
方案详情
文
Piper betle Linn. is a traditional plant associated with the Asian and southeast Asian cultures. Its use is also
recorded in folk medicines in these regions. Several of its medicinal properties have recently been proven. Phytochemical
analysis showed the presence ofmainly terpenes and phenols in betel leaves. These constituents vary in the different cultivars
of Piper betle. In this paper we have attempted to profile eight locally available betel cultivars using the recently developed
mass spectral ionization technique of direct analysis in real time (DART). Principal component analysis has also been employed
to analyze the DART MS data of these betel cultivars. The results show that the cultivars of Piper betle could be differentiated
using DART MS data.
方案详情

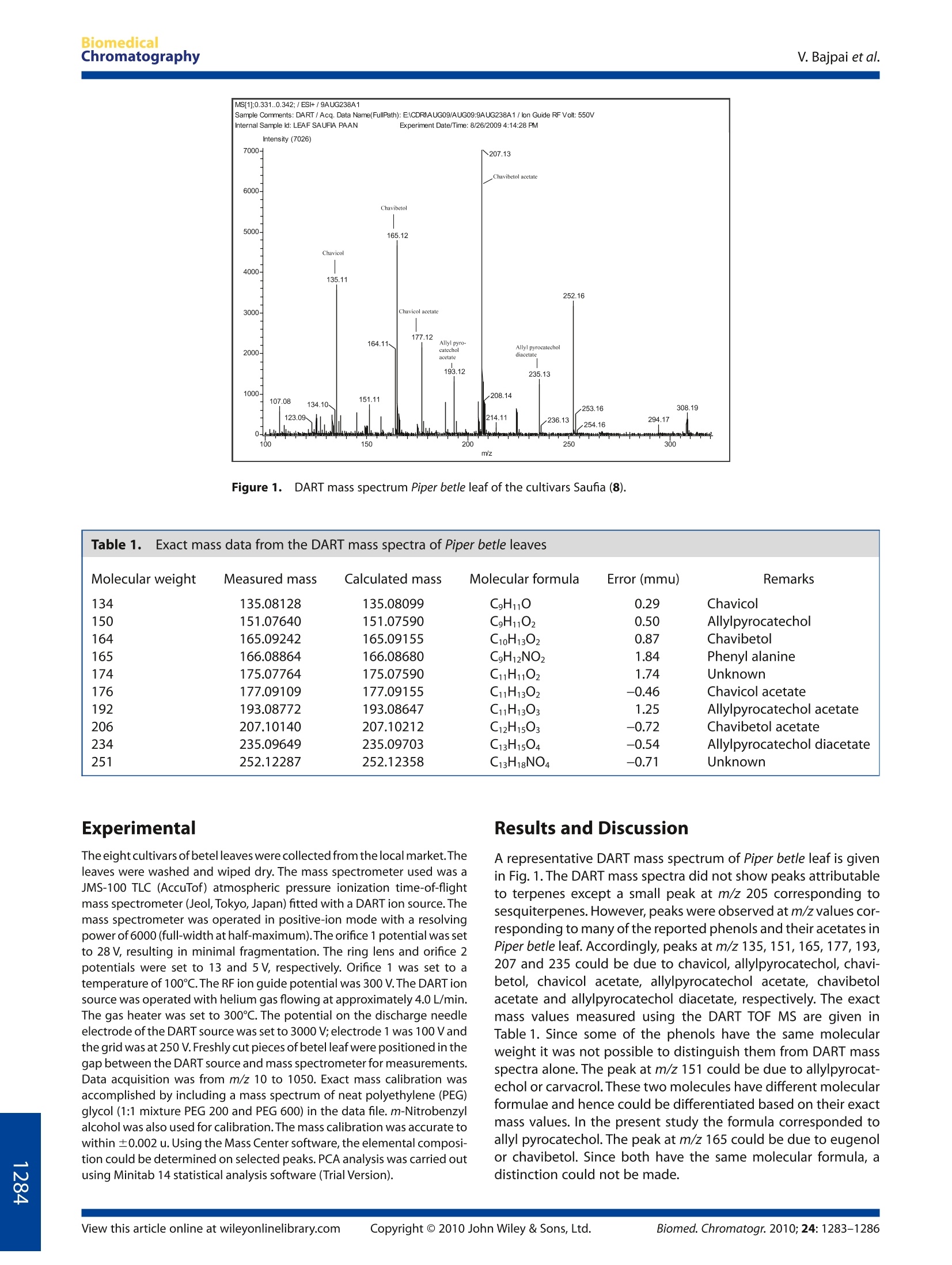
BiomedicalChromatographyResearch ArticleReceived 29 January 2010, Accepted 5 Mrach 2010 BiomedicalChromatographyV. Bajpai et al. Published online in Wiley Online Library: 1 June 2010 (wileyonlinelibrary.com) DOI 10.1002/bmc.1437 Profiling of Piper betle Linn. cultivarsby direct analysis in real time massspectrometric technique Vikas Bajpai, Deepty Sharma, Brijesh Kumar*and K. P. Madhusudanan ABSTRACT: Piper betle Linn. is a traditional plant associated with the Asian and southeast Asian cultures. Its use is alsorecorded in folk medicines in these regions. Several of its medicinal properties have recently been proven. Phytochemicalanalysis showed the presence of mainly terpenes and phenols in betel leaves. These constituents vary in the different cultivarsof Piper betle. In this paper we have attempted to profile eight locally available betel cultivars using the recently developedmass spectral ionization technique of direct analysis in real time (DART). Principal component analysis has also been employedto analyze the DART MS data of these betel cultivars. The results show that the cultivars of Piper betle could be differentiatedusing DART MS data. Copyright ◎ 2010 John Wiley & Sons, Ltd. Keywords: DARTMS; Piper betle L; cultivars; profiling; PCA Introduction Piper betle Linn. (betel vine) is a tropical plant closely related tothe common pepper and belongs to the family Piperaceae(Gunther, 1952). It is extensively grown in India,Sri Lanka, Malay-sia, Thailand, Taiwan and other southeast Asian countries and hasa long history of over 2000 years (Guha, 2006). Chewing of betelquid has been practised since ancient times and is prevalent inIndia, other Asian countries, the western pacific and amongmigrated communities in Africa, Europe and North America(Gupta and Ray, 2004). Betel quid generally consists of betel leaf(from the Piper betle L. vine), areca nut (from the Areca catechutree), catechu (a tannin-rich powder) and slaked lime (calciumhydroxide), to which tobacco is often added. There have beenmany reports that chewing betel quid causes oral cancer (IARC,1985; Sankaranarayanan, 1990; Moore et al.,2000; Avon, 2004). Betel leaf is an integral part of Asian, and, of course, Indianhistory. Its utility against various diseases can be traced in theancient vedic literature. An exhaustive list of its properties anduses is presented in a recent compilation (Warrier et al., 1995).Piper betle is aromatic, stimulant, carminative, astringent andantiseptic. Experimentally Piper betle leaves have been found tohave diverse pharmacological actions (Nadkarni, 1976), such asanti-inflammatory (Sarkar et al., 2008), anti-oxidant (Choudharyand Kale, 2002), radio-protective (Bhattacharya et al.,2005),andanti-allergic activities (Wirotesangthong et al., 2008). They havealso shown anti-microbial (Shitut etal., 1999), anti-fungal(Trakranrungsie et al., 2008), anti-amoebic (Sawangjaroen et al.,2006), hepatoprotective (Manigauha etal., 2009) anti-fertility(Sarkar et al., 2000) and anti-platelet activities (Lei et al., 2003). The chemical constituents of betel essential oil consist ofmainly terpenes and phenols (Atal et al.,1975; Balasubrahman-yam and Rawat, 1990; Rimando et al., 1986; Mohottalage et al.,2009). The characteristic flavour of betel is due to the betelphenols. The terpenoids include 1,8-cineole, cadinene, cam- phene,caryophylline, limonene, pinene, etc. Chavicol, allyl pyro-catechol, carvacrol,safrole, eugenol and chavibetol are the majorphenols found in Piper betle. Their acetates are also commonlyfound. Since the crop of Piper betle requires vegetative propagationand is widely cultivated, it is claimed to have 100 cultivars (lan-draces) based on regional and organoleptic considerations(Verma et al., 2004). Only isolated reports exist on the chemicalprofiling of these cultivars (Rawat et al., 1987; Rawat et al., 1989). Direct analysis in real time (DART) is a new ambient ionizationtechnique (Cody et al., 2005). Using a helium plasma DART ionizesatmospheric water and generates water clusters which in turnionize the sample held in the gas stream. The resulting spectraare relatively clean and simple. As DART ion source can ionizemolecules directly from the surface, plant products can be ana-lyzed directly without sample preparation (Haefliger and Jeckel-mann, 2007; Madhusudanan et al., 2008; Kim and Jang, 2009). Inview of the ease with which natural products can be analyzed byDARTMS, it was thought that DART MS could be a suitable tool forthe chemical profiling of the different landraces of Piper betleleaves. Locally available cultivars, namely Bangla (1),Desawari (2),Deshi (3), J. Green (4), J. White (5), Kalkatiya (6), Mahoba (7) andSaufia (8), of Piper betle were analyzed by DARTMS and the resultsare presented here. *Correspondence to: B. Kumar, Sophisticated Analytical Instrument Facility,Central Drug Research Institute (CSIR), Lucknow-226001, India. E-mail:brijesh_kumar@cdri.res.in Sophisticated Analytical Instrument Facility, Central Drug Research Institute,Lucknow-226001,India Abbreviations used: DART, direct analysis in real time; PCA,principal com-ponent analysis; TOF MS, time-of-flight mass spectrometry. Figure 1. DART mass spectrum Piper betle leaf of the cultivars Saufia (8). Molecular weight Measured mass Calculated mass Molecular formula Error (mmu) Remarks 134 135.08128 135.08099 C9H10 0.29 Chavicol 150 151.07640 151.07590 C9H1102 0.50 Allylpyrocatechol 164 165.09242 165.09155 C10H13O2 0.87 Chavibetol 165 166.08864 166.08680 C9H12NO2 1.84 Phenylalanine 174 175.07764 175.07590 C11H1102 1.74 Unknown 176 177.09109 177.09155 C11H1302 -0.46 Chavicol acetate 192 193.08772 193.08647 C11H1303 1.25 Allylpyrocatechol acetate 206 207.10140 207.10212 C12H15O: -0.72 Chavibetol acetate 234 235.09649 235.09703 C13H1504 -0.54 Allylpyrocatechol diacetate 251 252.12287 252.12358 C13H18NO4 -0.71 Unknown Experimental The eight cultivars of betel leaves were collected from the local market.Theleaves were washed and wiped dry. The mass spectrometer used was aJMS-100 TLC (AccuTof) atmospheric pressure ionization time-of-flightmass spectrometer (Jeol, Tokyo, Japan) fitted with a DART ion source. Themass spectrometer was operated in positive-ion mode with a resolvingpower of 6000 (full-width at half-maximum).The orifice 1 potential was setto 28 V, resulting in minimal fragmentation. The ring lens and orifice 2potentials were set to 13 and 5V, respectively. Orifice 1 was set to atemperature of 100℃. The RF ion guide potential was 300 V.The DART ionsource was operated with helium gas flowing at approximately 4.0 L/min.The gas heater was set to 300℃. The potential on the discharge needleelectrode of the DART source was set to 3000 V;electrode 1 was 100 Vandthe grid was at 250 V. Freshly cut pieces of betel leaf were positioned in thegap between the DART source and mass spectrometer for measurements.Data acquisition was from m/z 10 to 1050. Exact mass calibration wasaccomplished by including a mass spectrum of neat polyethylene (PEG)glycol (1:1 mixture PEG 200 and PEG 600) in the data file. m-Nitrobenzylalcohol was also used for calibration. The mass calibration was accurate towithin ±0.002 u. Using the Mass Center software, the elemental composi-tion could be determined on selected peaks. PCA analysis was carried outusing Minitab 14 statistical analysis software (Trial Version). Results and Discussion A representative DART mass spectrum of Piper betle leaf is givenin Fig. 1. The DART mass spectra did not show peaks attributableto terpenes except a small peak at m/z 205 corresponding tosesquiterpenes. However, peaks were observed at m/z values cor-responding to many of the reported phenols and their acetates inPiper betle leaf. Accordingly, peaks at m/z 135, 151,165,177,193,207 and 235 could be due to chavicol, allylpyrocatechol, chavi-betol, chavicol acetate, allylpyrocatechol acetate, chavibetolacetate and allylpyrocatechol diacetate,respectively. The exactmass values measured using the DART TOF MS are given inTable 1. Since some of the phenols have the same molecularweight it was not possible to distinguish them from DART massspectra alone. The peak at m/z 151 could be due to allylpyrocat-echol or carvacrol.These two molecules have different molecularformulae and hence could be differentiated based on their exactmass values. In the present study the formula corresponded toallyl pyrocatechol. The peak at m/z 165 could be due to eugenolor chavibetol. Since both have the same molecular formula, adistinction could not be made. There were differences in the spectra of the different cultivars,as shown in Table 2. A peak at m/z 135 corresponding to chavicolwas seen only in the cultivars Saufia. The data shows that allylpy-rocatechol is present in all cultivars except Desawari, whereaschavibetol is not present in Deasawari and Mahoba cultivars. Italso shows that chavicol acetate is present only in Bangla andSaufia cultivars. The peaks at m/z 193, 207, 235 and 252 arecommon to all cultivars. It is not easy to identify and differentiate all the componentsindependently based on their mass spectral data. MS-basedchemical profiling generates complex data sets which needsophisticated software to enable interpretation.Visualization is a (2),Deshi(3),J. Green (4), J. White (5), Kalkatiya (6), Mahoba (7) and Saufia (8) cultivars of Piper betle leaves m/z Piper betle cultivars 1 2 3 5 6 7 8 104 118 × — — 132 135 151 × × × × × 163 — × 165 × × 166 二 175 × × — 177 — 193 × × 205 — — 207 × × 235 252 × a 二,Absent; x, present. key aspect as the data contained a number of variables. Multi-variate analysis such as principal component analysis (PCA) canbe used to reduce the dimensionality and to allow independentclassification of cases. PCA groups the samples solely on informa-tion on the measured data and does not need any extra knowl-edge about the sample, and therefore can be used to summarizeand visualize the structure of the data. The mass spectral data(five sets) for all the eight Piper betle leaves were subjected to PCAusing 14 variables (abundances of ions at m/z 104, 115, 123,150,151, 165,175,193,207,235,252, 308,324, 352). The PCA scoreplot clearly brings out the relationship among all the betel dataand all the eight sets of betel cultivars are clearly separated(Fig.2). It seems that Saufia and Bangla are entirely different fromall other cultivars, whereas pairs of J. White and Desawari aremuch closer to each other. Similarly, Mahoba and Kalkatiya aresimilar but Deshi appears between J. White/Desawari andMahoba/Kalkatiya pairs. It is evident from this study that PCAeffectively served the purpose and all the betel cultivars could bedifferentiated by this method. Conclusion The DART MS of the leaves Piper betle could be recordedwithout any sample preparation. The abundances of the charac-teristic betel phenols were different in the eight cultivars. Princi-pal component analysis showed the expected grouping of thecultivars. Acknowledgements Grateful acknowledgement is made to SAIF, CDRI (CSIR),Lucknow where the mass spectrometric studies were carriedout. Vikas Bajpai and Ms Deepty are thankful to CSIR, New Delhifor research grant NWP0045. Acknowledgement is also due toDr Y.S. Prabhakar, scientist MPC Division, CDRI for his help andsuggestions. Score Plot of m/z 104, …, m/z 352 Figure 2. PCA score plot of the abundances of the various ions in the DART massspectra of the leaves of various betel cultivars. References Atal CK, Dhar KI and Singh J. The chemistry of Indian piper species. Lloydia1975;38:256-262. Avon SL. Oral mucosal lesions associated with use of quid. Journal of theCanadian Dental Association 2004;70(4): 244-248. Balasubrahmanyam VR and Rawat AKS. Studies on morphology andchemistry of Piper betle L. Journal of Plantation Crops 1990; 18(2):78-87. Bhattacharya S, Subrahmanian M, Roychoudhury S, Bauri AK, Kamat JP,Chattopadhyay S and Bandopadhyay SDK. Radioprotective propertyof the ethanolic extract of Piper betle leaf. Journal of RadiationResearch (Tokyo) 2005; 6: 165-171. Choudhary D and Kale RK. Antioxidant and non-toxic properties of Piperbetle leaf extract in vitro and in vivo studies. Phytotherapy Research2002;16:461-466. Cody RB, Laramee JA and Durst HD. Versatile new ion source for theanalysis of materials in open air under ambient conditions. AnalyticalChemistry 2005;77:2297-2302. Guha P. Betel Leaf: The neglected green gold of India. Journal of HumanEcology 2006;19(2):87-93. Gunther E. The Essential Oils, Vol. 5. Van Nostrand: New York, 1952; 160-161. Gupta PC and Ray CS. Epidemiology of betel quid usage. Annals Academyof Medicine Singapore 2004;33(suppl):31S-36S. Haefliger OP and Jeckelmann N. Direct mass spectrometric analysis offlavors and fragrances in real applications using DART. Rapid Commu-nications in Mass Spectrometry 2007;21(8):1361-1366. IARC. Tobacco Habits Other than Smoking; Betel Quid and Areca-nutChewing;and Some Related Nitrosamines. IARC Monographs on theEvaluation of the Carcinogenic Risk of Chemicals to Humans, Vol. 37.IARC Press: Lyon, 1985. Kim HJ and Jang YP. Direct analysis of curcumin in turmeric by DART-MS.Phytochemical Analysis 2009; 20:372-377. ( Lei D,Chan CP, Wang YJ, Wang TM, Lin BR, Huang C, Lee JJ, Chen HM, JengJH and Chang MC. Antioxidative and antiplatelet effects of aqueous inflorescence P iper b etle extract. Journal of Agricultural and Food Chemistry 2003; 5 1:2083-2088. ) Madhusudanan KP, Banerjee S, Suman PS. Khanuja SPS and Chatto-padhay SK. Analysis of hairy root culture of Rauvolfia serpentina usingdirect analysis in real-time mass spectrometric technique. BiomedicalChromatography 2008;22:596-600. ( Manigauha A, Patel S , Ali H, Chandy A and Maheshwari U. S tudy of t he effect of phytochemical constituents of Piper be t le leaves extracts on liver disorders by in vitro model. Journal of Pharmacy Research2009; 2(3):353-356. ) Mohottalage S, Tabacchi R and Guerin PM. Components from Sri LankanPiper betle L. leaf oil and their analogues showing toxicity against the housefly, Musca domestica. Flavour and Fragrance Journal 2009;22(2):130-138. Moore SR, Johnson NW, Pierce AM and Wilson DE. The epidemiology ofmouth cancer: a review of global incidence. Oral Diseases 2000; 6:65-74. Nadkarni KM. Indian Materia Medica, 3rd edn. Popular Prakashan:Bombay, 1976;960-964. Rawat AKS, Shome U and Balasubrahmanyam VR. Analysis of the VolatileConstituents of Piper betle L. Cultivars - aChemosystematic Approach,Leeuwenberg AJM (compiler), OAI, 1987;104-108. Rawat AKS, Tripathi RD, Khan AJ and Balasubrahmanyam VR. Essential oilcomponents as markers for identification of Piper betle L. cultivars,Biochemical Systematics and Ecology 1989; 17(1): 35-38. Rimando AM, Han BH, Park JH and Cantoria MC. Studies on the constitu-ents of Philippine Piper betle Linne leaves. Archives of PharmacologicalResearch 1986;9(2): 93-97. Sankaranarayanan R. Oral cancer in India: An epidemiological and clinicalreview.Oral Surgery, Oral Medicine, Oral Pathology 1990; 69:325-330. Sarkar D, Saha P, Gamre S, Bhattacharjee S, Hariharan C, Ganguly S, Sen R,Mandal G, Chattopadhyay S, Majumdar S and Chatterjee M. Anti-inflammatory effect of allylpyrocatechol in LPS-induced macroph-ages is mediated by suppression of iNOS and COX-2 via the NF-KBpathway.International Immunopharmacology 2008; 8: 1264-1271. Sarkar M, Gangopadhyay P, Basak B, Chakraborty K, Banerji J, Adhikary Pand Chatterjee A. The reversible antifertility effect of Piper betle Linn.on Swiss albino male mice. Contraception 2000; 62:271-274. Sawangjaroen N, Phongpaichit S, Subhadhirasakul S, Visutthi M, SrisuwanN and Thammapalerd N. Anti-amoebic activity of some medicinalplants used by AIDS patients in southern Thailand. ParasitologyResearch 2006;98:588-592. Shitut S, Pandit V and Mehta BK. The antimicrobial efficiency of Piper betleLinn. leaf (stalk) against human pathogenic bacteria and phytopatho-genic fungi. Central European Journal of Public Health 1999; 7: 137-139. Trakranrungsie N, Chatchawanchonteera A and Khunkitti W. Ethnoveteri-nary study for antidermatophytic activity of Piper betle, Alpiniagalanga and Allium ascalonicum extracts in vitro. Research in VeterinaryScience 2008;84:80-84. Verma A, Kumar N and Ranade SA. Genetic diversity amongst landraces ofa dioecious vegetatively propagated plant, betelvine (Piper betle L.).Journal of Biosciences 2004;29(3):319-328. Warrier PK, Nambiar VPK and Ramankutty C(eds). Indian Medicinal Plants:a Compendium of 500 Species,Arya Vaidya Sala, Kottakal, Kerala.OrientLongman: India, 1995. ( Wirotesangthong M , I n agaki N , Tanaka H , T h anakijcharoenpath W and N agai H . I n hibitory e f fects of Piper betle on p r oduction of allergic m ediators by bone marrow-derived mast cells an d lun g epithelial cells. International Immunopharmacology 2008;8:453-457. ) Biomed. Chromatogr. opyright ◎ John Wiley & Sons, Ltd. View this article online at wileyonlinelibrary.comCopyright ◎ John Wiley & Sons, Ltd.Biomed. Chromatogr.
确定



还剩2页未读,是否继续阅读?
华质泰科生物技术(北京)有限公司为您提供《蒌叶栽培品种中形态差异检测方案(液质联用仪)》,该方案主要用于林产品中植物生理检测,参考标准--,《蒌叶栽培品种中形态差异检测方案(液质联用仪)》用到的仪器有
相关方案
更多








