
On-line measurement of dissolved O2 in shake-flasks was realized via immobilized sensor spots containing a fluorophore with an O2 dependent luminescent decay time. An unaffected sensor signal during 80 autoclaving cycles suggests multi-usage of sensor equipped shake-flasks. The sensor had a response time of 6 s. Quantification of gas-liquid mass transfer revealed maximum kLa values of 150 h−1, from which maximum O2 transfer capacity of 33 mM h−1 was calculated. Liquid volume and shaking frequency have a strong influence on kLa. Exemplified by cultivations of Corynebacterium glutamicum the importance of shaking rate for O2 supply of bacterial cultures
is shown. Sampling of microbial cultures with intermittent shaking of a few minutes can cause O2 limitation. Based on the results of this work a simple and straightforward tool is now available for accurate O2 sensing in shake-flasks, which are widely used in microbial cultivations.
方案详情

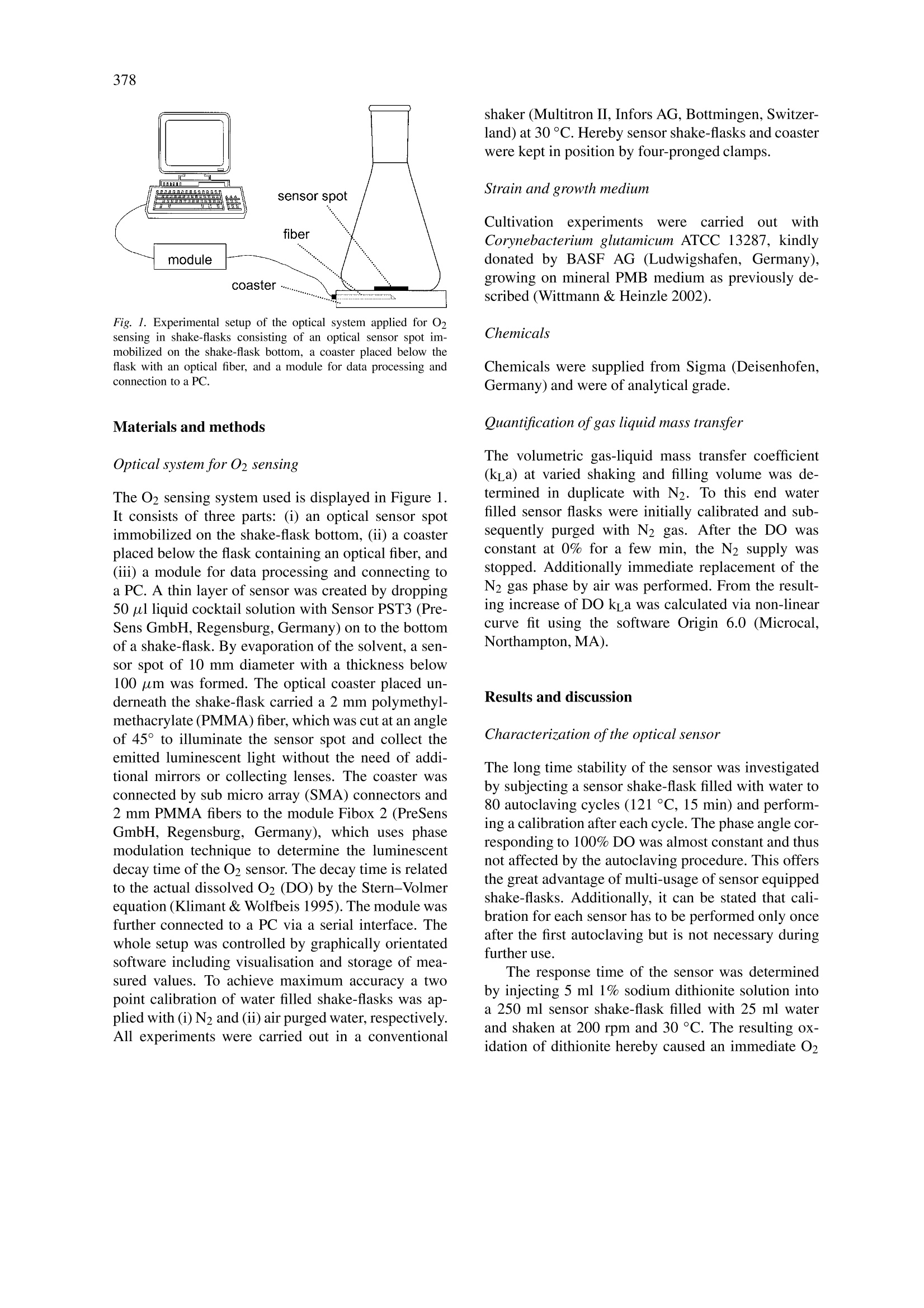
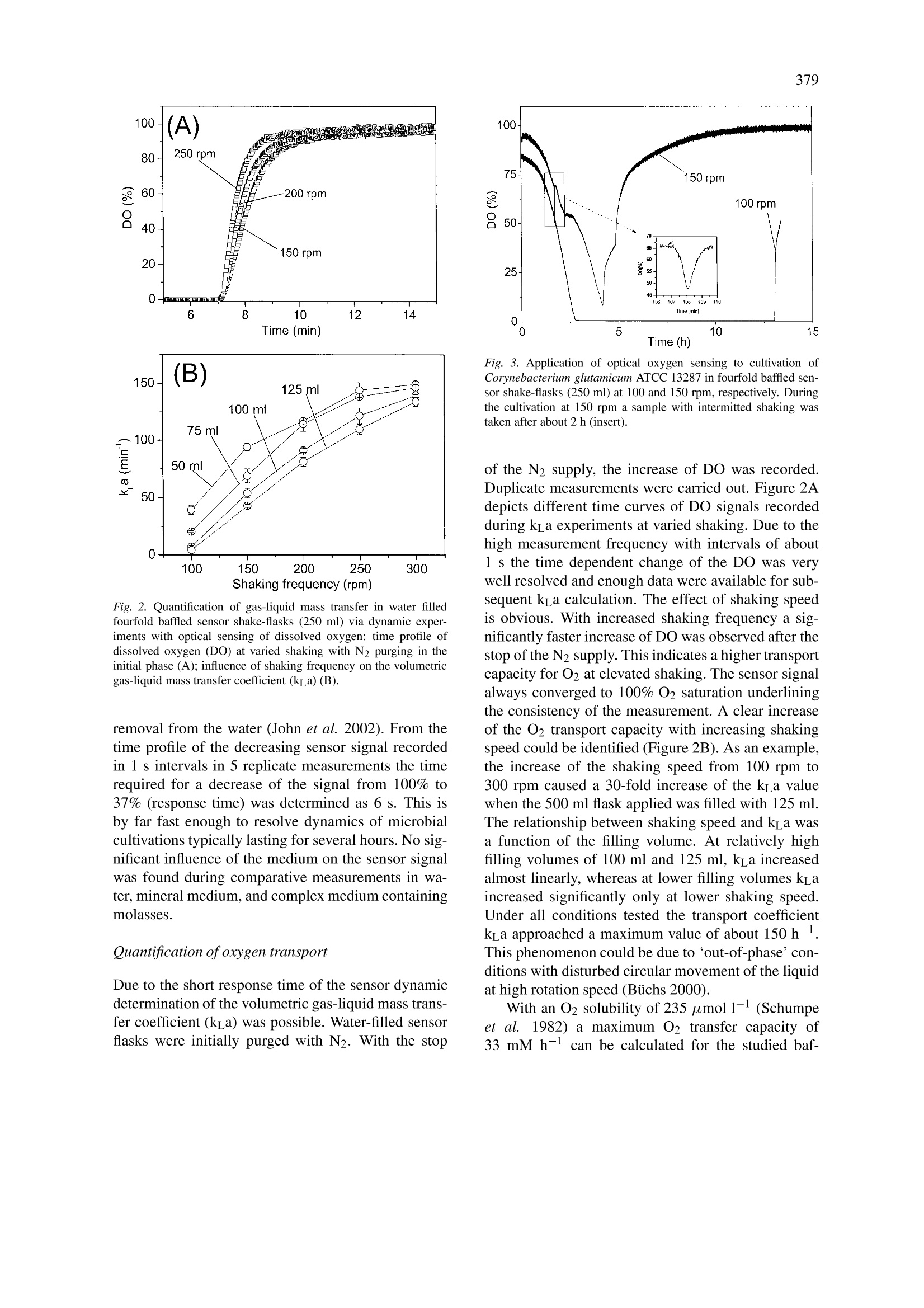
Biotechnology Letters 25: 377-380,2003.377@ 2003 Kluwer Academic Publishers. Printed in the Netherlands. 378 Characterization and application of an optical sensor for quantification ofdissolved O2 in shake-flasks Christoph Wittmann1,*, Hyung Min Kiml, Gernot John?& Elmar Heinzlel Biochemical Engineering, Saarland University, Am Stadtwald, 66123 Saarbriicken, Germany PreSens GmbH, 93053 Regensburg, Germany *Author for correspondence (Fax: +49-681-302-4572; E-mail: c.wittmann@mx.uni-saarland.de) Received 21 November 2002; Revisions requested 29 November 2002; Revisions received 24 December 2002; Accepted 24 December 2002 Key words: Corynebacterium glutamicum, gas-liquid mass transfer, optical sensor, oxygen, shake-flask Abstract On-line measurement of dissolved O2 in shake-flasks was realized via immobilized sensor spots containing afluorophore with an O2-dependent luminescent decay time. An unaffected sensor signal during 80 autoclavingcycles suggests multi-usage of sensor equipped shake-flasks. The sensor had a response time of 6 s. Quantificationof gas-liquid mass transfer revealed maximum k_a values of 150 h-l, from which maximum O2 transfer capacityof 33 mM h- was calculated. Liquid volume and shaking frequency have a strong influence on k a. Exemplifiedby cultivations of Corynebacterium glutamicum the importance of shaking rate for O2 supply of bacterial culturesis shown. Sampling of microbial cultures with intermittent shaking of a few minutes can cause O2 limitation.Based on the results of this work a simple and straightforward tool is now available for accurate O2 sensing inshake-flasks, which are widely used in microbial cultivations. Introduction O2 supply is one of the major issues in the cultiva-tion of aerobic organisms. For shake-flask cultureswhich are widely applied in academic and indus-trial research (Biichs 2000) a sufficient O2 supply isusually assumed, despite adequate methods for realmonitoring of dissolved O2 are missing (Tolosa et al.2002). Techniques for on-line O2 sensing in shake-flasks are therefore highly desired. Approaches withO2 probes have disadvantages such as a change of ac-tual flow conditions due to the insertion of the probe(Tunac 1989). A promising approach for on-line O2sensing is provided by fluorogenic compounds quan-titatively related to O2 concentration via quenching orluminescent decay time (Klimant & Wolfbeis 1995).Sensors based on this principle have been successfullyapplied to dissolved O2 measurement in bacterial cul-tivations in microtiter plates (John et al. 2002). Tolosaet al. (2002) recently applied optical O2 sensing toshake-flasks by attaching thin sensor layers to theflask bottom. Despite their approach seems very valu- able for shake-flask cultures, response time or longtime stability, crucial to evaluate the quality and ap-plicability of optical O2 sensing, were not discussed.Additionally the used sensor exhibited relatively highinaccuracy at higher DO values and increase of sig-nal noise after single autoclaving. The present workdescribes optical sensing for dissolved O2 quantifi-cation in shake-flasks, including (i) a thorough val-idation of the used sensor with respect to practicalapplication in cultivation experiments,(ii) gas-liquidmass transfer investigations under variation of shak-ing speed and filling volume and (iii) application tocultivations exemplified for Corynebacterium glutam-icum, an industrially relevant amino acid producingbacterium. Fig. 1. Experimental setup of the optical system applied for O2sensing in shake-flasks consisting of an optical sensor spot im-mobilized on the shake-flask bottom, a coaster placed below theflask with an optical fiber, and a module for data processing andconnection to a PC. Materials and methods Optical system for O2 sensing The O2 sensing system used is displayed in Figure 1It consists of three parts: (i) an optical sensor spotimmobilized on the shake-flask bottom, (ii) a coasterplaced below the flask containing an optical fiber, and(iii) a module for data processing and connecting toa PC. A thin layer of sensor was created by dropping50 ul liquid cocktail solution with Sensor PST3 (Pre-Sens GmbH, Regensburg,Germany) on to the bottomof a shake-flask. By evaporation of the solvent, a sen-sor spot of 10 mm diameter with a thickness below100 um was formed. The optical coaster placed un-derneath the shake-flask carried a 2 mm polymethyl-methacrylate (PMMA) fiber, which was cut at an angleof 45° to illuminate the sensor spot and collect theemitted luminescent light without the need of addi-tional mirrors or collecting lenses. The coaster wasconnected by sub micro array (SMA) connectors and2 mm PMMA fibers to the module Fibox 2 (PreSensGmbH, Regensburg, Germany), which uses phasemodulation technique to determine the luminescentdecay time of the O2 sensor. The decay time is relatedto the actual dissolved O2 (DO) by the Stern-Volmerequation (Klimant & Wolfbeis 1995). The module wasfurther connected to a PC via a serial interface. Thewhole setup was controlled by graphically orientatedsoftware including visualisation and storage of mea-sured values. To achieve maximum accuracy a twopoint calibration of water filled shake-flasks was ap-plied with (i) N2 and (ii) air purged water, respectively.All experiments were carried out in a conventional shaker (Multitron II, Infors AG, Bottmingen, Switzer-land) at 30 °C. Hereby sensor shake-flasks and coasterwere kept in position by four-pronged clamps. Strain and growth medium Cultivationn experimentsWereecarriedoutwithCorynebacterium glutamicum ATCC 13287, kindlydonated by BASF AG (Ludwigshafen, Germany),growing on mineral PMB medium as previously de-scribed (Wittmann & Heinzle 2002). Chemicals Chemicals were supplied from Sigma (Deisenhofen,Germany) and were of analytical grade. Quantification of gas liquid mass transfer The volumetric gas-liquid mass transfer coefficient(kLa) at varied shaking and filling volume was de-termined in duplicate with N2. To this end waterfilled sensor flasks were initially calibrated and sub-sequently purged with N2 gas. After the DO wasconstant at 0% for a few min, the N2 supply wasstopped. Additionally immediate replacement of theN2 gas phase by air was performed. From the result-ing increase of DO kLa was calculated via non-linearcurve fit using the software Origin 6.0 (Microcal,Northampton, MA). Results and discussion Characterization ofthe optical sensor The long time stability of the sensor was investigatedby subjecting a sensor shake-flask filled with water to80 autoclaving cycles (121°C, 15 min) and perform-ing a calibration after each cycle. The phase angle cor-responding to 100% DO was almost constant and thusnot affected by the autoclaving procedure. This offersthe great advantage of multi-usage of sensor equippedshake-flasks. Additionally, it can be stated that cali-bration for each sensor has to be performed only onceafter the first autoclaving but is not necessary duringfurther use. . The response time of the sensor was determinedby injecting 5 ml 1% sodium dithionite solution intoa 250 ml sensor shake-flask filled with 25 ml waterand shaken at 200 rpm and 30°C. The resulting ox-idation of dithionite hereby caused an immediate O2 Fig. 2. Quantification of gas-liquid mass transfer in water filledfourfold baffled sensor shake-flasks (250 ml) via dynamic exper-iments with optical sensing of dissolved oxygen: time profile ofdissolved oxygen (DO) at varied shaking with N2 purging in theinitial phase (A); influence of shaking frequency on the volumetricgas-liquid mass transfer coefficient (kLa) (B). removal from the water (John et al. 2002). From thetime profile of the decreasing sensor signal recordedin 1 s intervals in 5 replicate measurements the timerequired for a decrease of the signal from 100% to37% (response time) was determined as 6 s. This isby far fast enough to resolve dynamics of microbialcultivations typically lasting for several hours. No sig-nificant influence of the medium on the sensor signalwas found during comparative measurements in wa-ter, mineral medium, and complex medium containingmolasses. Quantification ofoxygen transport Due to the short response time of the sensor dynamicdetermination of the volumetric gas-liquid mass trans-fer coefficient (kLa) was possible. Water-filled sensorflasks were initially purged with N2. With the stop Fig. 3. Application of optical oxygen sensing to cultivation ofCorynebacterium glutamicum ATCC 13287 in fourfold baffled sen-sor shake-flasks (250 ml) at 100 and 150 rpm, respectively. Duringthe cultivation at 150 rpm a sample with intermitted shaking wastaken after about 2h (insert). of the N2 supply, the increase of DO was recorded.Duplicate measurements were carried out. Figure 2Adepicts different time curves of DO signals recordedduring kLa experiments at varied shaking. Due to thehigh measurement frequency with intervals of about1 s the time dependent change of the DO was verywell resolved and enough data were available for sub-sequent kLa calculation. The effect of shaking speedis obvious. With increased shaking frequency a sig-nificantly faster increase of DO was observed after thestop of the N2 supply. This indicates a higher transportcapacity for O2 at elevated shaking. The sensor signalalways converged to 100% O2 saturation underliningthe consistency of the measurement. A clear increaseof the O2 transport capacity with increasing shakingspeed could be identified (Figure 2B). As an example,the increase of the shaking speed from 100 rpm to300 rpm caused a 30-fold increase of the kLa valuewhen the 500 ml flask applied was filled with 125 ml.The relationship between shaking speed and kLa wasa function of the filling volume. At relatively highfilling volumes of 100 ml and 125 ml, kLa increasedalmost linearly, whereas at lower filling volumes kLaincreased significantly only at lower shaking speed.Under all conditions tested the transport coefficientkLa approached a maximum value of about 150 h-.This phenomenon could be due to‘out-of-phase'con-ditions with disturbed circular movement of the liquidat high rotation speed (Buichs 2000). With an O2 solubility of 235 umol 1-1 (Schumpeet al.1982) a maximum O2 transfer capacity of33 mM h-1 can be calculated for the studied baf- fled shake-flasks, which is in the range of moderatelystirred tank reactors (Bourne et al. 1992) or deep-well microplates (Duetz et al. 2000). For baffledshake-flasks, a low speed of rotation is generally rec-ommended to avoid moistening of the plug, whichmight cause reduced gas permeability through the plugand increased contamination risk (Biichs 2000). It be-comes clear from the present work that such a lowspeed of rotation is linked to significantly reduced O2transfer. The experiments at varied volume revealed aclear dependence of kL a on the applied liquid volume.With increasing volume the ka decreased, wherebythe clearest trend down to about 0.1 h- was found atslowest shaking of 100 rpm. The kLa decrease withincreasing volume became less pronounced at highershaking frequency. At 300 rpm k_a was rather sim-ilar at about 150 h-1 for all volumes tested. For allk a measurements a good agreement between the twoduplicates was achieved. Application to shake-flask cultivation The application of the O2 sensor to microbial culti-vations is demonstrated for Corynebacterium glutam-icum ATCC 13287. Figure 3 depicts the time courseof DO during cultivation of this strain at 100 rpm and150 rpm, respectively. Marked differences in the O2profiles were observed depending on shaking speed.At 150 rpm C. glutamicum was sufficiently suppliedwith O2 over the whole cultivation, whereas O2 wasdepleted within 2.5 h with reduced shaking.O2 limita-tion at 100 rpm lasted for about 10 h. The results showthe high impact of shaking rate on O2 supply of theculture. After 2 h cultivation at 150 rpm a samplingof the culture was carried out involving shaking inter-mittence for about 45 s. Due to this the DO droppedfrom 65% down to 45% (detailed in Figure 3). Ex-trapolating this curve clearly implies that O2 limitationof the culture would result after about 2 min.Such atime interval is typically exceeded during sampling,where the shake-flask is removed from the shaker,placed under a sterile hood for sampling and sub-sequently put back. This is even more important, ifseveral shake-flasks are sampled at the same time. Thisunderlines the high probability of O2 limitation duringsampling in shake-flask cultures. Insufficient O2 sup-ply during shake-flask cultivation or during sampling from a shake-flask might have a significant influenceon the obtained results due to the sensitivity of var-ious microorganisms towards O2 limitation (Hewittet al. 2000, Nakano & Hullet 1997). Therefore thedeveloped approach of on-line O2 sensing seems veryvaluable to remove much of uncertainty surroundingshake-flask experimentation. Acknowledgements We would like to thank Christian Huber and Chris-tian Krause for help during the experimental work andcritical and helpful comments on the manuscript. References ( Bourne JR, Zurita E, Heinzle E (1992) Bioreactor scale-up for t h eoxygen-sensitive culture Bacillus subtilis: the influence o f stirrershaft geometry. Biotechnol. Prog. 8: 580-582. ) Biichs J (2000) Introduction to advantages and problems of shakencultures. Biochem. Eng.J.7:91-98. ( Duetz WA, Ruedi L, Hermann R, O’Connor K, Buchs J, WitholtB ( 2000) Methods fo r intense a e ration, growth, storage, a n dreplication o f bacterial s trains in microtiter p lates. Appl. E nviron.Microbiol . 66:2641-2646. ) ( Hewitt CJ, Nebe-Von Caron G, Axelsson B, McFarlane CM, Nienow A W (2000) Studies related to the scale-up o f high- cell-density E. coli fed-batch fermentations using multiparameterflow c ytometry: effect o f a changing microenvironment wit h re- spect to glucose and dissolved oxygen concentration. Biotechnol. Bioeng. 70:381-390. ) ( John G T, Klimant I , Wittmann C, H e inzle E (2002) I n tegrated o p- tical sensing o f d issolved oxygen in mi c rotiter plates - A nov e l tool f or microbial cultivation. Biotechnol. Bi o eng., in press. ) ( K limant I , Wolfbeis OS ( 1 995) Oxygen-sensitive luminescent ma-terials based on s ilicone-soluble r uthenium d i imine complexes.Anal. Chem.67: 3 160-3166. ) ( Maier U, Buchs J ( 2 001) Characterization of the g as-liquid masstransfer in shaking bioreactors. Biochem . Eng. J.7: 99-106. ) Nakano MM, Hulett FM (1997) Adaptation of Bacillus subtilis to oxygen limitation. FEMS Microbiol. Lett. 157: 1-7. ( Schumpe A , Q u icker G , D e ckwer WD (1982) Gas solubilities inmicrobial culture media. Adv. Biochem. Eng. 24: 1- 3 8. ) ( Tolosa L, Kostov Y , Harms P, Rao G (2002) Non-invasive measure-ment of dissolved oxygen in s h ake-flasks. Biotechnol. B ioeng.80:594-597. ) ( Tunac J B ( 1 989) High-aeration ca p acity shake-flask sy s tem. J. Ferm. E ng. 68: 157-159. ) ( Wittmann C, H e inzle E (2002) G e nealogy pr o filing through strain improvement using metabolic network analysis -Metabolic flu x g enealogy of several generations of lysine producing corynebac- t eria. Appl. Environ. Microbiol. 68:5843-5859. )
确定


还剩2页未读,是否继续阅读?
科艺仪器有限公司为您提供《摇瓶中溶解氧检测方案(氧分析仪)》,该方案主要用于其他中含量分析检测,参考标准--,《摇瓶中溶解氧检测方案(氧分析仪)》用到的仪器有光学O2、pH 和 CO2测量仪
推荐专场















