方案详情
文
In the past few decades calcium sulfate based building products have become materials of choice for various interior construction purposes, The basis of gypsum technology is the ability of gypsum to transform into various calcium sulfates (hemihydrale, anhydrite) when being heated. When hemihydrate or anhydrite is mixed with water calcium sulfale dihydrate is formed again due to re-hydralion. During re-hydration of hemihydrate, the following exothermic reaction occurs:
方案详情

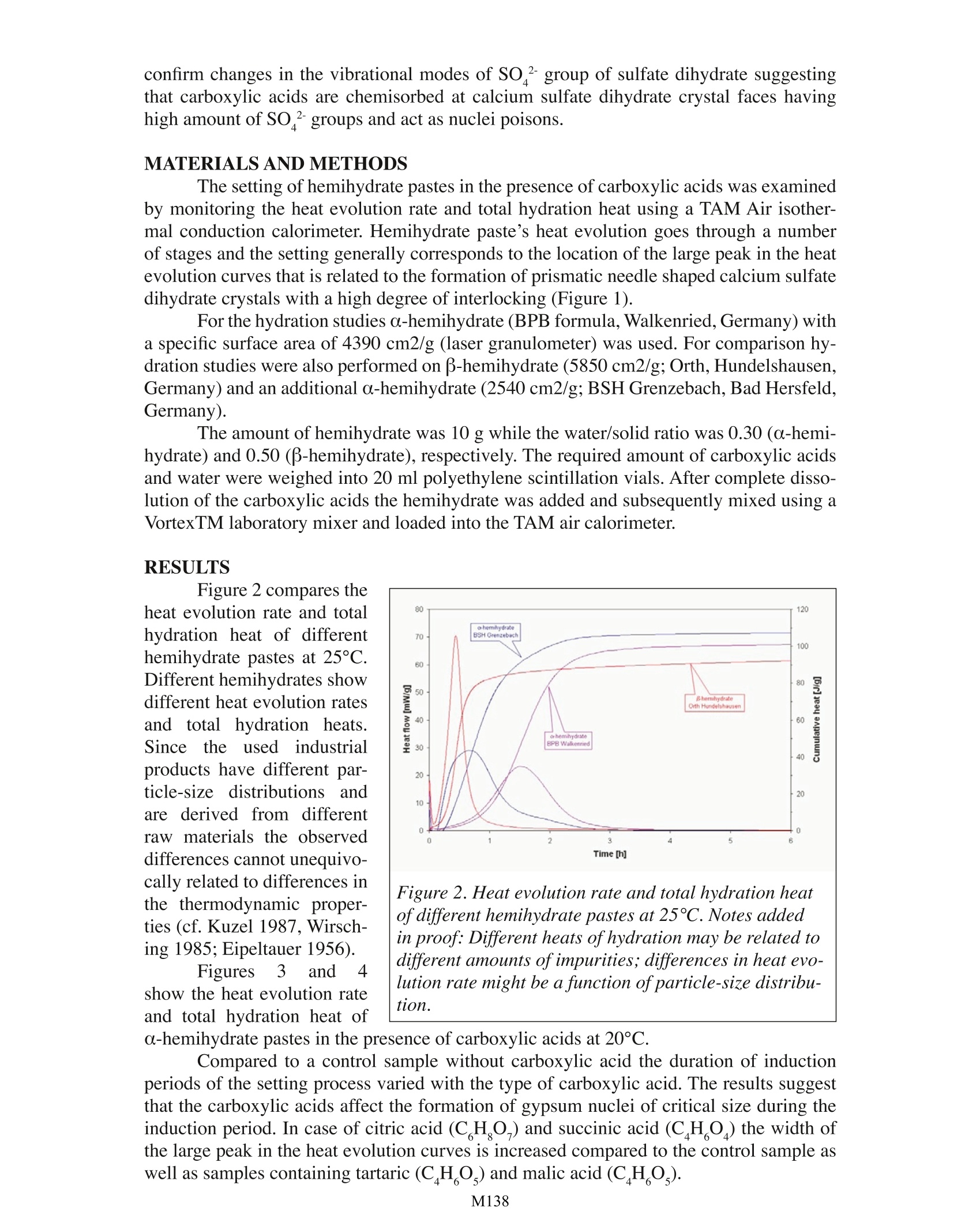
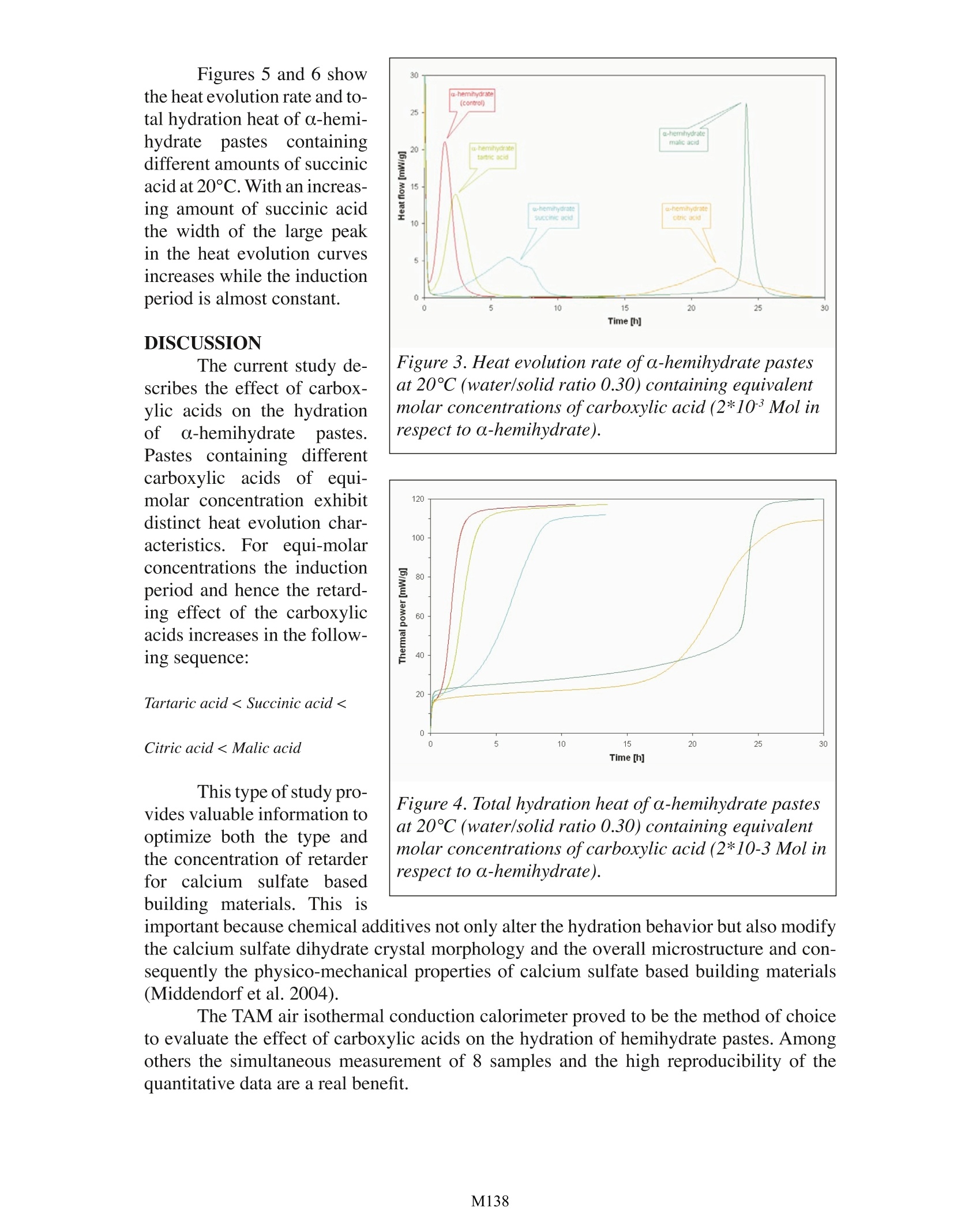
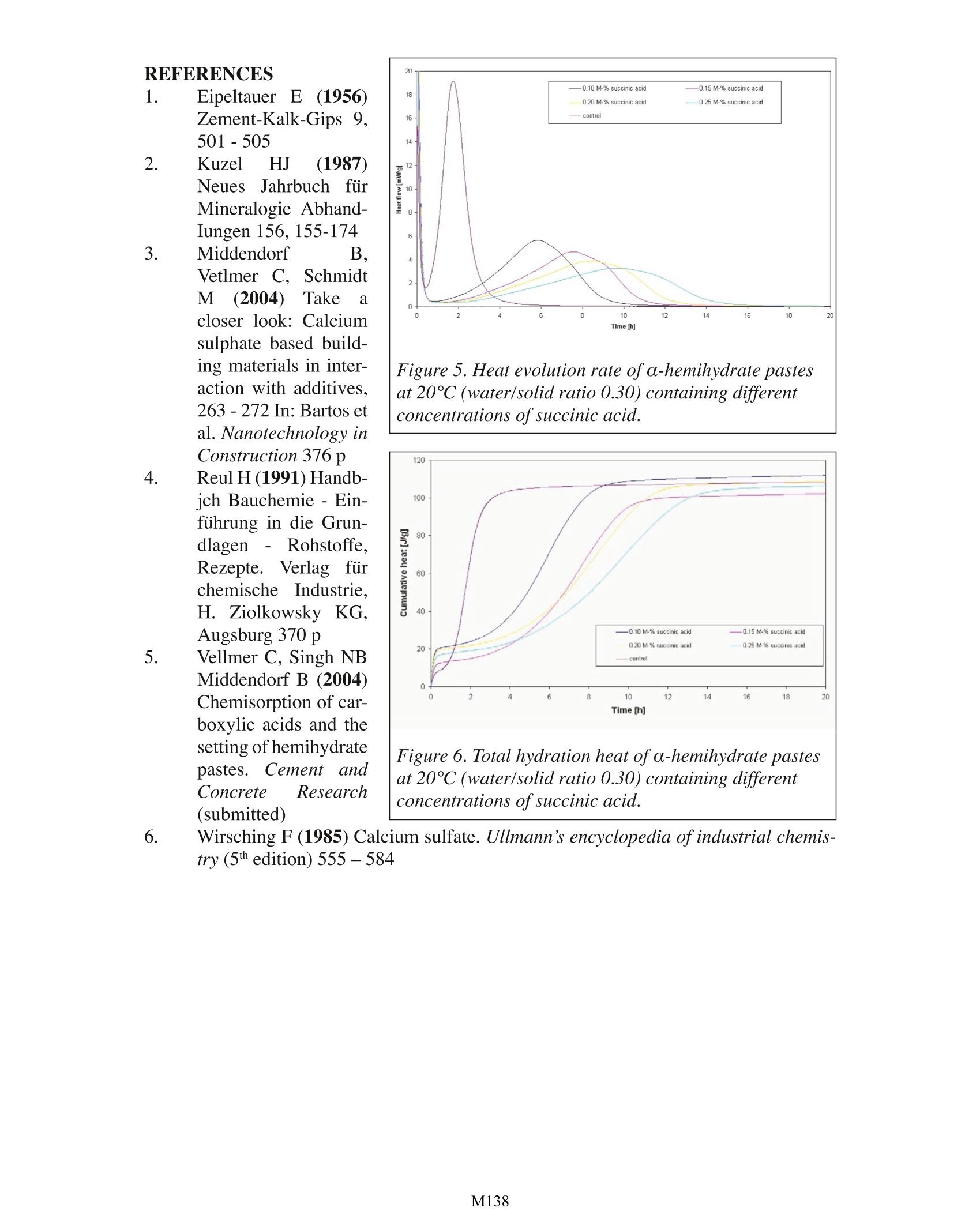
Effect of Carboxylic Acids on the Hydration ofCalcium Sulfate Hemihydrate Pastes Carsten VellmerUniversity of Kassel Department of Structural Materials Kassel, Germany TA Instruments, 109 Lukens Drive, New Castle, DE 19720, USA INTRODUCTION In the past few decades calcium sulfate based building products have become mate-rials of choice for various interior construction purposes, The basis of gypsum technologyis the ability of gypsum to transform into various calcium sulfates (hemihydrale, anhydrite)when being heated. When hemihydrate or anhydrite is mixed with water calcium sulfaledihydrate is formed again due to re-hydralion.During re-hydration of hemihydrate, the fol-lowing exothermic reaction occurs: CaSO 1/2H,O+3/2H,0=> CaSO 2H,0+ Heat Depending on the industrial production process, hemihydrate occurs in two differentforms (a- and B-hemihydrate). Autoclave processes result in well formed transparent crys-tals with sharp crystal outlines ( o-hemihydrate). Directly fired rotary kilns or indirectlyheated kettles produce flaky particles made up of small crystals (B-hemihydrate). The twodifferent forms vary in their application characteristics (Wirsching 1985). For technical application the setting ofcalcium sulfate based building products is accelerated((anhydrite) Orretarded (hemihydrate) bya variety of chemical addi-tives (Reul 1991). Widelyused retarders are carboxylicacids and their salts. Retard-ers may affect the rate ofdissolution of hemihydrate,change saturation or supersaturation, poison growinggypsum nuclei of criticalsize, slow down the growthof dihydrate crystals,,etc.However, Fourier TransformInfrared (FTIR) spcctroscopystudies (Vellmer et al. 2004) Figure 1. Scanning electron microscope (SEM) image ofhardened a-hemihydrate paste. confirm changes in the vibrational modes of SO’group of sulfate dihydrate suggestingthat carboxylic acids are chemisorbed at calcium sulfate dihydrate crystal faces havinghigh amount of SO ?groups and act as nuclei poisons. MATERIALS AND METHODS The setting of hemihydrate pastes in the presence of carboxylic acids was examinedby monitoring the heat evolution rate and total hydration heat using a TAM Air isother-mal conduction calorimeter. Hemihydrate paste’s heat evolution goes through a numberof stages and the setting generally corresponds to the location of the large peak in the heatevolution curves that is related to the formation of prismatic needle shaped calcium sulfatedihydrate crystals with a high degree of interlocking (Figure 1). For the hydration studies a-hemihydrate (BPB formula, Walkenried, Germany) witha specific surface area of 4390 cm2/g (laser granulometer) was used. For comparison hy-dration studies were also performed on β-hemihydrate (5850 cm2/g; Orth, Hundelshausen,Germany) and an additional o-hemihydrate (2540 cm2/g; BSH Grenzebach, Bad Hersfeld,Germany). The amount of hemihydrate was 10 g while the water/solid ratio was 0.30 (o-hemi-hydrate) and 0.50 (B-hemihydrate), respectively.The required amount of carboxylic acidsand water were weighed into 20 ml polyethylene scintillation vials. After complete disso-lution of the carboxylic acids the hemihydrate was added and subsequently mixed using aVortexTM laboratory mixer and loaded into the TAM air calorimeter. RESULTS Figure 2 compares theheat evolution rate and totalhydration heat of differenthemihydrate pastes at 25°C.Different hemihydrates showdifferent heat evolution ratesand total hydration heats.Since the used industrialproducts have different par-ticle-size distributions andare (derived from differentraw materials the observeddifferences cannot unequivo-cally related to differences inthe thermodynamic proper-ties (cf. Kuzel 1987,Wirsch-ing 1985; Eipeltauer 1956). Figures 3 and 4show the heat evolution rateand total hydration heat of Figure 2. Heat evolution rate and total hydration heatof different hemihydrate pastes at 25℃. Notes addedin proof: Different heats ofhydration may be related todifferent amounts ofimpurities; differences in heat evo-lution rate might be a function of particle-size distribu-tion. o-hemihydrate pastes in the presence of carboxylic acids at 20°℃. Compared to a control sample without carboxylic acid the duration of inductionperiods of the setting process varied with the type of carboxylic acid. The results suggestthat the carboxylic acids affect the formation of gypsum nuclei of critical size during theinduction period. In case of citric acid (C H O,) and succinic acid (CH ) the width ofthe large peak in the heat evolution curves is increased compared to the control sample aswell as samples containing tartaric (CH,O,) and malic acid (CH,O.). Figures 5 and 6 showthe heat evolution rate and to-tal hydration heat of o-hemi-hydrate pastes containingdifferent amounts of succinicacid at 20°C. With an increas-ing amount of succinic acidthe width of the large peakin the heat evolution curvesincreases while the inductionperiod is almost constant. DISCUSSION The current study de-scribes the effect of carbox-ylic acids on the hydrationof a-hemihydrate pastes.Pastes containing differentcarboxylic acids of equi-molar concentration exhibitdistinct heat evolution char-Cacteristics. For equi-molarconcentrations the inductionperiod and hence the retard-ing effect of the carboxylicacids increases in the follow-ing sequence: Tartaric acid
确定

还剩2页未读,是否继续阅读?
TA仪器为您提供《半水硫化钙中羧酸对其水化作用的影响检测方案(差示扫描量热)》,该方案主要用于基础有机原料中羧酸对其水化作用的影响检测,参考标准--,《半水硫化钙中羧酸对其水化作用的影响检测方案(差示扫描量热)》用到的仪器有NANO等温差示扫描量热仪
推荐专场
相关方案
更多
该厂商其他方案
更多
















