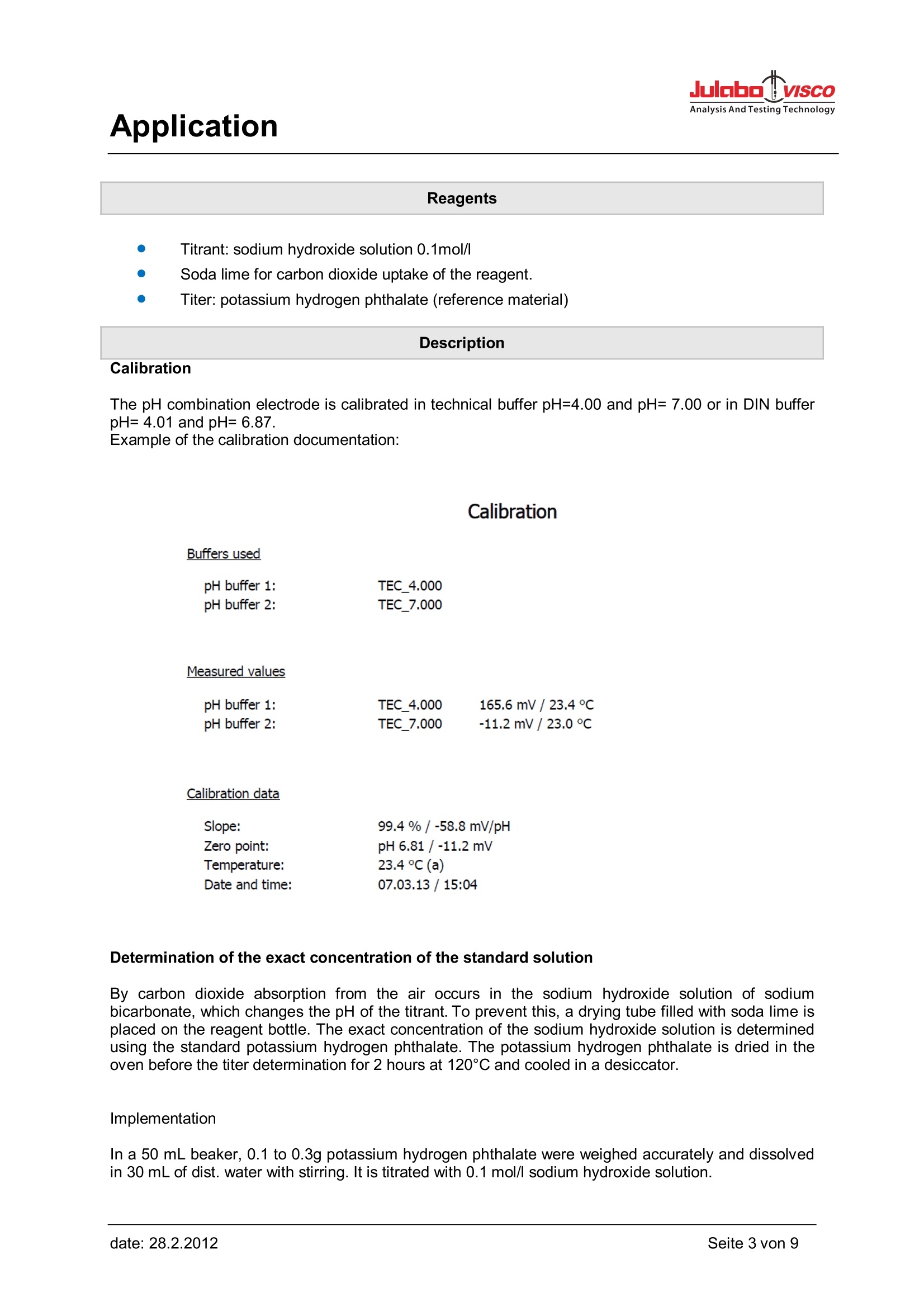
Determination of the exact concentration of the standard solution
By carbon dioxide absorption from the air occurs in the sodium hydroxide solution of sodium bicarbonate, which changes the pH of the titrant. To prevent this, a drying tube filled with soda lime is placed on the reagent bottle. The exact concentration of the sodium hydroxide solution is determined using the standard potassium hydrogen phthalate. The potassium hydrogen phthalate is dried in the oven before the titer determination for 2 hours at 120°C and cooled in a desiccator.
方案详情

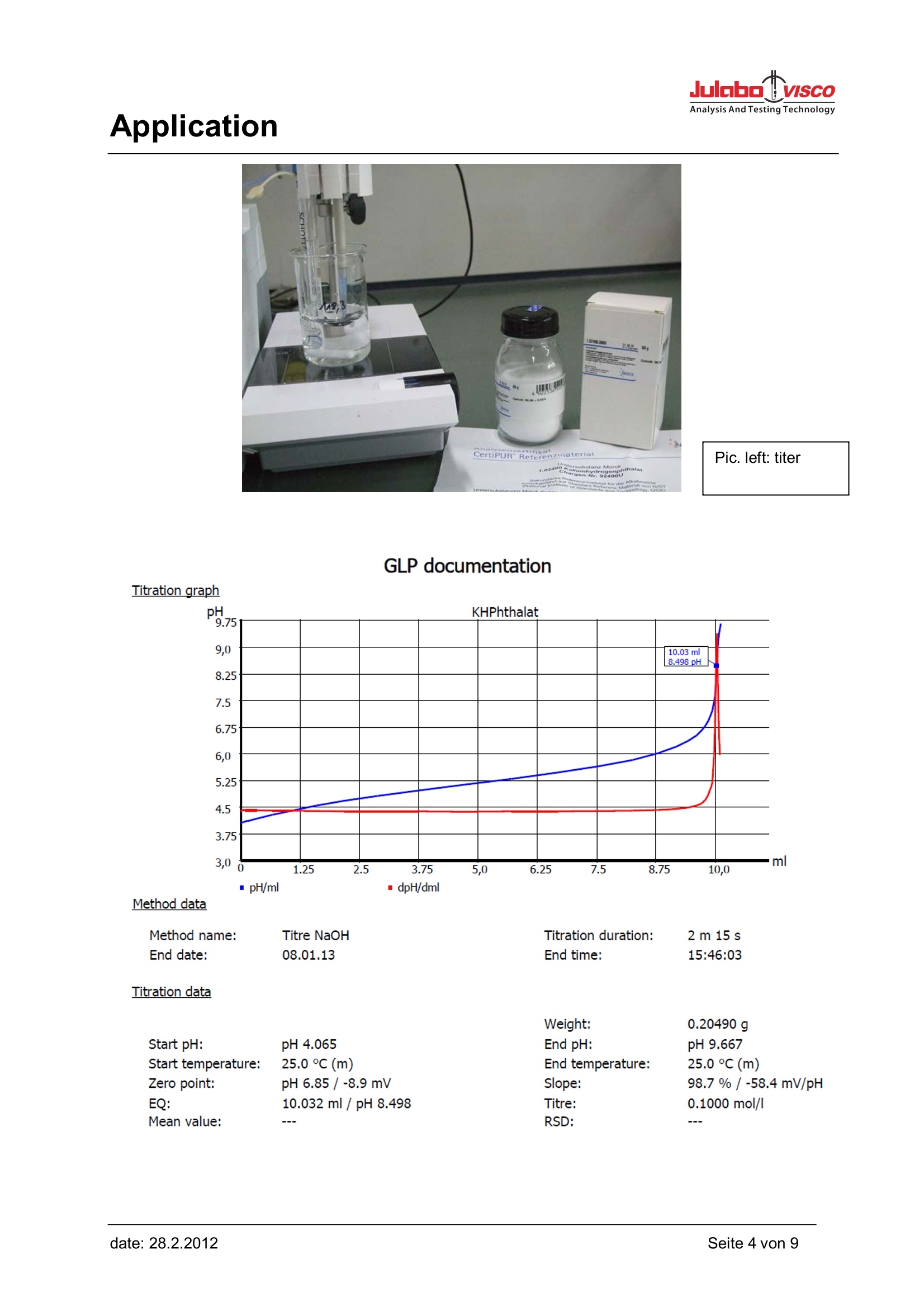
Julabo VISCOAnalysis And Testing Technology Application Application Use This method is used fort he quantitative determination of total acidity in fruit juice. Here, the citric acidas the main use as a reference. Molecular weight citric acid M= 192.13 g/mol Appliances Titrator: TL 6000/7000 (TL 6000/7000 M2/20) consists of Basic device Magnetic stirrer TM 235 20 mL Exchange unit WA 20, with nrown glass bottle for titrant complete And pH combination electrode A 162 DIN ID Electrodes Electrode:A 162 DINID Calibration: DIN buffer pH= 4.00 and pH=7.00 Application Reagents Titrant: sodium hydroxide solution 0.1mol/l Soda lime for carbon dioxide uptake of the reagent. Titer: potassium hydrogen phthalate (reference material) Description Calibration The pH combination electrode is calibrated in technical buffer pH=4.00 and pH= 7.00 or in DIN bufferpH= 4.01 and pH= 6.87. Example of the calibration documentation: Calibration Buffers used pH buffer 1: TEC 4.000 pH buffer 2: TEC 7.000 Measured values pH buffer 1: TEC 4.000 165.6 mV/23.4°℃ pH buffer 2: TEC 7.000 -11.2mV/23.0°C Calibration data Slope: 99.4%/-58.8 mV/pH Zero point: pH 6.81 /-11.2 mV Temperature: 23.4°C(a) Date and time: 07.03.13/15:04 Determination of the exact concentration of the standard solution By carbon dioxide absorption from the air occurs in the sodium hydroxide solution of sodiumbicarbonate, which changes the pH of the titrant. To prevent this, a drying tube filled with soda lime isplaced on the reagent bottle. The exact concentration of the sodium hydroxide solution is determinedusing the standard potassium hydrogen phthalate. The potassium hydrogen phthalate is dried in theoven before the titer determination for 2 hours at 120°C and cooled in a desiccator. Implementation In a 50 mL beaker, 0.1 to 0.3g potassium hydrogen phthalate were weighed accurately and dissolvedin 30 mL of dist. water with stirring. It is titrated with 0.1 mol/l sodium hydroxide solution. Pic. left: titer GLP documentation Titration graph pH/ml mcdpH/dml Method data Method name: Titre NaOH Titration duration: 2 m 15 s End date: 08.01.13 End time: 15:46:03 Titration data Weight: 0.20490 g Start pH: pH 4.065 End pH: pH 9.667 Start temperature: 25.0 ℃ (m) End temperature: 25.0°C (m) Zero point: pH 6.85 /-8.9 mV Slope: EQ: 10.032 ml/pH 8.498 Titre: 0.1000 mol/l Mean value: RSD: Application Calculation formula Titre:(W*F2)/((EQ1-B)*M*F1)-> WAMol (M):204.22000Weight (W):0.2049 g (m)Factor 2 (F2):1000.0000Blank value (B):0.0000 mlFactor 1 (F1):1.0000Statistics: The titration parameters are described under ,,method“. Titration of the sample Into a 50mL beaker 5 -25mL fruit juice must be pipetted accurately and mixed with 20mL of dist.Water with stirring. It is titrated with 0.1 mol/l sodium hydroxid solution. graninl 00.m Pic.left: preparation af the sample Application Pic. left: titration of the fruitiuice Application Reaction equation: Citric acid is a tribasic acid. There a three moles of sodium hxdroxide required to neutralize one moleof citric acid completely: Result example: GLP documentation Titration graph 12.18 mlpH 8.200 · pH/ml Method data Method name: Orange Juice Titration duration: 1m 57 s End date: 08.03.13 End time: 12:19:40 Titration data Sample ID: Granini 2 Pattern: 10.000 ml Start pH: pH 3.853 End pH: pH 8.235 Start temperature: 23.3℃(a) End temperature: 23.8°C(a) Zero point: pH6.81 /-11.3 mV Slope: 99.6%/-58.9 mV/pH EP1: 12.179ml / pH 8.200 Acidity: 7.80 g/l Calculation formula Acidity: (EP1-B)*T*M*F1/(V*F2) Mol (M): 64.04000 Blank value (B): 0.0000 ml Titre(T): 0.10000000 (m) Factor 1 (F1): 1.0000 Pattern (V): 10.000 ml (m) Factor 2 (F2): 1.0000 Statistics: Off Application Method Example Method data overall viewMethod name:Orange JuiceCreated at: 03/08/13 12:06:41Method type:Automatic titrationLast modification: 03/08/13 12:16:39Measured value:pHDamping settings: NoneTitration mode:End pt.Documentation: GLPLinear steps:0.040 mlMeasuring speed / drift:Normal:minimum holding time: 02 smaximum holding time:15 sMeasuring time: 02 sDrift: 20 mV/minInitial waiting time:0 sTitration direction:IncreasePretitration:OffEndpoint 1:pH 8.200delta endpoint 1: pH 1.000Endpoint delay 1: 5sEndpoint 2:Off Dosing parameter Dosingspeed: 65.00 % Filling speed: 30 s Maximum dosing volume: 50.00 ml Unit values Unit size: 20ml Unit ID: 10039117 Reagent: NaOH Batch ID: no entry Concentration [mol/]: 0.01000 Determined at: 03/08/13 20:03:29 Expire date: Opened/compounded: Test according ISO 8655: 03/19/12 Last modification: 03/08/1312:03:32 Application Notes If you have any questions on the application, you can feel free to contact us.. JULABO TECHNOLOGY(Beijing)Co.,LTD地址:北京市朝阳区酒仙桥东路1号院M8号楼C厅3层301室: Room 301,C hall,M8 office building,No.1 jiuxianqiao east road ,Chaoyang District,Beijing,ChinamTel.:4008092068Fax : +86 (0)1064390585E-Mail : info@julabo.cn优莱博技术(北京)有限公司③Web: www.julabo.cn, www.julabo-visco.com Seite von date: date: eite von
确定






还剩7页未读,是否继续阅读?
优莱博技术(北京)有限公司为您提供《检测果汁饮料中的总酸值》,该方案主要用于果蔬汁类及其饮料中理化分析检测,参考标准--,《检测果汁饮料中的总酸值》用到的仪器有Chemtron CAT2-16全自动电位滴定仪、ChemTron PREMIUM多功能旋转粘度计、ChemTron CON600 专家型台式电导率计、ChemTron DenDi 台式便携一体密度仪、ChemTron P8000 系列旋光仪、ChemTron VTR-80高低温磁力搅拌反应装置、Chemtron CAT 7500容量法卡式水份滴定仪
推荐专场
电导率仪、电导仪、盐度计
更多
该厂商其他方案
更多