方案详情
文
磷酸锆钠NZP在核废料应用中极具潜力的主体基材,其热物理性能是评价这些混合物稳定性的必要参数。本文描述了一些包含碱性土族元素的NZP型混合物合成和量热计测量结果,还描述了自主研发的室温下落式量热计用于低温量热测量。下落式量热计采用标准参考材料进行校准,仪器工作温度范围为373~873K。本文还讨论了这些材料焓值增加与温度的关系以及化学组分对材料性能的影响。
方案详情

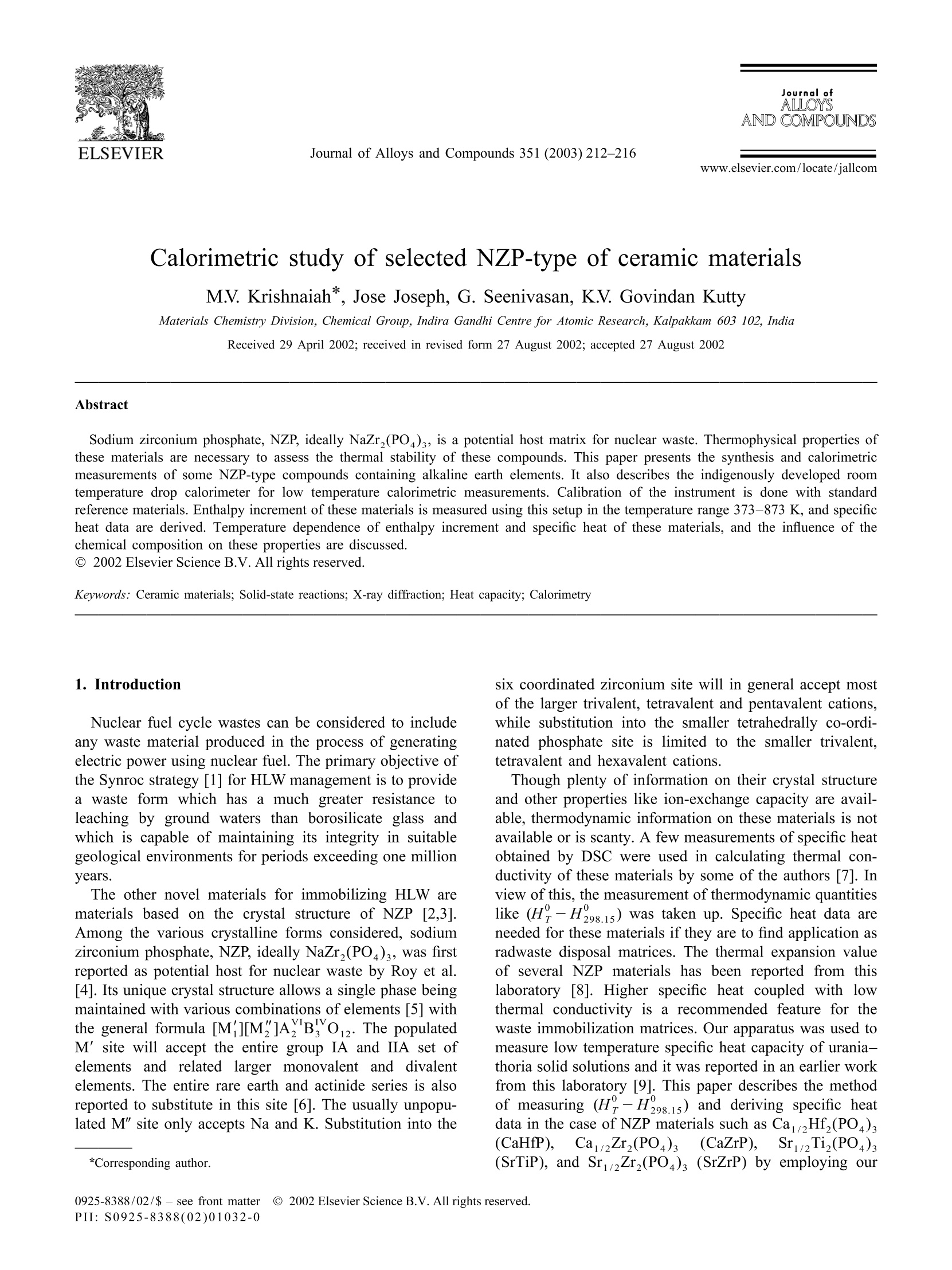
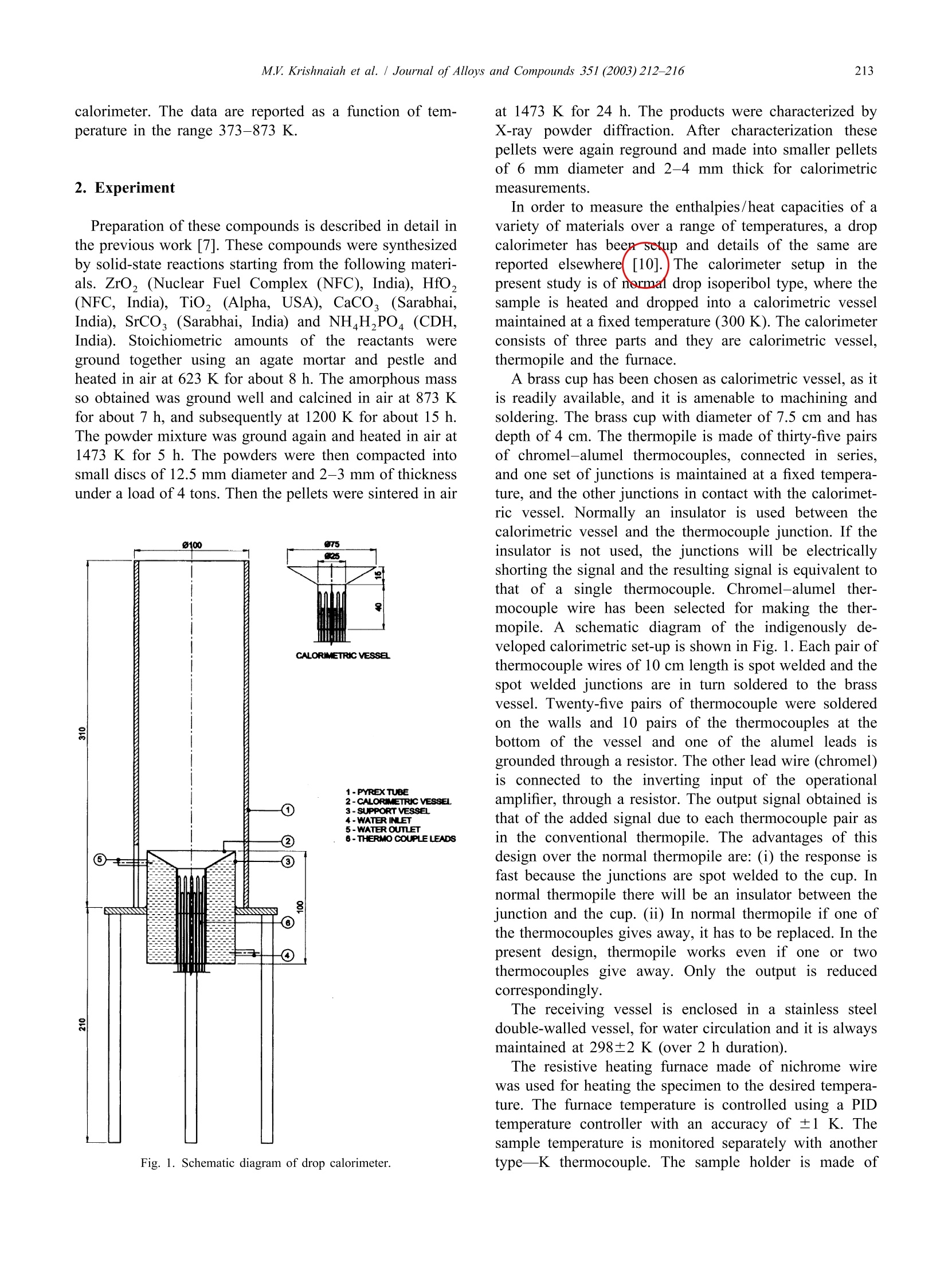
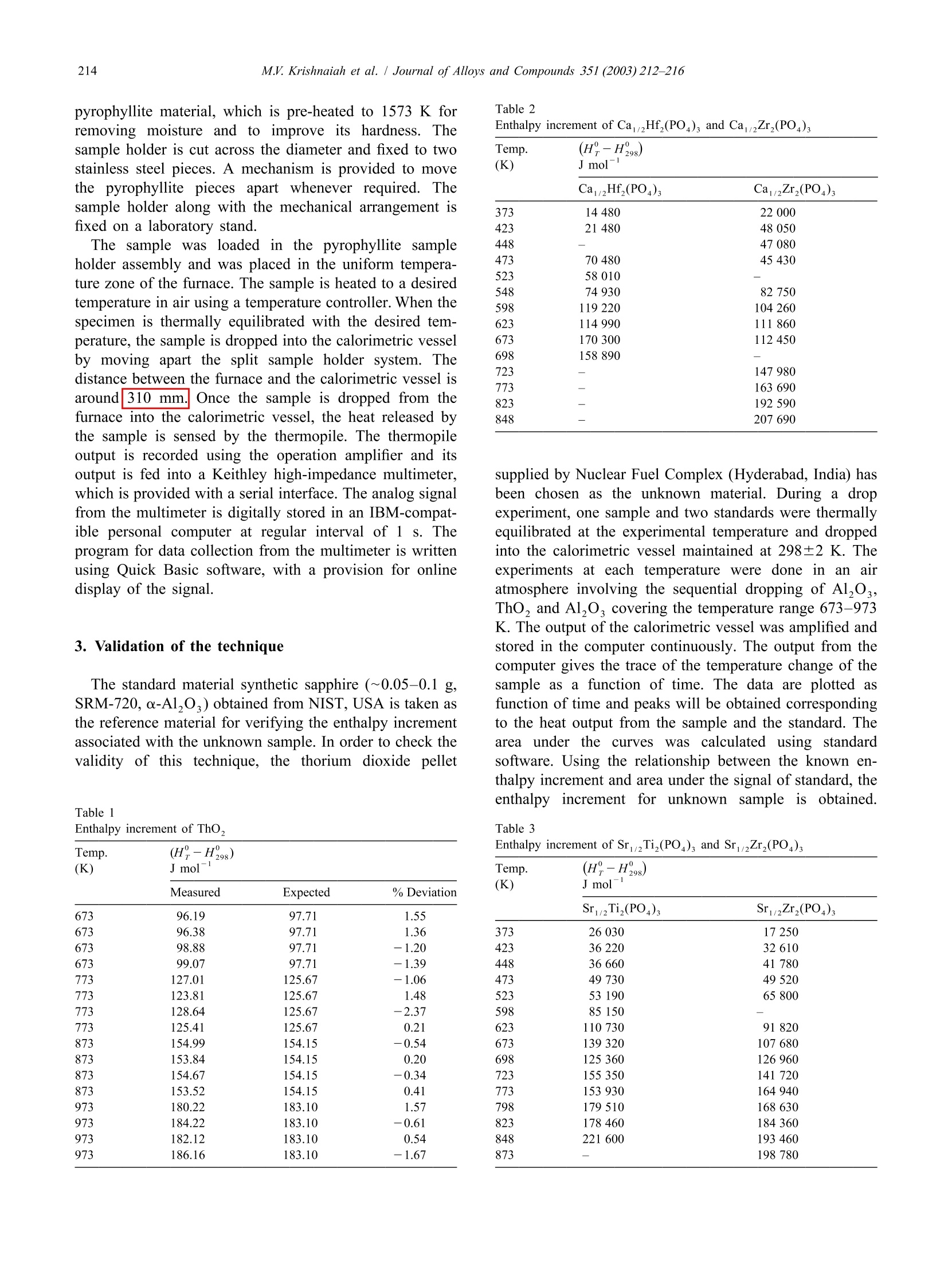
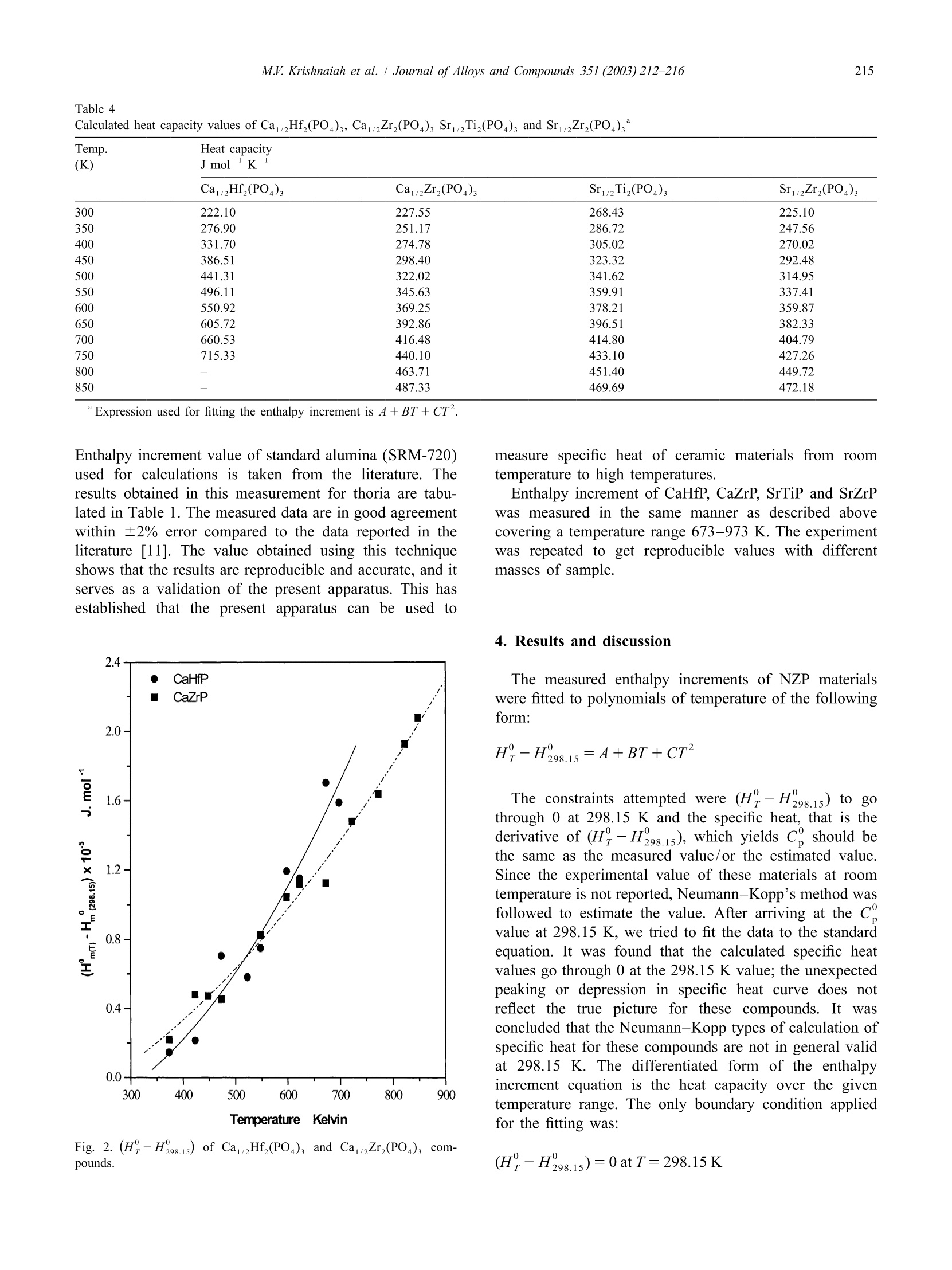
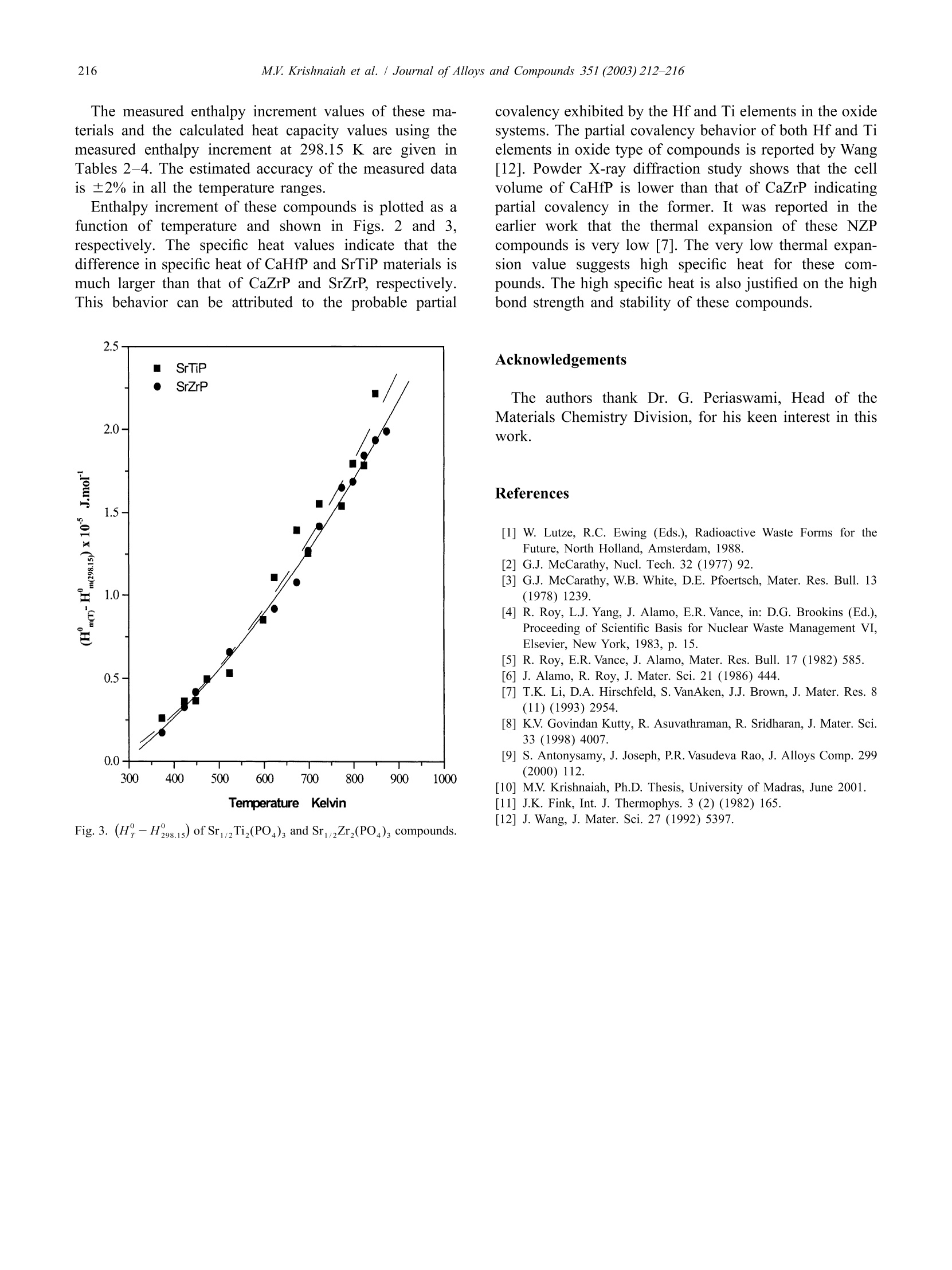
Journal ofALLOYSAND COMPOUNDSJournal of Alloys and Compounds 351 (2003) 212-216 213M.V. Krishnaiah et al./ Journal of Alloys and Compounds 351 (2003)212-216 www.elsevier.com/locate/jallcom Calorimetric study of selected NZP-type of ceramic materials M.V. Krishnaiah*, Jose Joseph, G. Seenivasan, K.V. Govindan Kutty Materials Chemistry Division, Chemical Group, Indira Gandhi Centre for Atomic Research, Kalpakkam 603 102, IndiaReceived 29 April 2002; received in revised form 27 August 2002; accepted 27 August 2002 Abstract Sodium zirconium phosphate, NZP, ideally NaZr,(PO), is a potential host matrix for nuclear waste. Thermophysical properties ofthese materials are necessary to assess the thermal stability of these compounds. This paper presents the synthesis and calorimetricmeasurements of some NZP-type compounds containing alkaline earth elements. It also describes the indigenously developed roomtemperature drop calorimeter for low temperature calorimetric measurements. Calibration of the instrument is done with standardreference materials. Enthalpy increment of these materials is measured using this setup in the temperature range 373-873 K, and specificheat data are derived. Temperature dependence of enthalpy increment and specific heat of these materials, and the influence of thechemical composition on these properties are discussed. C 2002 Elsevier Science B.V. All rights reserved. Keywords: Ceramic materials; Solid-state reactions; X-ray diffraction; Heat capacity; Calorimetry 1. Introduction Nuclear fuel cycle wastes can be considered to includeany waste material produced in the process of generatingelectric power using nuclear fuel. The primary objective ofthe Synroc strategy [1] for HLW management is to providea waste form which has a much greater resistance toleaching by ground waters than borosilicate glass andwhich is capable of maintaining its integrity in suitablegeological environments for periods exceeding one millionyears. The other novel materials for immobilizing HLW arematerials based on the crystal structure of NZP [2,3].Among the various crystalline forms considered, sodiumzirconium phosphate, NZP, ideally NaZr,(PO4)3, was firstreported as potential host for nuclear waste by Roy et al.[4]. Its unique crystal structure allows a single phase beingmaintained with various combinations of elements [5] withthe general formula [M][M"]AYBo. The populatedM’ site will accept the entire group IA and IIA set ofelements and related larger monovalent and divalentelements. The entire rare earth and actinide series is alsoreported to substitute in this site [6]. The usually unpopu-lated M" site only accepts Na and K. Substitution into the ( *Corresponding author. ) six coordinated zirconium site will in general accept mostof the larger trivalent, tetravalent and pentavalent cations,while substitution into the smaller tetrahedrally co-ordi-nated phosphate site is limited to the smaller trivalent,tetravalent and hexavalent cations. Though plenty of information on their crystal structureand other properties like ion-exchange capacity are avail-able, thermodynamic information on these materials is notavailable or is scanty. A few measurements of specific heatobtained by DSC were used in calculating thermal con-ductivity of these materials by some of the authors [7]. Inview of this, the measurement of thermodynamic quantitiesyr0like (H?-H298.15) was taken up. Specific heat data areneeded for these materials if they are to find application asradwaste disposal matrices. The thermal expansion valueof several NZP materials has been reported from thislaboratory [8]. Higher specific heat coupled with lowthermal conductivity is a recommended feature for thewaste immobilization matrices. Our apparatus was used tomeasure low temperature specific heat capacity of urania-thoria solid solutions and it was reported in an earlier workfrom this laboratory [9]. This paper describes the methodof measuring (HY-H298.15) and deriving specific heatdata in the case ofNZP materials such as Ca1/2Hf,(PO4)s(CaHfP),Ca1/2Zr,(PO4)3(CaZrP),Sr1/2Ti,(PO4)3(SrTiP), and Sr2Zr,(PO4)3 (SrZrP) by employing our ( 0925-8388/02/$ - see front matte x r @ 20 0 2 Els e vier Science B.V. All rights reserved.PII: S0925-8388(02)01032-0 ) calorimeter. The data are reported as a function of tem-perature in the range 373-873 K. 2. Experiment Preparation of these compounds is described in detail inthe previous work [7]. These compounds were synthesizedby solid-state reactions starting from the following materi-als. ZrO, (Nuclear Fuel Complex (NFC), India), HfO,(NFC, India), TiO, (Alpha, USA), CaCO, (Sarabhai,India), SrCO, (Sarabhai, India) and NHHPO4 (CDH,India). Stoichiometric amountsS(of the reactantsVwereground together using an agate mortar and pestle andheated in air at 623 K for about 8 h. The amorphous massso obtained was ground well and calcined in air at 873 Kfor about 7 h, and subsequently at 1200 K for about 15 h.The powder mixture was ground again and heated in air at1473 K for 5 h. The powders were then compacted intosmall discs of 12.5 mm diameter and 2-3 mm of thicknessunder a load of 4 tons. Then the pellets were sintered in air Fig. 1. Schematic diagram of drop calorimeter. at 1473 K for 24 h. The products were characterized byX-ray powder diffraction. After characterization thesepellets were again reground and made into smaller pelletsof 6 mm diameter and 2-4 mm thick for calorimetricmeasurements. A brass cup has been chosen as calorimetric vessel, as itis readily available, and it is amenable to machining andsoldering. The brass cup with diameter of 7.5 cm and hasdepth of 4 cm. The thermopile is made of thirty-five pairsof chromel-alumel thermocouples, connected in series,and one set of junctions is maintained at a fixed tempera-ture, and the other junctions in contact with the calorimet-ric vessel. Normally an insulator is used between thecalorimetric vessel and the thermocouple junction. If theinsulator is not used, the junctions will be electricallyshorting the signal and the resulting signal is equivalent tothat of a single thermocouple. Chromel-alumel ther-mocouple wire has been selected for making the ther-mopile. A schematic diagram of the indigenously de-veloped calorimetric set-up is shown in Fig.1. Each pair ofthermocouple wires of 10 cm length is spot welded and thespot welded junctions are in turn soldered to the brassvessel. Twenty-five pairs of thermocouple were solderedon the walls and 10 pairs of the thermocouples at thebottom of the vessel and one of the alumel leads isgrounded through a resistor. The other lead wire (chromel)is connected to the inverting input of the operationalamplifier, through a resistor. The output signal obtained isthat of the added signal due to each thermocouple pair asin the conventional thermopile. The advantages of thisdesign over the normal thermopile are: (i) the response isfast because the junctions are spot welded to the cup. Innormal thermopile there will be an insulator between thejunction and the cup.(ii) In normal thermopile if one ofthe thermocouples gives away, it has to be replaced. In thepresent design, thermopile works even if one or twothermocouples give away. Only the output is reducedcorrespondingly. The receiving vessel is enclosed in a stainless steeldouble-walled vessel, for water circulation and it is alwaysmaintained at 298±2 K (over 2 h duration). The resistive heating furnace made of nichrome wirewas used for heating the specimen to the desired tempera-ture. The furnace temperature is controlled using a PIDtemperature controller with an accuracy of ±1 K. Thesample temperature is monitored separately with anothertypeK thermocouple. The sample holder is made of pyrophyllite material, which is pre-heated to 1573 K forremoving moisture and to improve its hardness. Thesample holder is cut across the diameter and fixed to twostainless steel pieces. A mechanism is provided to movethe pyrophyllite pieces apart whenever required. Thesample holder along with the mechanical arrangement isfixed on a laboratory stand. 3. Validation of the technique The standard material synthetic sapphire (~0.05-0.1 g,SRM-720,a-Al,O,) obtained from NIST, USA is taken asthe reference material for verifying the enthalpy incrementassociated with the unknown sample. In order to check thevalidity of this technique, the thorium dioxide pellet Table 1Enthalpy increment of ThO, (K) Jmol Measured Expected % Deviation 673 96.19 97.71 1.55 673 96.38 97.71 1.36 673 98.88 97.71 -1.20 673 99.07 97.71 -1.39 773 127.01 125.67 -1.06 773 123.81 125.67 1.48 773 128.64 125.67 -2.37 773 125.41 125.67 0.21 873 154.99 154.15 -0.54 873 153.84 154.15 0.20 873 154.67 154.15 -0.34 873 153.52 154.15 0.41 973 180.22 183.10 1.57 973 184.22 183.10 -0.61 973 182.12 183.10 0.54 973 186.16 183.10 -1.67 Table 2Enthalpy increment of Ca1/2Hf,(PO4)s and Ca1/2Zr,(PO4) Temp. (H-H298) (K) J mol' Ca/Hf,(PO4)s CaZr,(PO4)s 373 14480 22000 423 21480 48050 448 47080 473 70480 45430 523 58010 548 74930 82750 598 119220 104260 623 114990 111860 673 170300 112 450 698 158890 723 147980 773 163 690 823 192590 848 207 690 supplied by Nuclear Fuel Complex (Hyderabad, India) hasbeen chosen as the unknown material. During a dropexperiment, one sample and two standards were thermallyequilibrated at the experimental temperature and droppedinto the calorimetric vessel maintained at 298±2 K. Theexperiments at each temperature were done in an airatmosphere involving the sequential dropping of Al,03,ThO, and A1,Os covering the temperature range 673-973K. The output of the calorimetric vessel was amplified andstored in the computer continuously. The output from thecomputer gives the trace of the temperature change of thesample as a function of time. The data are plotted asfunction of time and peaks will be obtained correspondingto the heat output from the sample and the standard. Thearea under the curves was calculated using standardsoftware. Using the relationship between the known en-thalpy increment and area under the signal of standard, theenthalpy incrementfor unknownnsamplee isobtained. Table 3Enthalpy increment of SrTi,(PO)s and SrZr,(PO)3 (K) J mol Sr1/2Ti,(PO4)3 Sri2Zr,(PO4)3 373 26030 17 250 423 36220 32610 448 36 660 41780 473 49730 49520 523 53190 65800 598 85150 623 110730 91820 673 139320 107680 698 125360 126960 723 155350 141720 773 153 930 164940 798 179510 168630 823 178460 184360 848 221 600 193 460 873 198 780 Table 4 Calculated heat capacity values of CaHf(PO)s, CaZr,(PO), SrTi,(PO), and SrZr,(PO) (K) J mol1K CaHf,(PO) Ca1/2Zr,(PO4)s SrTi,(PO4)s SrZr,(PO) 300 222.10 227.55 268.43 225.10 350 276.90 251.17 286.72 247.56 400 331.70 274.78 305.02 270.02 450 386.51 298.40 323.32 292.48 500 441.31 322.02 341.62 314.95 550 496.11 345.63 359.91 337.41 600 550.92 369.25 378.21 359.87 650 605.72 392.86 396.51 382.33 700 660.53 416.48 414.80 404.79 750 715.33 440.10 433.10 427.26 800 一 463.71 451.40 449.72 850 487.33 469.69 472.18 Expression used for fitting the enthalpy increment is A+BT + CT. Enthalpy increment value of standard alumina (SRM-720)used for calculations is taken from the literature. Theresults obtained in this measurement for thoria are tabu-lated in Table 1. The measured data are in good agreementwithin ±2% error compared to the data reported in theliterature [11]. The value obtained using this techniqueshows that the results are reproducible and accurate, and itserves as a validation of the present apparatus. This hasestablished that the present apparatus can be used to measure specific heat of ceramic materials from roomtemperature to high temperatures. Enthalpy increment of CaHfP, CaZrP, SrTiP and SrZrPwas measured in the same manner as described abovecovering a temperature range 673-973 K. The experimentwas repeated to get reproducible values with differentmasses of sample. 4. Results and discussion The measured enthalpy increments of NZP materialswere fitted to polynomials of temperature of the followingform: The constraints attempted were (H-H298.15) to gothrough 0 at 298.15 K and the specific heat, that is thederivative of (H-H298.15), which yields C, should bethe same as the measured value/or the estimated value.Since the experimental value of these materials at roomtemperature is not reported, Neumann-Kopp’s method wasfollowed to estimate the value. After arriving at the Cvalue at 298.15 K, we tried to fit the data to the standardequation. It was found that the calculated specific heatvalues go through 0 at the 298.15 K value; the unexpectedpeaking or depression in specific heat curve does notreflect the true picture for these compounds. It wasconcluded that the Neumann-Kopp types of calculation ofspecific heat for these compounds are not in general validat 298.15 K. The differentiated form of the enthalpyincrement equation is the heat capacity over the giventemperature range. The only boundary condition appliedfor the fitting was: Fig. 2. (H?-H298.15) of CaHf (PO )s and CaZr,(PO4)3 com-pounds. The measured enthalpy increment values of these ma-terials and the calculated heat capacity values using themeasured enthalpy increment at 298.15 K are given inTables 2-4. The estimated accuracy of the measured datais ±2% in all the temperature ranges. Enthalpy increment of these compounds is plotted as afunction of temperature and shown in Figs. 2 and 3,respectively. The specific heat values indicate that thedifference in specific heat of CaHfP and SrTiP materials ismuch larger than that of CaZrP and SrZrP, respectively.This behavior can be attributed to the probable partial Fig.3. (H?-H298.15) of Sr2Ti, (PO )s and SrZr,(PO4)s compounds. covalency exhibited by the Hf and Ti elements in the oxidesystems. The partial covalency behavior of both Hf and Tielements in oxide type of compounds is reported by Wang[12]. Powder X-ray diffraction study shows that the cellvolume of CaHfP is lower than that of CaZrP indicatingpartial covalency in the former. It was reported in theearlier work that the thermal expansion of these NZPcompounds is very low [7]. The very low thermal expan-sion value suggests high specific heat for these com-pounds. The high specific heat is also justified on the highbond strength and stability of these compounds. Acknowledgements The authors thank Dr. G. Periaswami, Head of theMaterials Chemistry Division, for his keen interest in thiswork. References ( [1 ] W. L utze, R .C. E w ing (E d s.), R a dioactive W a ste Fo r ms for theFuture, North Holland, Amsterdam, 1988. ) ( [2] G.J. McCarathy, Nucl. Tech. 32 (1977) 92. ) ( [3] G.J. McCarathy, W.B. W h ite, D.E. Pf o ertsch, Mater. Re s . Bu l l. 13( 1 978) 1239. ) [4] R. Roy, L.J. Yang, J. Alamo, E.R. Vance, in: D.G. Brookins (Ed.),Proceeding of Scientific Basis for Nuclear Waste Management VI,Elsevier, New York, 1983, p. 15. ( [5] R . R oy, E .R. Vance, J. A lamo, M a ter. R e s. B u ll. 1 7 ( 1 982) 585. [6] J. Alamo, R . R oy, J. M ater. S ci. 2 1 ( 1986) 444. ) ( [7] T.K. L i, D.A. H irschfeld, S. VanAken, J .J. Brown, J . M ater. R es. 8 (11)(1993)2954. ) ( [8] K.V. Govindan K u tty, R . Asuvathraman, R. Sridharan, J. Ma t er. Sc i . 33 (1998) 4007. ) [9] S. Antonysamy, J. Joseph, P.R. Vasudeva Rao, J. Alloys Comp. 299(2000) 112. ( [ 1 0] M .V. K rishnaiah, Ph.D. Thesis, University of Madras, June 2001. ) ( [11 ] J.K. Fink, Int. J . Thermophys . 3 (2) ( 1982) 165. ) ( [ 1 2] J. Wang, J . Mater. Sci. 27 ( 1 992) 5397. )
确定
还剩3页未读,是否继续阅读?
上海依阳实业有限公司为您提供《核废料中热容,热焓,比热容检测方案 》,该方案主要用于其他中热容,热焓,比热容检测,参考标准--,《核废料中热容,热焓,比热容检测方案 》用到的仪器有下落法中温量热仪/比热容测定仪
推荐专场
相关方案
更多
该厂商其他方案
更多
















