方案详情
文
德国飞驰的激光粒度仪A22内置超声波系统,能很好的分散带测的大密度样品,同时,可移动的测量池保证了有效检测通道的利用率,可获得更准确、重复性更好的数据,而且分辨率也很高。
方案详情

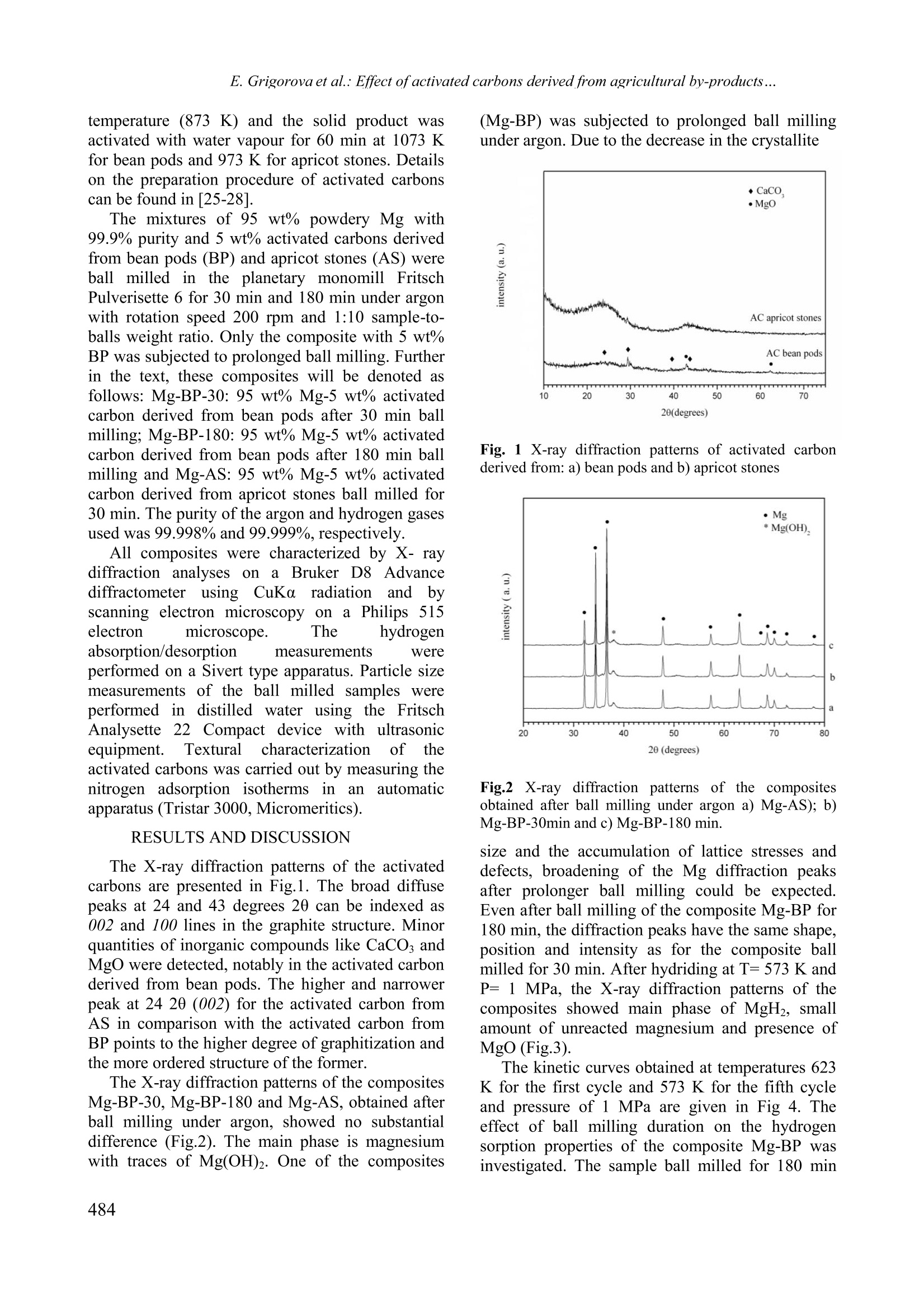
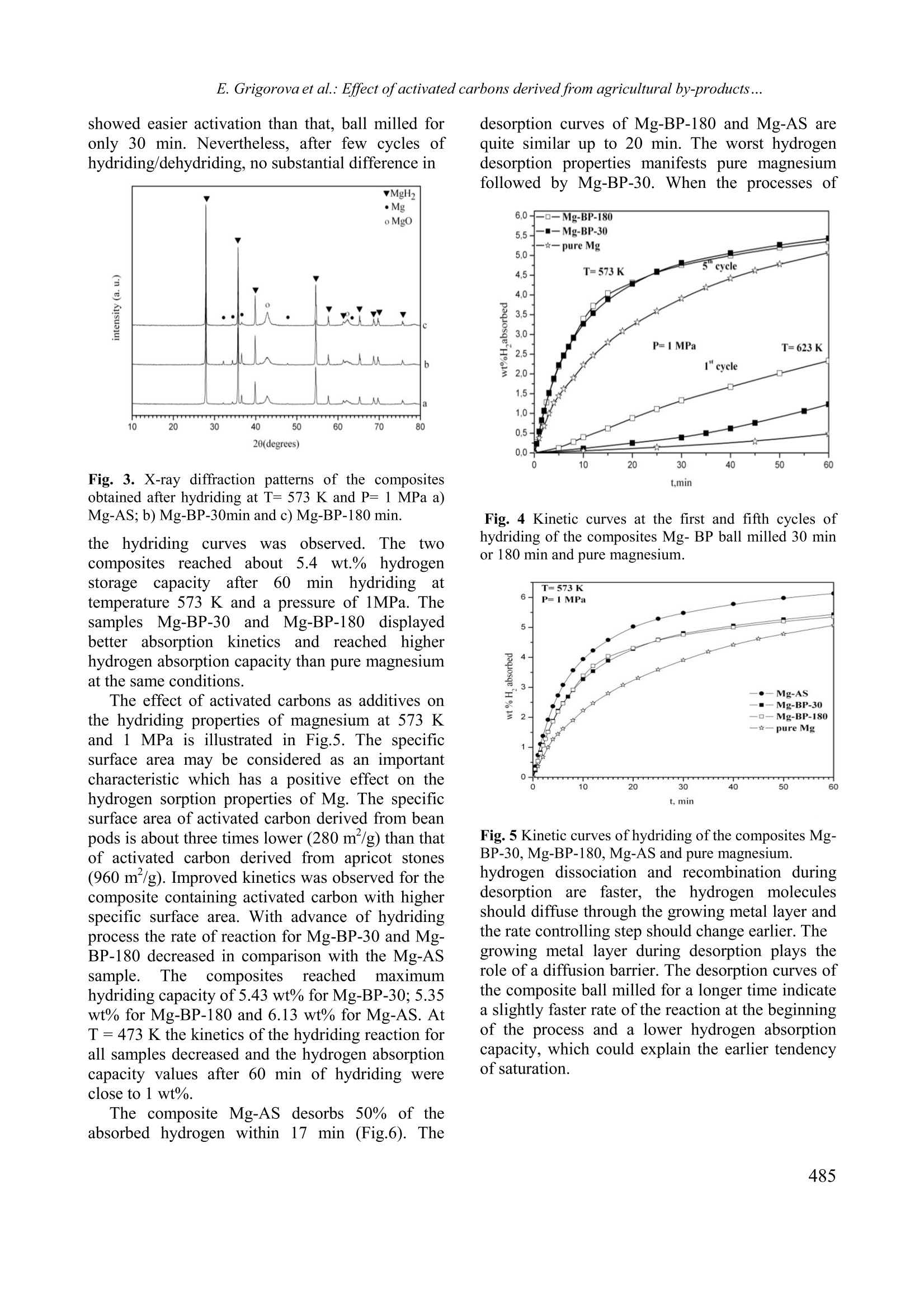
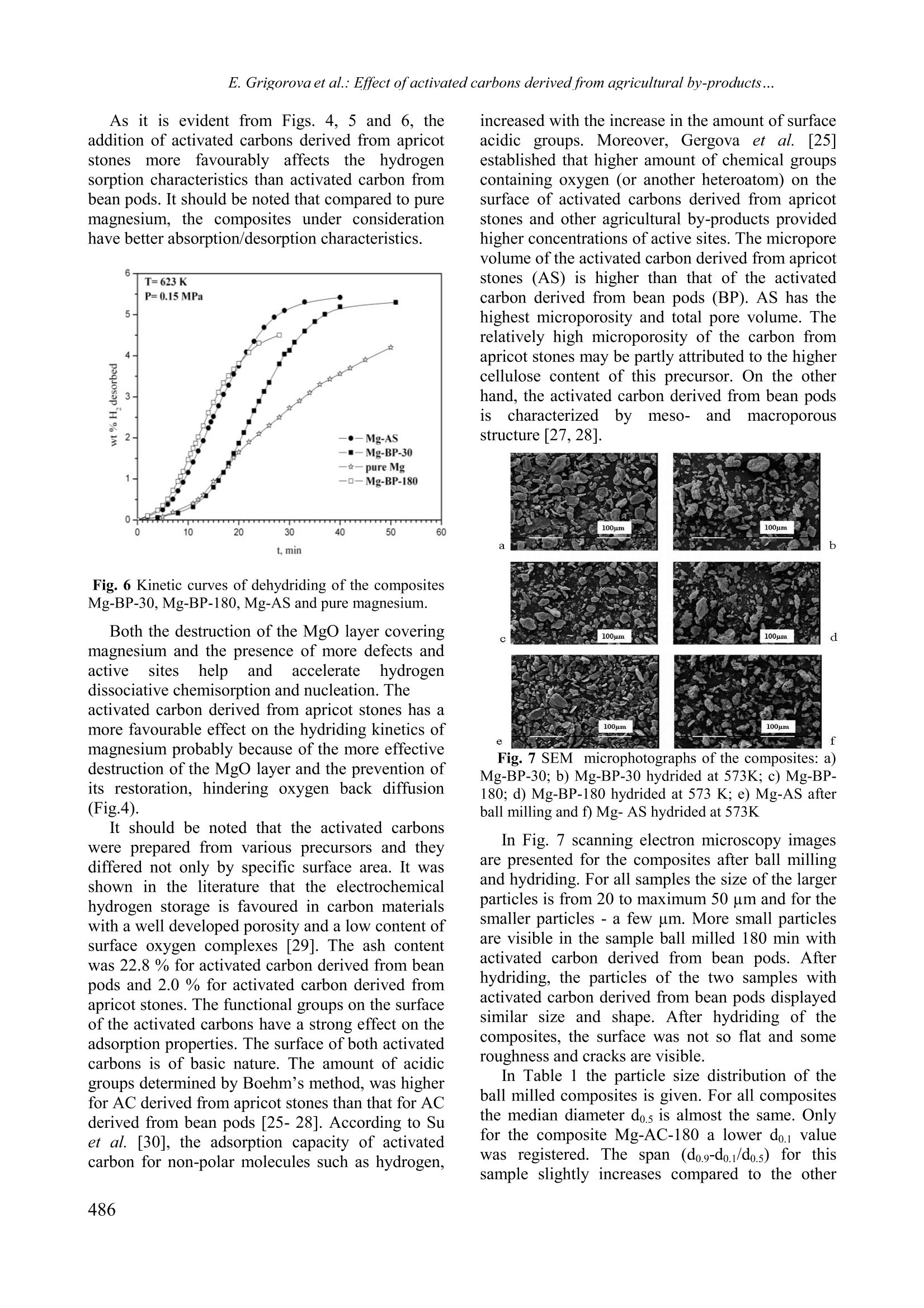
Bulgarian Chemical Communications, Volume 43, Number 4 (pp 483-488) 2011 E. Grigorova et al.: Effect ofactivated carbons derived from agricultural by-products... Effect of activated carbons derived from agricultural by-products on the hydrogenstorage properties of magnesium E. Grigorova , Ts. Mandzhukova", M. Khristov", P. Tzvetkov, B. Tsyntsarski Institute ofGeneral and Inorganic Chemistry,Bulgarian Academy of Sciences, bl.11, Acad. G. Bonchev str. 1113Sofia, Bulgaria Institute of Organic Chemistry, Bulgarian Academy ofSciences, bl.9, Acad. G. Bonchev str., 1113 Sofia, Bt iria Received February 15, 2011; Accepted April 4, 2011 The absorption-desorption characteristics towards hydrogen of the composites 95wt.% Mg-5wt.% activated carbonsderived from bean pods and apricot stones, obtained by ball milling in argon atmosphere were investigated. Hydridingof the composites was performed at temperatures 573 K and 473 K and pressure of 1 MPa and dehydriding - at T=623K and P =0.15 MPa. Absorption capacity values of 5.43 wt.% and 6.13 wt.% (at T=573 K and P=1 MPa) werereached for the composites containing activated carbons derived from bean pods and from apricot stones, respectively.The hydriding/dehydriding characteristics of the studied composites were compared with those of pure magnesium. Itwas established that the activated carbons derived from bean pods and apricot stones improved the hydrogen sorptionkinetics of magnesium. Keywords: hydrogen storage materials; metal hydrides; mechanochemical processing, gas-solid reactions. INTRODUCTION Magnesium iscconsideredas apromisingmaterial for hydrogensstorage due to itshightheoretical absorption capacityof ‘7.6 wt%.volumetric density, availability and low cost. Thelimitation of its practical application is due to twomain reasons: i) sluggish hydriding/dehydridingkinetics and ii) high temperatures of hydrogenabsorption and desorption. The mechanochemicaltreatment, along with the use of an appropriateadditive,could rresolve these drawbacks.Mechanical grinding in high-energy ball millsconsists of repetitive cold welding and fracturing.Its application for the preparation of hydrogenstorage materials leads to an increase in the specificsurface area and appearance of different defects,whichmodifyy thehydridingkofineticsof thecomposites. Several additivescor types tomagnesium based materials were used, such as: 3dmetals [1,2], metal oxides [3-7], intermetallic [8,9]and carbon containing compounds as: graphite [10-15,17,18,20-22,24], multi and single-walled carbonnanotubes [16-19, 24], carbon black [17,18, 23],nanodiamonds [23], amorphous carbon soot [23]and carbon nano-fibers [24]. Depending of thenature of the additive the mechanism of its catalyticeffect is different. Some aspects of the role of carbon containing additives on the hydrogen sorption properties ofmagnesium are not clarified in the literature. Thiswas discussed and well illustrated by V. Fuster etal.[201 Manyy authors agreed that: carboncontaininggadditivesimprovedthehydrogensorption properties of magnesium. Some of themhave proved that graphite protects magnesium fromoxidation and acts as a process controlling agentduring ball milling due to its lubricant properties.There are some controversial opinions about thecatalytic role of graphite on hydrogen sorptionproperties. As a conclusion it should be mentionedthat the effect of carbon containing additives tomagnesium is complex and not yet sufficientlyclarified. Activated carbons are often synthesized fromprecursors based on expensive and depleting fossilfuels. They can also be prepared from biomass oragricultural by-products with lower price. The se subject of tl presenttwork was toinvestigate the effect of activated carbons preparedfrom plant precursors as bean pods and apricotstones on the hydrogen sorption properties ofmagnesium. The hydriding/dehydridingcharacteristics of the studied compositesswerecompared with those of pure magnesium. EXPERIMENTAL Activated carbonsweree preparedby steampyrolysis from apricot stones and bean pods. Theraw material was heated up to the carbonization *To whom all correspondence should be sei C 2011 Bulgarian Academy of Sciences, Union of Chemists in Bulgaria temperature (873 K) and the solid product wasactivated with water vapour for 60 min at 1073 Kfor bean pods and 973 K for apricot stones. Detailson the preparation procedure of activated carbonscan be found in[25-281. The mixtures of 95 wt% powdery Mg with99.9% purity and 5 wt% activated carbons derivedfrom bean pods (BP) and apricot stones (AS) wereball milled in the planetary monomill FritschPulverisette 6 for 30 min and 180 min under argonwith rotation speed 200 rpm and 1:10 sample-to-balls weight ratio. Only the composite with 5 wt%BP was subjected to prolonged ball milling. Furtherin the text, these composites will be denoted asfollows: Mg-BP-30: 95 wt% Mg-5 wt% activatedcarbon derived from bean pods after 30 min ballmilling; Mg-BP-180: 95 wt%Mg-5wt% activatedcarbon derived from bean pods after 180 min ballmilling and Mg-AS: 95 wt% Mg-5 wt% activatedcarbon derived from apricot stones ball milled for30 min. The purity of the argon and hydrogen gasesused was 99.998% and 99.999%, respectively. All composites were characterized by X-raydiffraction analyses on a Bruker D8 Advancediffractometer usinggcCuKaradiationnaand bbyscanning electron microscopy on a Philips 515electron microscope. The hydrogenabsorption/desorption measurements wereperformed on a Sivert type apparatus. Particle sizemeasurements of the ball milled samples wereperformed iin(distilled water using the FritschAnalysette22 Compact device with ultrasonicequipment. Texturalcharacterizationnof theactivated carbons was carried out by measuring thenitrogen adsorption isotherms incanaautomaticapparatus (Tristar 3000, Micromeritics). RESULTS AND DISCUSSION The X-ray diffraction patterns of the activatedcarbons are presented in Fig.1. The broad diffusepeaks at 24 and 43 degrees 20 can be indexed as002 and 100 lines in the graphite structure. Minorquantities of inorganic compounds like CaCOs andMgO were detected, notably in the activated carbonderived from bean pods. The higher and narrowerpeak at 24 20 (002) for the activated carbon fromAS in comparison with the activated carbon fromBP points to the higher degree of graphitization andthe more ordered structure of the former. The X-ray diffraction patterns of the compositesMg-BP-30,Mg-BP-180 and Mg-AS, obtained afterball milling under argon, showed no substantialdifference (Fig.2). The main phase is magnesiumwith traces of Mg(OH)2. One of the composites (Mg-BP) was subjected to prolonged ball millingunder argon. Due to the decrease in the crystallite Fig. 1 X-ray diffraction patterns of activated carbonderived from: a) bean pods and b) apricot stones Fig.2 X-ray diffraction patterns of the compositesobtained after ball milling under argon a) Mg-AS); b)Mg-BP-30min and c)Mg-BP-180 min. size and the accumulation of lattice stresses anddefects, broadening of the Mg diffraction peaksafter prolonger ball milling could be expected.Even after ball milling of the composite Mg-BP for180 min, the diffraction peaks have the same shape,position and intensity as for the composite ballmilled for 30 min. After hydriding at T= 573 K andP= 1 MPa, the X-ray diffraction patterns of thecomposites showed main phase of MgH, smallamount of unreacted magnesium and presence ofMgO (Fig.3). The kinetic curves obtained at temperatures 623K for the first cycle and 573 K for the fifth cycleand pressure of 1 MPa are given in Fig 4. Theeffect of ball milling duration on the hydrogensorption properties of the composite Mg-BP wasinvestigated. The sample ball milled for 180 min showed easier activation than that, ball milled foronly 30 min. Nevertheless, after few cycles ofhydriding/dehydriding, no substantial difference in Fig. 3. X-ray diffraction patterns of the compositesobtained after hydriding at T= 573 K and P= 1 MPa a)Mg-AS;b)Mg-BP-30min and c) Mg-BP-180 min. thehydriding curveswasobserved.The twocomposites reached about55.4 wt.%hydrogenstorage capacity after 60 min hydridingattemperature 573 K and a pressure of 1MPa. Thesamples Mg-BP-30 and Mg-BP-180 displayedbetter absorption kinetics and reachehded higherhydrogen absorption capacity than pure magnesiumat the same conditions. The effect of activated carbons as additives onthe hydriding properties of magnesium at 573 Kand 1 MPa is illustrated in Fig.5. The specificsurface area may be considered as an importantcharacteristic which has a positive effect on thehydrogen sorption properties of Mg. The specificsurface area of activated carbon derived from beanpods is about three times lower (280 m /g) than thatof activated carbon derived from apricot stones(960 m /g). Improved kinetics was observed for thecomposite containing activated carbon with higherspecific surface area. With advance of hydridingprocess the rate of reactio?p-3n for Mg-BP-30 and Mg-BP-180 decreased in comparison with the Mg-ASsample.The composites reached maximumhydriding capacity of 5.43 wt% for Mg-BP-30; 5.35wt% for Mg-BP-180 and 6.13 wt% for Mg-AS. AtT=473 K the kinetics of the hydriding reaction forall samples decreased and the hydrogen absorptioncapacity values after 60 min of hydriding wereclose to 1 wt%. The composite Mg-AS desorbs 50% of theabsorbed hydrogen within 17 min (Fig.6). The desorption curves of Mg-BP-180 and Mg-AS arequite similar up to 20 min. The worst hydrogendesorption properties manifests pure magnesiumfollowed by Mg-BP-30. When the processes of Fig. 4 Kinetic curves at the first and fifth cycles ofhydriding of the composites Mg- BP ball milled 30 minor 180 min and pure magnesium. Fig. 5 Kinetic curves of hydriding of the composites Mg- BP-30,Mg-BP-180, Mg-AS and pure magnesium.hydrogen dissociation and recombination duringdesorption1 are faster, the hydrogen moleculesshould diffuse through the growing metal layer andthe rate controlling step should change earlier. Thegrowing metal layer during desorption plays therole of a diffusion barrier. The desorption curves ofthe composite ball milled for a longer time indicatea slightly faster rate of the reaction at the beginningof the process and a lower hydrogen absorptioncapacity, which could explain the earlier tendencyofsaturation. As it is evident from Figs. 4, 5 and 6, theaddition of activated carbons derived from apricotstones; more f1avourably affectssthehydrogensorption characteristics than activated carbon frombean pods. It should be noted that compared to puremagnesium, the composites under considerationhave better absorption/desorption characteristics. Fig.6 Kinetic curves of dehydriding of the compositesMg-BP-30,Mg-BP-180, Mg-AS and pure magnesium. Both the destruction of the MgO layer coveringmagnesium and the presence of more defects andactivesiteshelp andaccelerateehydrogendissociative chemisorption and nucleation.Theactivated carbon derived from apricot stones has amore favourable effect on the hydriding kinetics ofmagnesium probably because of the more effectivedestruction of the Mgo layer and the prevention ofits restoration, hindering oxygen back diffusion(Fig.4). It should be noted that the activated carbonswere prepared from various precursors and theydiffered not only by specific surface area. It wasshown in the literature that the electrochemicalhydrogen storage is favoured in carbon materialswith a well developed porosity and a low content ofsurface oxygen complexes [29]. The ash contentwas 22.8% for activated carbon derived from beanpods and 2.0 % for activated carbon derived fromapricot stones. The functional groups on the surfaceof the activated carbons have a strong effect on theadsorption properties. The surface of both activatedcarbons is of basic nature. The amount of acidicgroups determined by Boehm’s method, was higherfor AC derived from apricot stones than that for ACderived from bean pods [25- 28]. According to Suet al. [30], the adsorption capacity of activatedcarbon for non-polar molecules such as hydrogen, increased with the increase in the amount of surfaceacidics groups. Moreover, Gergova et al. [25]established that higher amount of chemical groupscontaining oxygen (or another heteroatom) on thesurface of activated carbons derived from apricotstones and other agricultural by-products providedhigher concentrations of active sites. The microporevolume of the activated carbon derived from apricotstones (AS) is higher than that of the activatedcarbon derived from bean pods (BP). AS has thehighest microporosity and total pore volume. Therelatively high microporosity of the carbon fromapricot stones may be partly attributed to the highercellulose content of this precursor. On the otherhand, the activated carbon derived from bean podsischaracterizedbymeso-2and macroporousstructure [27,28]. Fig. 7 SEM microphotographs of the composites: a)Mg-BP-30; b) Mg-BP-30 hydrided at 573K; c) Mg-BP-180; d) Mg-BP-180 hydrided at 573 K; e) Mg-AS afterball milling and f) Mg-AS hydrided at 573K In Fig. 7 scanning electron microscopy imagesare presented for the composites after ball millingand hydriding. For all samples the size of the largerparticles is from 20 to maximum 50 um and for thesmaller particles -a few um. More small particlesare visible in the sample ball milled 180 min withactivated carbon derived from bean pods. Aftertxocanhydriding, the particles of the two samples withactivated carbon derived from bean pods displayedsimilar size and shape. After hydriding of thecomposites, the surface was not so flat and someroughness and cracks are visible. In Table 1 the particle size distribution of theball milled composites is given. For all compositesthe median diameter do.5 is almost the same. Onlyfor the composite Mg-AC-180 a lower do.1 valuewas registered. The span (do.-do.i/do.5) for thissample slightly increases compared to the other composites ball milled for 30 min which have thesame particle size distribution. These results are ingoodagreementwith thescanning electronmicroscopy images showed in Fig. 6. The d0.9 valuefor all composites is about 50 um. The increase inthe duration of ball milling of Mg-BP lead to someTable 1. Particles size distribution, measured in water.The do. and do.9 is the maximal diameter of 10 and 90% of the particles. The dos is the median diameter. Sample do.1 do.5 do.9 span (um) (um) (um) do.9-do.1/d0.5 Mg-BP-30 8.5 22.6 52.1 Mg-BP-180 5.1 20.9 53.8 Mg-AS 8.6 22.6 49.9 Mg-BP: 95wt% Mg-5 wt% activated carbon derivedfrom bean pods, ball mill 30 min and 180 min; Mg-AS:95wt% Mg-5wt% activated carbon derived from apricotstones. diminution of the particle size and probably tobetter reduction of the oxide layer on the surface.These facts could explain the easier activationduring the hydriding processand the betterhydrogen desorption properties of the sample ballmilled for 180 min. Despite their close particle sizevalues, the composites ball milled for 30 mindisplayed different hydrogen sorption properties.These observations confirmed the assumption thatthe carbon containing additives to magnesium notonly acted as process controlling agents to diminishcold welding during ball milling, but their effectappears to be much more complex. CONCLUSIONS The irole of new (carbonbasedadditivessynthesized from agricultural by-products on thehydrogen sorption characteristics of magnesiumwas studied. The 95wt% Mg-55 wt% activatedcarbon derived from apricot stones reached themaximum value of hydrogen absorption capacity of6.13 wt% at 573 K and 1 MPa after 60 min ofhydriding. The composite containing activatedcarbon derived from apricot stones showed a morepronouncedpositive effectonthe hydrogensorption characteristics of magnesium. Ball millingfor 180 min under argon for the composite Mg-BP-180 lead to easier activation, but after few cycles ofhydriding/dehydriding, the absorption kinetics ofthe two samples ball milled for a longer or a shortertime did not show any difference. The higher specific surface area of the activatedcarbon0 aadditive reflected favourably(on thehydrogen sorption kinetics. Some other characteristics of activated carbons, such as surfacechemistryand porosity, alsoaffectedtheseprocesses. It was established that the activatedcarbons derived from bean pods and apricot stonesimproved thehydrogensorption1 kineticsofmagnesium. Ackinowledgement: The financialsupportofBulgarian Ministry ofScience and Education, theNational Science Fund of Bulgaria under contractsYS No02 -220/2008 and National Centre for NewMaterials UNION, No DCVP-02/2/2009 is highlyappreciated. ( REFERENCES ) 1.E. Yu. Ivanov, I. G. Konstanchuk, A. A. Stepanovand V. V. Boldyrev, Dokl. Akad. Nauk SSSR 286,385 (1986). 2.C.X. Shang, M. Bououdina, Y. Song, Z.X. Guo, Int.J. Hydrogen Energ. 29,73 (2004). 3. M. Khrussanova, M. Terzieva, P. Peshev, Int. J.Hydrogen Energ. 15, 799 (1990). 4.Z. Dehouche, T. Klassen, W. Oelerich, J. Goyette,T.K. Bose, R. Schulz, J. Alloys Compd. 347, 319(2002). 5. J.-L. Bobet, F.J. Castro, B. Chevalier, Scr. Mater.52, 33 (2005). 6. F.J. Castro, J.-L. Bobet, J. Alloys Compd. 366, 303(2004). 7.G. Barkhordarian, T. Klassen, R. Bormann, Scr.Mater. 49, 213 (2003). 8. M. Khrussanova, J.-L. Bobet, M. Terzieva, BChevalier, D. Radev, P. Peshev, B. Darriet, J. AlloysCompd. 307,283 (2000). 9.R. Vijay, R. Sundaresan, M. P. Maiya, S. SrinivasaMurthy, Y. Fu, H.-P. Klein and M. Groll, J. AlloysCompd. 384, 283 (2004). ( 10. C. X. Shang, Z. X. Guo, J. Power S ources 1 29, 7 3 (2004). ) 11. S. Bouaricha, J.-P. Dodelet, D. Guay, J. Huot, R.Schulz, J. Mater. Res. 16,2893 (2001). 12. J.-L. Bobet, E. Grigorova, M. Khrussanova, MKhristov, P. Stefanov, P. Peshev, J. Alloys Compd.366,298 (2004). 13. C. Iwakura, H. Inoue, S. G. Zhang, S. Nohara, J.Alloys Compd.293-295, 653 (1999). ( 14. S. Bouaricha, J . P . D odelet, D . Guay, J. H u ot, S. B oily,R. Schulz, J. Alloys Compd. 3 07,226(2000). ) 15. S. S. Ruggeri, L. Roue, G. Liang,J. Huot, R. Schulz,J.Alloys Compd.343, 170 (2002). 16. F.X. Wang, X.P. Gao, Z.W. Lu, S.H. Ye, J.Q. Qu,F.Wu, H.T. Yuan, D.Y. Song, J. Alloys Compd. 370,326 (2004). 17. C.Z. Wu, P. Wang, X. Yao, C. Liu, D.M. Chen,G.Q. Lu, H.M. Cheng, J. Alloys Compd. 414, 259(2006). 18. Z.G. Huang, Z.P. Guo, A. Calka, D. Wexler, H. K.Liu,J. Alloys Compd. 427,94(2007). 19. C. S. Goh, J. Wei, L. C. Lee, M. Gupta, Compos.Sci. Technol. 68, 1432 (2008) ( 20. V. Fuster, G . U r retavizcaya, F . J. Castro, J . Alloys C ompd. 481,673 (2009). ) ( 21. C. Milanese, A. Girella,S. Garroni, G. B runi, V.Berbenni, P. M atteazzi, A. Marini, Int. J. HydrogenEnerg. 35, 9027 (2010). ) ( 22. E. Grigorova, M . Khristov, M . K h russanova, Ce n t.Eur. J. Chem. 8 , 737 (2010) DOI: 1 0.2478/s11532- 010-0040-0. ) ( 23. Tony S passov, Z latina Zlatanova, Maya Spassova, Stanislava Todorova, Int. J. Hydrogen Energ. 35, 10396(2010). ) ( 24. M.A. Lillo-Rodenas, Z.X. Guo, K.F. A g uey-Zinsou, D. Cazorla-Amoros, Carbon 46, 1 26(2008). ) ( 25. K. Gergova, N. Petrov and S. Eser, Carbon 32, 693 (1994). ) 26. B. Cabal, T. Budinova, C. O. Ania, B. Tsyntsarski, J.B. Parra, B. Petrova, J. Hazard. Mater. 161, 1150(2009). ( 27. B . P etrova, T . B udinova, B . Tsyntsarski, N. Pe t rov, G. Bardarska, C . Ania, J . P a rra, Bulg. C hem.. Commun. 42,141 (2010). ) ( 28. N. Petrov, T . Budinova,B. Tsyntsarski, B. Petrova, D . Teodosiev, N . B oncheva, Bulg. Chem. Commun 42, 19(2010). ) 29. M.J. Bleda-Martinez, J.M. Perez, A. Linares-Solano,E. Morallon, D. Cazorla-Amoros, Carbon 46, 1053(2008). 30. W. Su, Y. Zhou, L. Wei, Y. Sun, L. Zhou,Newcarbon mater. 22,135 (2007). ( 31. A. Nithya, S. Rajendran, Bulg. Chem. Commun., 42119(2010). ) BJIIAHIIE HA IOBABKATA OT AKTIBHI BBIJIEHI IIOJIVHEHI OTCEJICKOCTOIIAHCKM IIPOIVKTM BBPXV CBOCTBATA HA MATHE3MY3A CBXPAHEHME HA BOIOPOI E. TpuropoBa *, IIB. MaHIKyKOBa, M. XpucToB, IIeTbp IIBeTKOB n Boiko IInHIapck’ “Ihcmumym no o6ua u HeopeahuyHa xuMu, Bbneapcka akaoemua Ha Haykume, yn. AKao. I. Bonye6, 6nok 11, Codua 1113 6ncmumym no opeaHuyHa xuMua c yehmbp no dumoxuMug, Bbneapcka akaoemua ha haykume, yn. Akao. I. Bonyee6nok 9, Codun1113 IIocTbIIHJIa Ha 15 beBpyapH, 2011 r.; IpHeTa Ha 4 anpHJI, 2011 r. (Pe3IoMe) I3cJIeIBaHM caaa6cop6nMoHHO-Iecop6nHoHHHTexapaKTepHCTHKH IIO OTHoIIeHHe Ha BoIopoIHaKOMII03MTMTe 95 Mac.% Mg- 5 Mac.% aKTMBeH BbIJIeH, CMHTe3MpaH OT KaiiceBM KOCTHJIKM HJIM 606eHM IIIyIIIyJIK,IIOJyyeHM ype3 MexaHoaKTMBMpaHe IoI aproH. IIpoIecbT Ha XIppaHe e M3BbpIIeH IIpM TeMIlepaTypa 573 K 473K H HaJISraHe iMPa, a Ha IexiippaHe - IpH T =623KM P= 0.15 MPa. IocTHTHaTHTe CTOMHOCTM Haa6cop6IIHOHHHA KaIaIHTeT ca 5.43 Mac.% 3a KoMII03MTa, CbIbpxaI aKTMBeH BBIJIeH OT 6060BM IIIyIIIyJIKM M 6.13Mac.% 33aa KKOMIIO3MTa, CBIbpxaIlaKTMBeH BbIJIeH OT KaiiceBl KOCTHJIKM (IpT=573KMP=1MPa).XapakTepHcTHKMTe Ha XHIppaHe/IexHIpupaHe Ha H3cJIeBaHHTe KOMIIO3MTM ca cpaBHeHM c Te3 Ha 4MCTMAMarHe3ui. ycTaHoBeHo e, ye aKTMBHTe BbIJIeHM, IIOJIyyeHM OT 606oBM ⅢIyIIIyJIKM M KaicMeBM KOCTMJIKM, IIOI06pABaTcop6IHOHHITe xapaKTepMCTMKM Ha MarHe3MA IIO OTHOIIIeHHe Ha BoIopoIa
确定



还剩4页未读,是否继续阅读?
北京飞驰科学仪器有限公司为您提供《镁和活性炭中镁和活性炭的研磨细度检测方案(激光粒度仪)》,该方案主要用于其他中镁和活性炭的研磨细度检测,参考标准--,《镁和活性炭中镁和活性炭的研磨细度检测方案(激光粒度仪)》用到的仪器有德国FRITSCH(飞驰)A22大量程纳米激光粒度仪、德国FRITSCH P6 单罐行星式高能球磨机/仪
推荐专场
相关方案
更多
该厂商其他方案
更多