) A single class of olfactory neuronsmediates behavioral responses to a Drosophila sex pheromone. Nature 446:542-546. 17. Schlief ML, Wilson RI (2007) Olfactory processing and behavior downstream fromhighly selective receptor neurons. Nat Neurosci 10:623-630. 18. Xu P, Atkinson R, Jones DN, Smith DP (2005) Drosophila OBP LUSH is required foractivity of pheromone-sensitive neurons. Neuron 45:193-200. 19. Hall JC (1978) Behavioral analysis in Drosophila mosaics. Results Prob/ Cell Differ 9:259-305. 20. Herrada G, Dulac C (1997) A novel family of putative pheromone receptors inmammals with a topographically organized and sexually dimorphic distribution.Cell 90:763-773. 21. Kimchi T, Xu J, Dulac C (2007) A functional circuit underlying male sexual behavior inthe female mouse brain. Nature 448:1009-1014. 22. van der Goes van Naters W, Carlson JR (2007) Receptors and neurons for fly odors inDrosophila. Curr Bio/ 17:606-612. 23. Dulac C, Wagner S (2006) Genetic analysis of brain circuits underlying pheromonesignaling. Annu Rev Genet 40:449-467. 24. Cooks RG, Ouyang Z, Takats Z, Wiseman JM (2006) Detection technologies: Ambientmass spectrometry. Science 311:1566-1570. 25. Cody RB, Laramee JA, Durst HD (2005) Versatile new ion source for the analysisof materials in open air under ambient conditions.Anal Chem 77:2297-2302. 26. McEwen CN, McKay RG, Larsen BS (2005) Analysis of solids, liquids, and biologicaltissues using solids probe introduction at atmospheric pressure on commercial LC/MSinstruments.Anal Chem 77:7826-7831. 27. PierceCY,etal.(2007)Ambient generation of fatty acid methyl ester ions from bacterial wholecells by direct analysis in real-time (DART) mass spectrometry. Chem Commun 807-809. 28. Vail T, Jones PR, Sparkman OD (2007) Rapid and unambiguous identification ofmelamine in contaminated pet food based on mass spectrometry with four degrees ofconfirmation. JAnal Toxico/ 31:304-312. 29. Foley B, Chenoweth SF, Nuzhdin SV, Blows MW (2007) Natural genetic variation incuticular hydrocarbon expression in male and female Drosophila melanogaster. Ge-netics 175:1465-1477. 30. Antony C, Jallon J (1982) The chemical basis for sex recognition in Drosophila mela-nogaster.J Insect Physio/ 28:873-880. 31. Pechine JM, Perez F, Antony C, Jallon JM (1985) A further characterization of Drosoph-ila cuticular monoenes using a mass spectrometry method to localize double bonds incomplex mixtures. Anal Biochem 145:177-182. 32. Brieger G, Butterworth FM (1970) Drosophila melanogaster: Identity of male lipid inreproductive system. Science 167:1262. 33. Reed B, Zhang Y, Chait BT, Kreek MJ (2003) Dynorphin A(1-17)biotransformation instriatum of freely moving rats using microdialysis and matrix-assisted laser desorption/ionization mass spectrometry. J Neurochem 86:815-823 34. Baseski HM, Watson CJ, Cellar NA, Shackman JG, Kennedy RT (2005) Capillary liquidchromatography with MS3 for the determination of enkephalin in microdialysissamples from the striatum of anesthetized and freely moving rats. J Mass Spectrom40:146-153. www.pnas.org/cgi/doi/pnas.NASSMay vol.| no. ww.pnas.org/cgi/doi/pnas.ew et al.
确定

还剩4页未读,是否继续阅读?
华质泰科生物技术(北京)有限公司为您提供《果蝇中清醒状态的表皮烃类化合物检测方案(液质联用仪)》,该方案主要用于其他中清醒状态的表皮烃类化合物检测,参考标准--,《果蝇中清醒状态的表皮烃类化合物检测方案(液质联用仪)》用到的仪器有
相关方案
更多









