方案详情
文
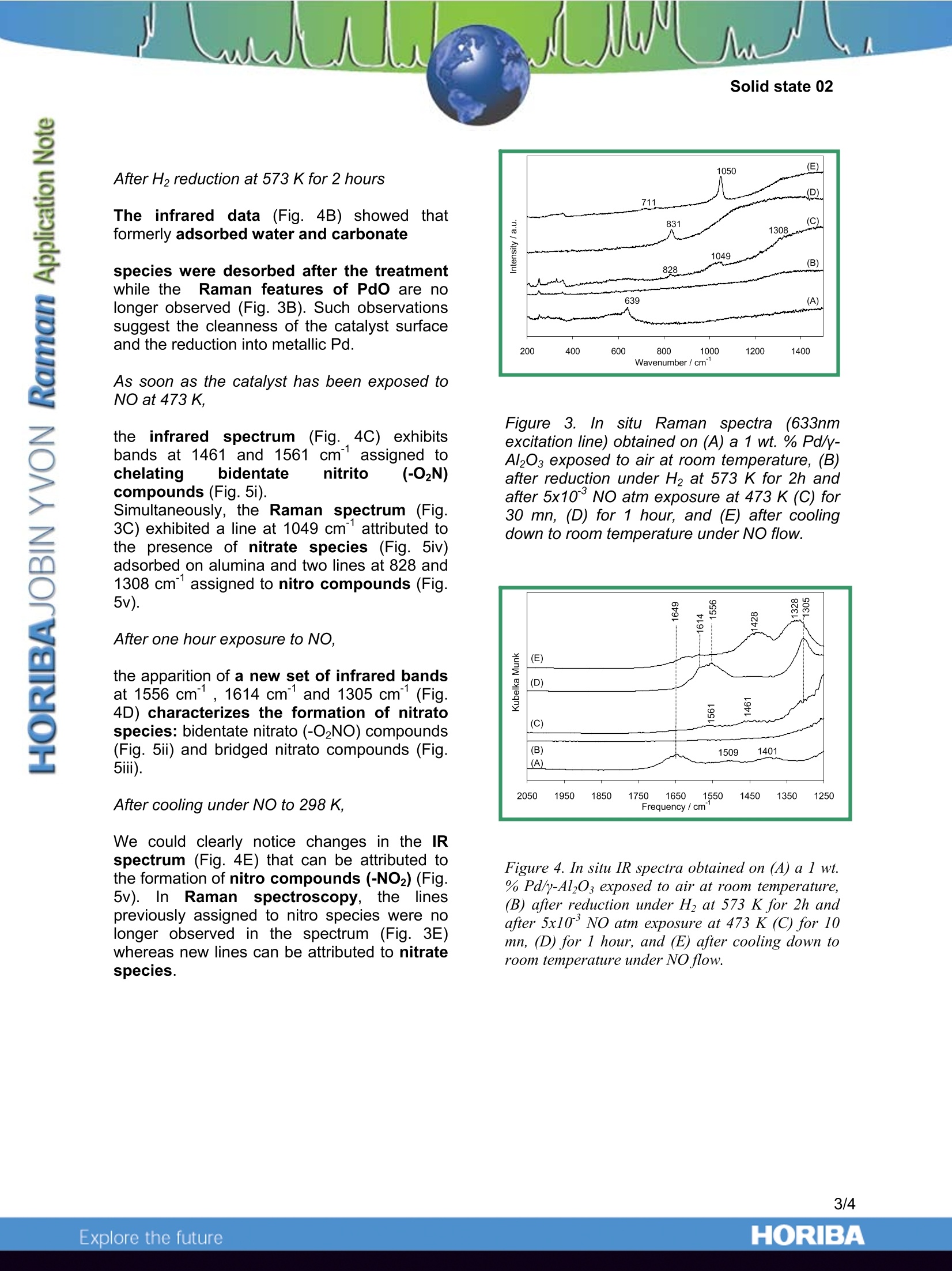
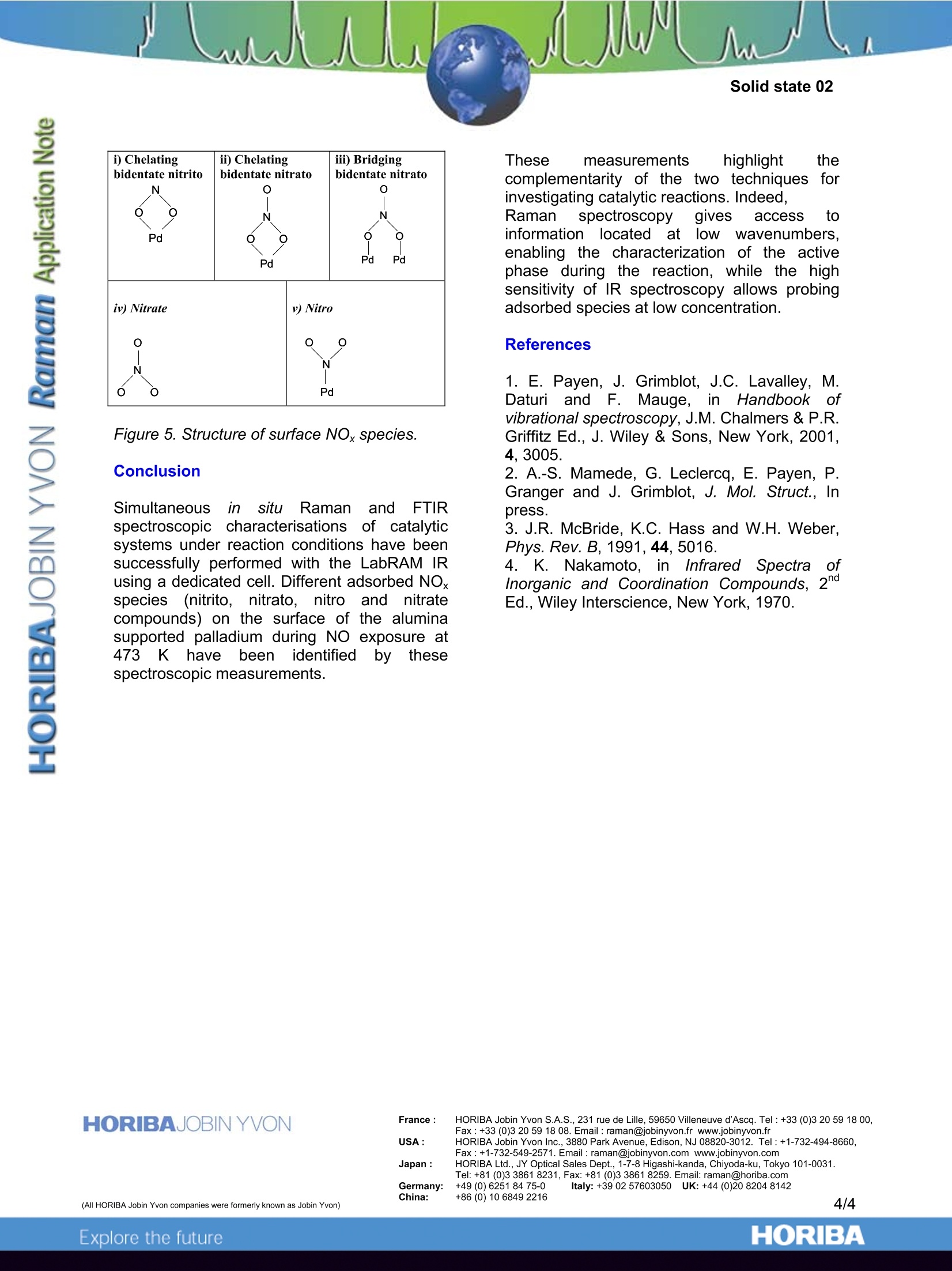
Simultaneous in situ Raman and FTIR spectroscopic characterisations of catalytic systems under reaction conditions have been successfully performed with the LabRAM IR using a dedicated cell. Different adsorbed NOx species (nitrito, nitrato, nitro and nitrate compounds) on the surface of the alumina supported palladium during NO exposure at 473 K have been identified by these spectroscopic measurements.
These measurements highlight the complementarity of the two techniques for investigating catalytic reactions. Indeed, Raman spectroscopy gives access to information located at low wavenumbers, enabling the characterization of the active phase during the reaction, while the high sensitivity of IR spectroscopy allows probing adsorbed species at low concentration.
方案详情

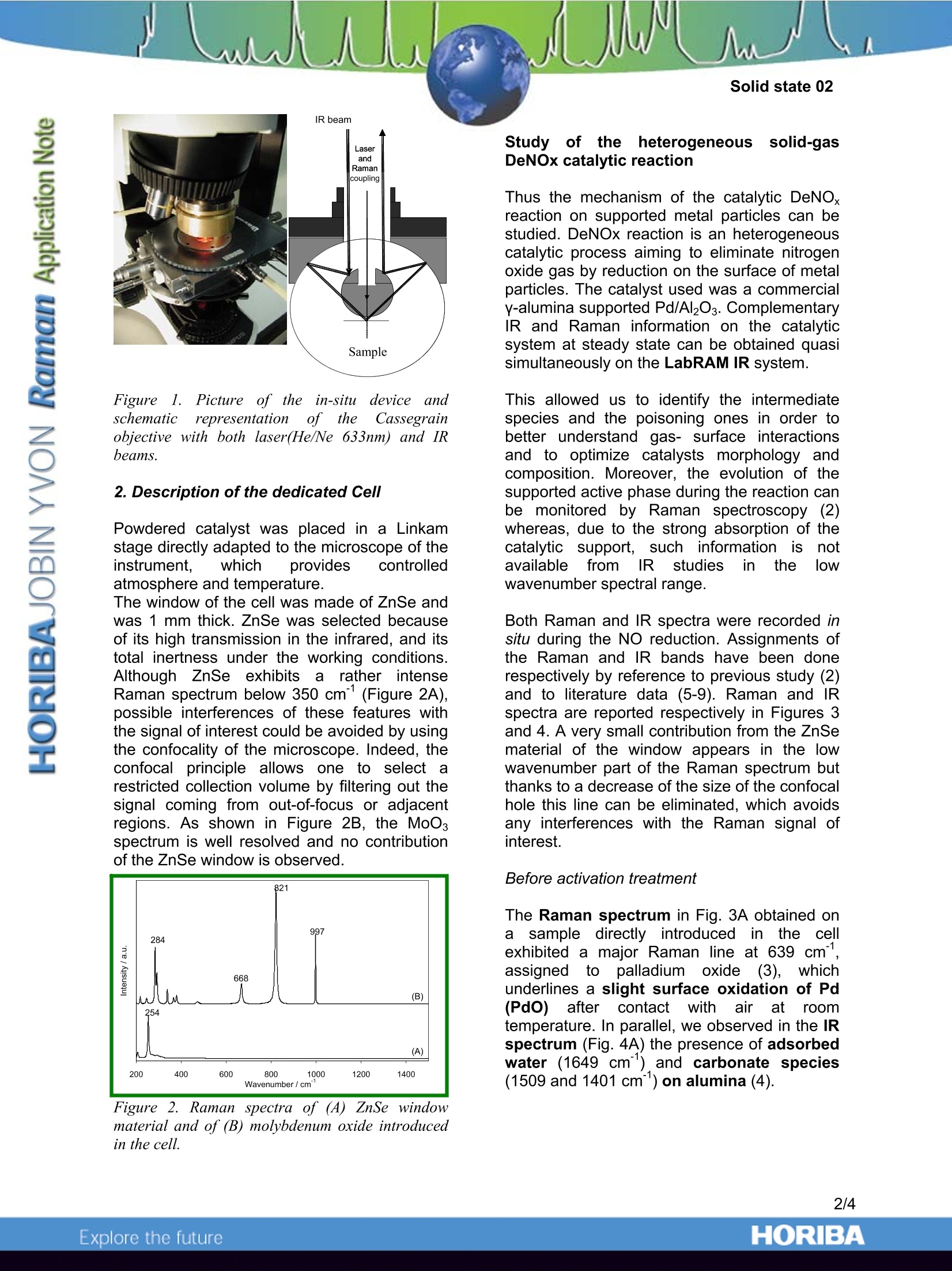
In Situ Characterisation of Heterogeneous Catalytic Reactions byRaman and IR Vibrational Spectroscopies on a single Instrument -HORIBA Jobin Yvon S.A.S., 59650 Villeneuve d'Ascq, France -SenslR, Danbury, CT 06810, USA -Laboratoire de Catalyse de Lille, UMR 8010, Bat. C3, USTL, 59655 Villeneuve d'Ascq, France Complementary IR and Ramanspectroscopies for the study of catalyticreactions A catalyst is a chemical species able toincrease the speed of a thermodynamicallyallowed reaction. If many reactions are likely tooccur, a well selected catalyst may selectivelyincrease the speed of a given reaction at theexpense of the other reactions. Catalysts aretherefore of great interest to the industry asthey allow to improve yields and to better tunethe properties of the final product. Vibrational spectroscopic characterisation cantake place all along the catalyst life from thecontrol of the synthesis of the precursor to thecontrol of the catalyst regeneration via theanalysis of the surface reaction mechanism. Tostudy catalytic reactivity, the strengths ofRaman include the higher spatial resolutionand the ability to probe the low wavenumberspectral range where most of the activephases (metal oxides or sulfides) have theircharacteristic lines. Thus Raman spectroscopyhas been applied in heterogeneous catalysisfor the characterization of bulk and supportedoxides. As a complementary technique, FTIRhas beenmoreewidelyusedfforthecharacterisation of the surface acidity of thecatalysts through the adsorption of probemolecules (1). TheeHFTIR/Raman1 combination microprobesystem, using SameSpottechnology enablessimultaneous examination of one sample areaby both techniques. A single microscope issimultaneously coupled to a Ramanspectrograph and an infrared interferometer.While the spatial resolution is quite differentbecause of the diffraction limit imposed by thewavelength of the probing radiation, the laserand IR beams coincide at the sample, thusproviding information on essentially the samematerial area. Taking advantage of the different informationavailable from the two techniques, this newtechnology enables the characterisation of on-going heterogeneous catalytic processes byusing a dedicated cell directly fitted to themicroscope stage. The ability to monitor thevery same reaction via both techniques in aquasi-simultaneous approach provides full andcomplementary vibrational information aboutthe mechanisms involved at the surface of thecatalyst during a catalytic reaction. This new technological improvement enablesus, as an example of application, to follow boththe modification of the molecular structure ofthe active phase by Raman spectroscopy witha 633nm laser excitation line and the nature ofthe differentSsurface adsorbedl reactionintermediates by IR spectroscopy during thereaction of NO decomposition at 473 K on analumina supported palladium. Simultaneous IR and Raman study of acatalytic reaction: Experimental 1. In situ Raman andFTIR quasisimultaneous spectroscopic measurements Raman and FTIR spectroscopicmeasurements were performed on a LabRAMIR spectrometer.. This instrument is acombined dispersive Raman andFT-IRmicroscope. An 2all-reflectingobjective(Cassegrain/Schwarzschild type) as shown inFig. 1 enables both Raman and FTIR spectrato be acquired. Indeed, this optical system hasbeen specifically designed and optimised toprovide an additional unique functionality to theLabRAM IR : the collection of Raman and IRspectra from the same spot without any needfor switching objectives. This greatly facilitatesthe operation and handling of the samples. Figurel. Picture of theein-situL(device andschematicrepresentationrof thecCassegrainobjective with both laser(He/Ne 633nm) and IRbeams. 2. Description of the dedicated Cell Powdered catalyst was placed in a Linkamstage directly adapted to the microscope of theinstrument, which provides controlledatmosphere and temperature. The window of the cell was made of ZnSe andwas 1 mm thick. ZnSe was selected becauseof its high transmission in the infrared, and itstotal inertness under the working conditions.Although ZnSe exhibitss alrather intenseRaman spectrum below 350 cm°(Figure 2A),possible interferences of these features withthe signal of interest could be avoided by usingthe confocality of the microscope. Indeed, theconfocal principle allows one to select arestricted collection volume by filtering out thesignal coming from out-of-focus or adjacentregions. As shown in Figure 2B, the MoOsspectrum is well resolved and no contributionof the ZnSe window is observed. Figure 2. Raman spectra of (A) ZnSe windowmaterial and of (B) molybdenum oxide introducedin the cell. Studyyof1the1heterogeneous solid-gasDeNOx catalytic reaction Thus the mechanism of the catalytic DeNOxreaction on supported metal particles can bestudied. DeNOx reaction is an heterogeneouscatalytic process aiming to eliminate nitrogenoxide gas by reduction on the surface of metalparticles. The catalyst used was a commercialy-alumina supported Pd/Al2O3. ComplementaryIR and Raman information on the catalyticsystem at steady state can be obtained quasisimultaneously on the LabRAM IR system. This allowed us to identify the intermediatespecies and the poisoning ones in order tobetter understand gas- surface interactionsand to optimize catalysts morphology andcomposition. Moreover,the evolution of thesupported active phase during the reaction canbe monitored by Raman spectroscopy (2)whereas, due to the strong absorption of thecatalytic support,, such information isS1notavailablee tfrom IR studies in the lowwavenumberspectral range. Both Raman and IR spectra were recorded insitu during the NO reduction. Assignments ofthe Raman and IR bands have been donerespectively by reference to previous study (2)and to literature data (5-9). Raman and IRspectra are reported respectively in Figures 3and 4. A very small contribution from the ZnSematerial of the window appears in the lowwavenumber part of the Raman spectrum butthanks to a decrease of the size of the confocalhole this line can be eliminated, which avoidsany interferences with the Raman signal ofinterest. Before activation treatment The Raman spectrum in Fig. 3A obtained onasample directly introduced in the cellexhibited a major Raman line at 639 cm,assigneddttoo palladium?oxide(3), whunderlines a slight surface oxidation of Pd(PdO)after contactwith airr atroomtemperature. In parallel, we observed in the IRspectrum (Fig. 4A) the presence of adsorbedwater (1649 cm") and carbonate species(1509 and 1401 cm) on alumina (4). After H2 reduction at 573 K for 2 hours The infrared data (Fig. 4B) showed thatformerly adsorbed water and carbonate species were desorbed after the treatmentwhile theeFRaman features of Pdo are nolonger observed (Fig. 3B). Such observationssuggest the cleanness of the catalyst surfaceand the reduction into metallic Pd. As soon as the catalyst has been exposed toNO at 473 K. the infrared spectrum (Fig. 4C) exhibitsbands at 1461 and 1561 cm assigned tochelating bidentate nitrito 1 (-O2N)compounds (Fig. 5i). Simultaneously, the Raman spectrum (Fig.3C) exhibited a line at 1049 cmattributed tothe presence of nitrate species (Fig. 5iv)adsorbed on alumina and two lines at 828 and1308 cm°assigned to nitro compounds (Fig.5v). After one hour exposure to NO, the apparition of a new set of infrared bandsat 1556 cm, 1614 cm and 1305 cm(Fig.4D) characterizes the formation of nitratospecies: bidentate nitrato (-O2NO) compounds(Fig. 5ii) and bridged nitrato compounds (Fig.5iii). After cooling under NO to 298 K, We could clearly notice changes in the IRspectrum (Fig. 4E) that can be attributed tothe formation of nitro compounds (-NO2) (Fig.5v). In Ramanspectroscopy,l, thee linespreviously assigned to nitro species were nolonger observed in the spectrum (Fig. 3E)whereas new lines can be attributed to nitratespecies. Figure 3. In situ Raman spectra((633nmexcitation line) obtained on (A) a 1 wt. % Pd/y-Al2O3 exposed to air at room temperature, (B)after reduction under H2 at 573 K for 2h andafter 5x10° NO atm exposure at 473 K (C) for30 mn, (D) for 1 hour, and (E) after coolingdown to room temperature under NO flow. Figure 4. In situ IR spectra obtained on(A) a l wt.% Pd/y-Al203 exposed to air at room temperature,(B) after reduction under H2 at 573 K for 2h andafter 5x10’ NO atm exposure at 473 K (C) for 10mn, (D) for 1 hour, and (E) after cooling down toroom temperature under NO flow. i) Chelatingbidentate nitritoNOPd ii) Chelatingbidentate nitratoO OPd iii) Bridgingbidentate nitratoOPd Pd iv) Nitrate o v) Nitro oN Pd Figure 5. Structure of surface NOx species. Conclusion Simultaneous in situuRaman andFTIRspectroscopic characterisations: of catalyticsystems under reaction conditions have beensuccessfully performed with the LabRAM IRusing a dedicated cell. Different adsorbed NOxspecies(nitrito, nitrato, nitroandnitratecompounds) on the surface of the aluminasupported palladium during NO exposure at3 K have t identified byythese 473 beenspectroscopic measurements. These measurements highlight thecomplementarity of the two techniques forinvestigating catalytic reactions. Indeed,Ramanspectroscopy’ gives access toinformationlocated at lowwavenumbers.enabling the characterization of the activephase during the reaction, while the highsensitivity of IR spectroscopy allows probingadsorbed species at low concentration. References 1. E. Payen, J. Grimblot, J.C. Lavalley, M.Daturi and F. IMauge,in Handbook ofvibrational spectroscopy, J.M. Chalmers & P.R.Griffitz Ed., J. Wiley & Sons, New York, 2001,4, 3005. 2. A.-S. Mamede, G. Leclercq, E. Payen, P.Granger and J. Grimblot, J. Mol. Struct., Inpress. 3. J.R. McBride, K.C. Hass and W.H. Weber,Phys. Rev. B, 1991, 44, 5016. 4. K. Nakamoto, in Infrared SpectraofInorganic and Coordination Compounds, 2Ed., Wiley Interscience, New York, 1970. ORIBAExplore the future ORIBAExplore the future Simultaneous in situ Raman and FTIR spectroscopic characterisations of catalytic systems under reaction conditions have been successfully performed with the LabRAM IR using a dedicated cell. Different adsorbed NOx species (nitrito, nitrato, nitro and nitrate compounds) on the surface of the alumina supported palladium during NO exposure at 473 K have been identified by these spectroscopic measurements.These measurements highlight the complementarity of the two techniques for investigating catalytic reactions. Indeed, Raman spectroscopy gives access to information located at low wavenumbers, enabling the characterization of the active phase during the reaction, while the high sensitivity of IR spectroscopy allows probing adsorbed species at low concentration.
确定

还剩2页未读,是否继续阅读?
HORIBA(中国)为您提供《ZnSe,脱硝反应中反应监控检测方案(激光拉曼光谱)》,该方案主要用于其他中反应监控检测,参考标准--,《ZnSe,脱硝反应中反应监控检测方案(激光拉曼光谱)》用到的仪器有
相关方案
更多
该厂商其他方案
更多









