方案详情
文
the analysis of industrial and laboratory-prepared ...
方案详情

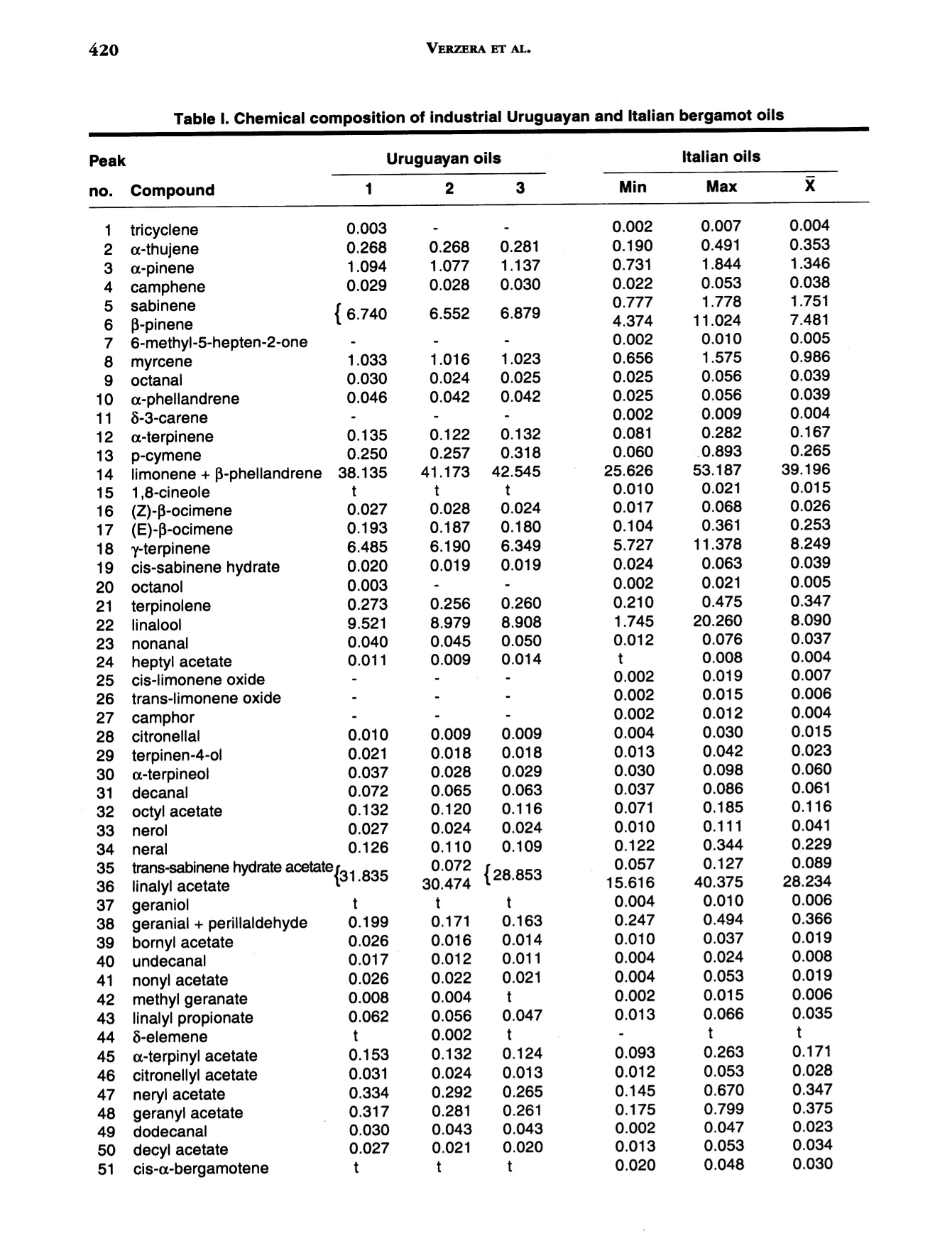
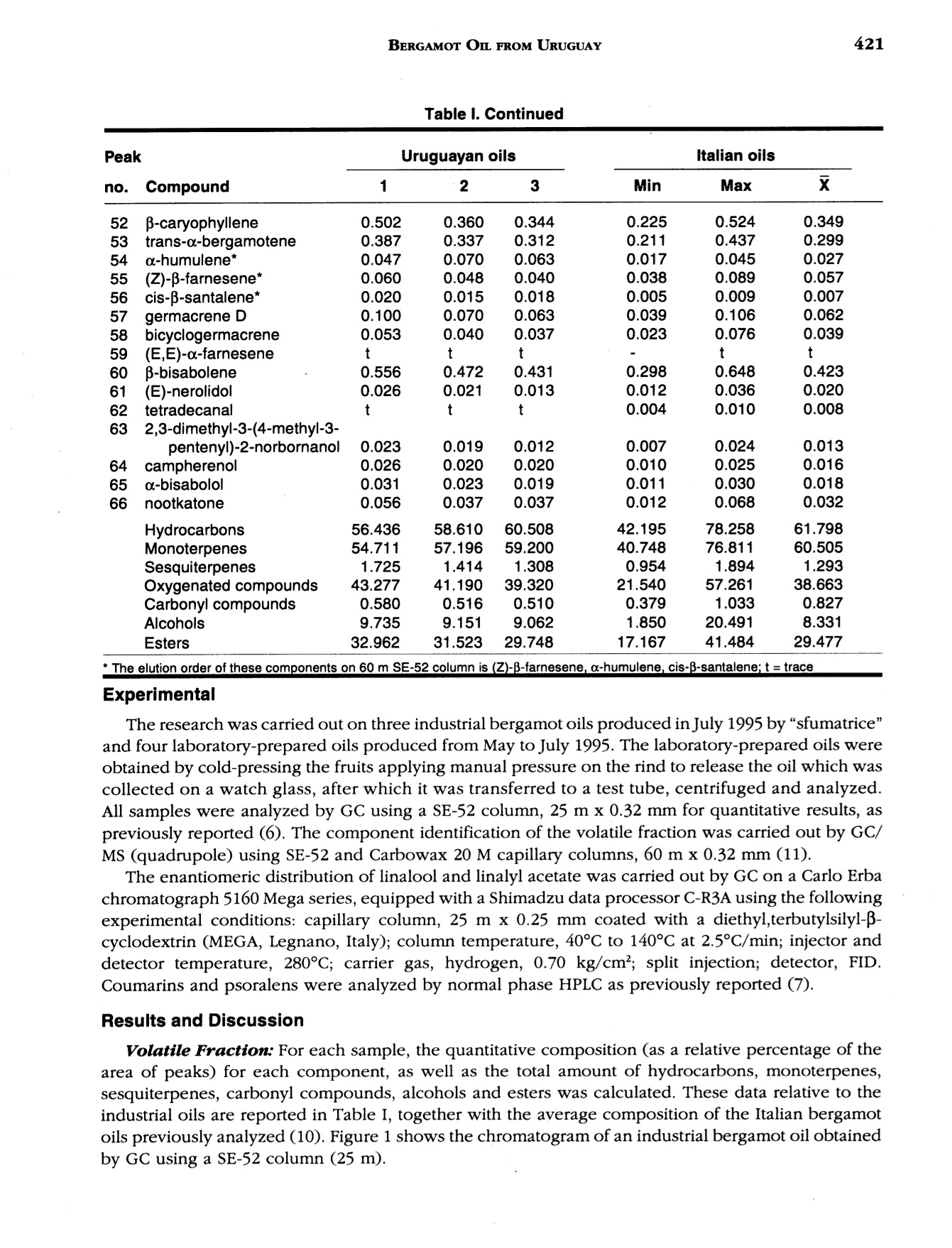
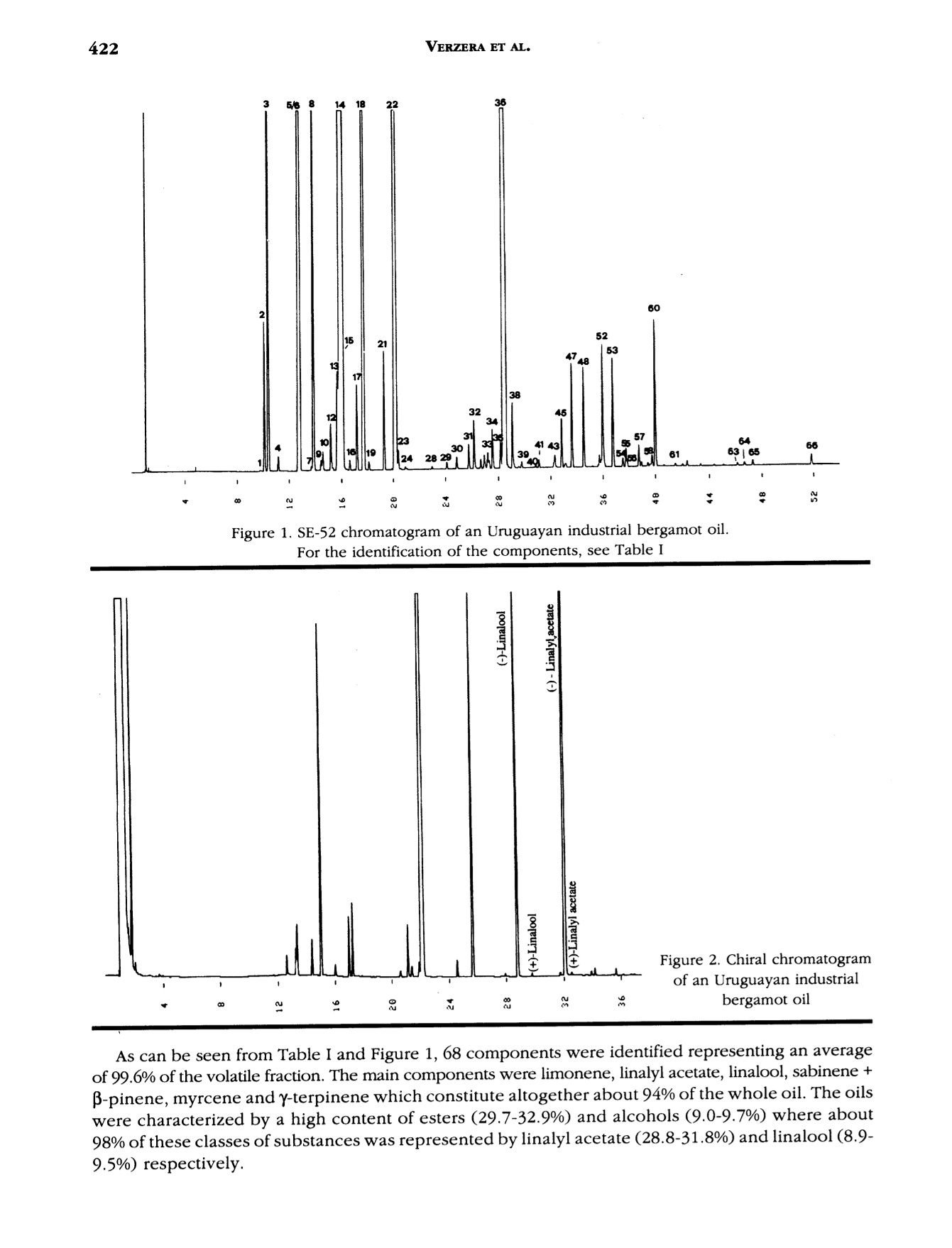
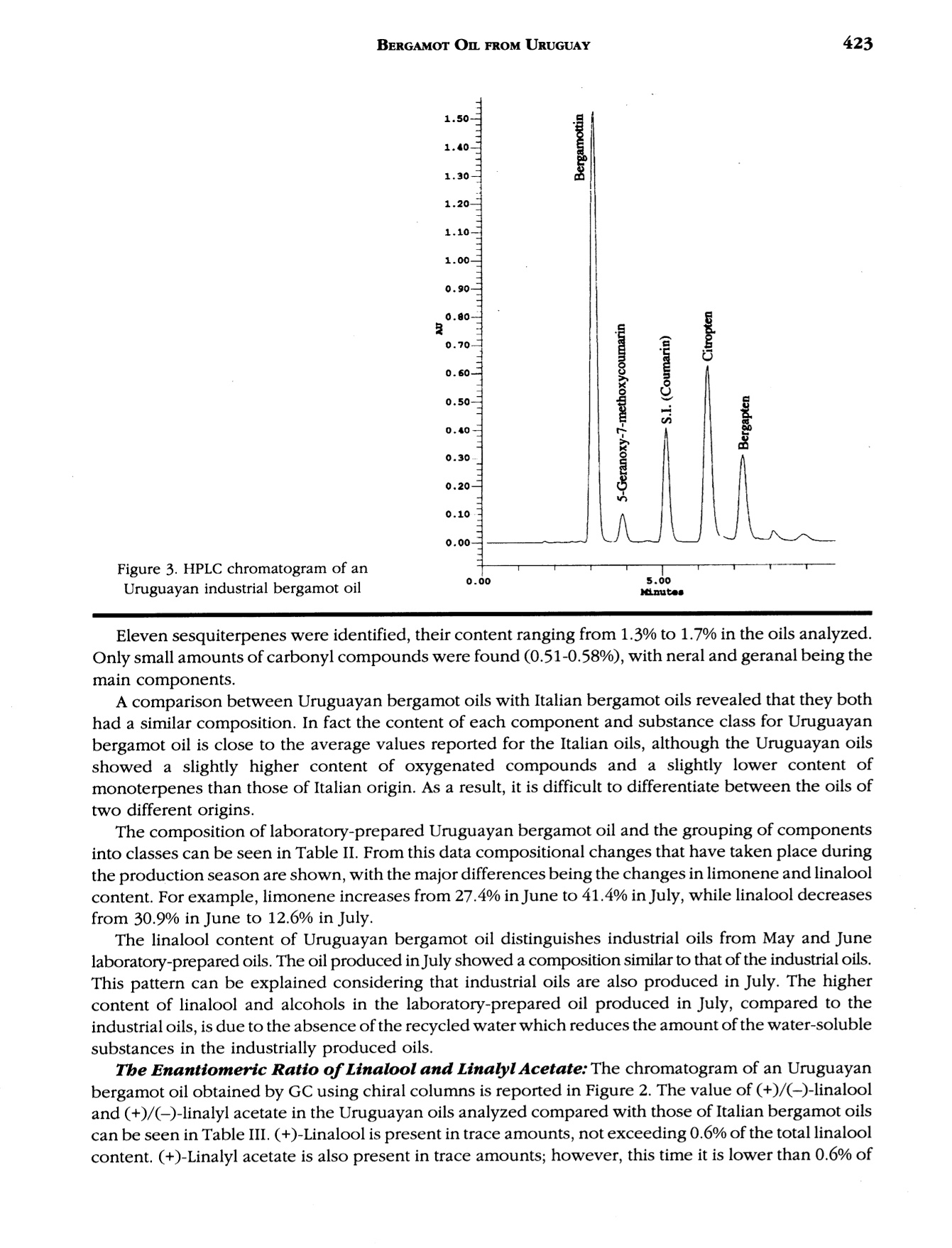
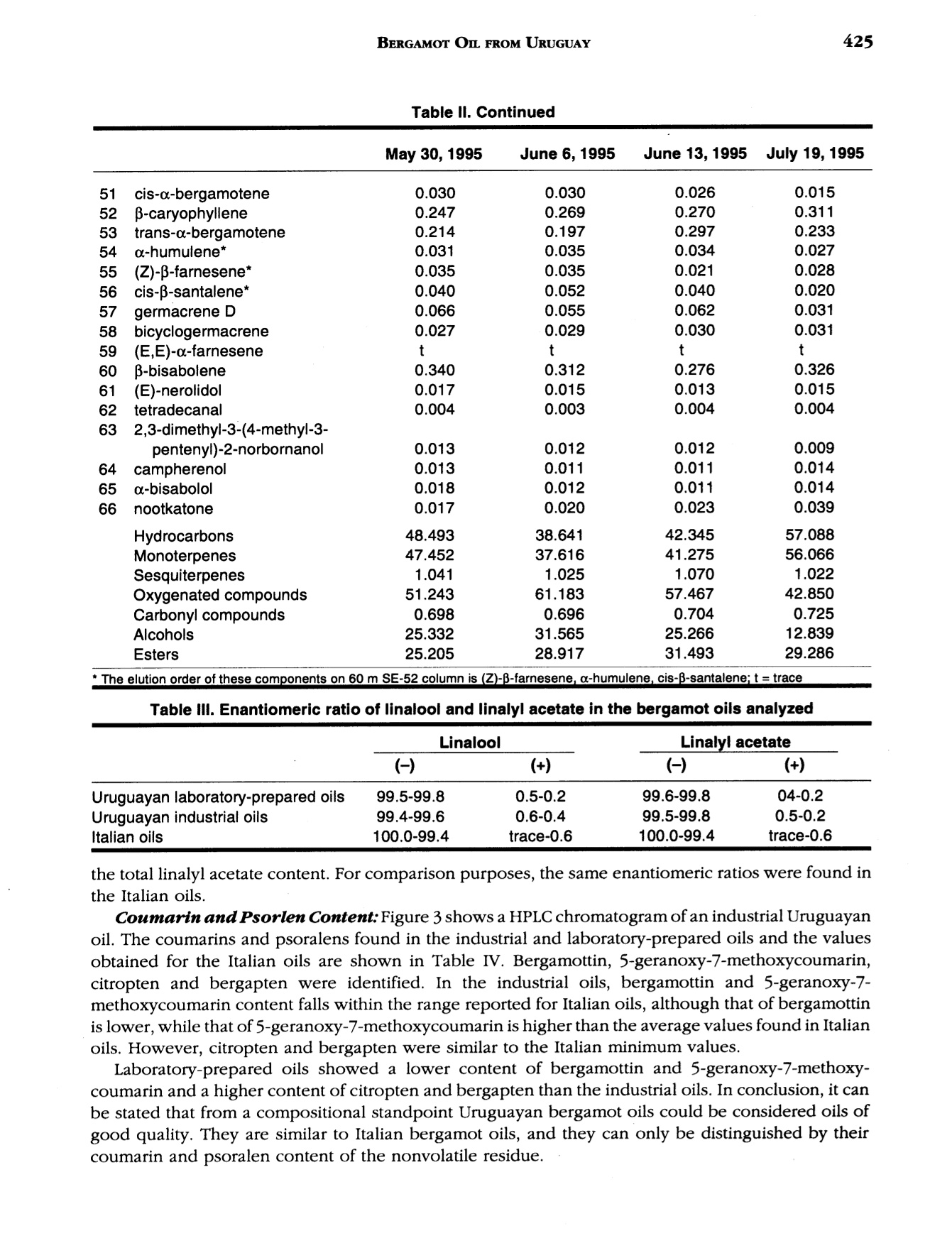
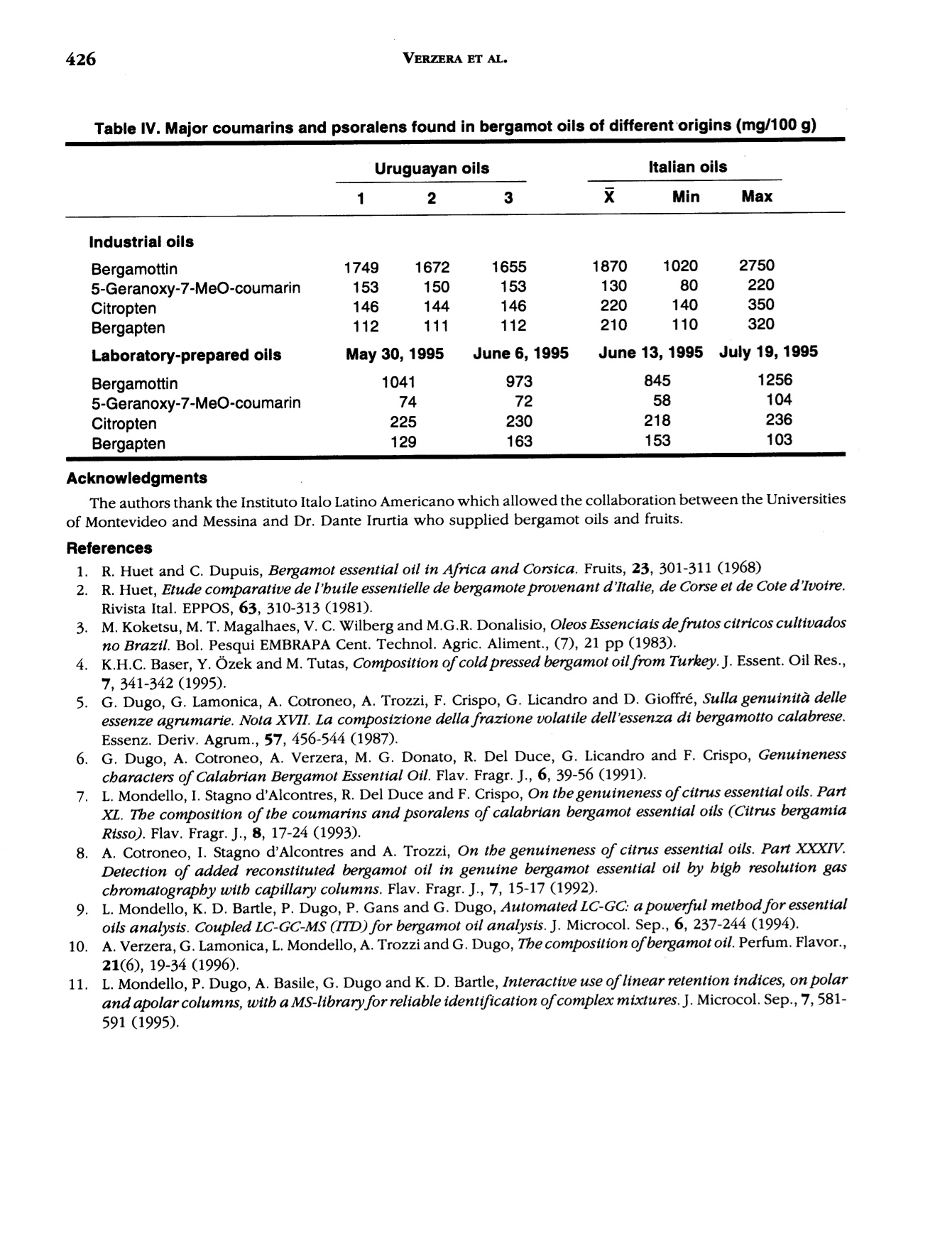
J. Essent. Oil Res., 9, 419-426 (Jul/Aug 1997) 420VERZERA ET AL. Received: Marcb1996Revised: June 19961041-2905/97/0004-0419$04.00/0—@1997 Allured Publishing Corp RESEARCH REPORT Uruguayan Essential Oils. Part V.Composition of Bergamot Oil Eduardo Dellacassa,Daniel Lorenzo and Patrick Moyna Catedra de Farmacognosia y Productos Naturales, Facultad de QuimicaUniversidad de la Republica, Montevideo, Uruguay Antonella Verzera* and Antonella Cavazza Dipartimento Farmaco-chimico, Facolta di Farmacia, Universita di Messina, Italy Abstract The analysis of industrial and laboratory-prepared bergamot oils produced inUruguay during the 1995 production season was carried out by GC and GC/MS. Thecomposition of the Uruguayan bergamot oils were compared with those of Italianbergamot oils. They were found to be very similar; however, they could bedifferentiated from their psoralen and coumarin contents. The enantiomeric ratio oflinalool and linalyl acetate was also studied by GC using a β-cyclodextrin column.The results were similar to that found in Italian oils. Key Word Index Citrus bergamia, Rutaceae, bergamot oil, essential oil composition, linalool,linalyl acetate, enantioselective GC, coumarins, psoralens. Introduction Citrus bergamia is mainly cultivated in Italy and therefore most of the bergamot oil on the marketis of Italian origin. Because of the great interest in bergamot oil, which is widely used in the food andcosmetic industries, bergamot cultivation has expanded to the Ivory Coast, Brazil, Argentina, Corsica,Guinea, Turkey and Cameroon. Some information about the composition of these oils is reported inthe literature (1-4). In Uruguay, bergamot has been cultivated in a limited area of about 3 h since 1980 using plantingstock of Italian origin. Recently the fruit has been processed by local industries using“birillatrice"machines for the juice and"sfumatrice”machines for the oil. The yield of the oil is about 4%. This paper reports the results relating to industrial and laboratory-prepared bergamot oils producedin Uruguay during the 1995 production season. The volatile fraction, the nonvolatile residue and thelinalool and linalyl acetate enantiomeric ratio were analyzed in order to define the composition and toevaluate the quality of Uruguayan bergamot oil. Its composition was then compared with that of thepreviously analyzed Italian bergamot oil (5-10). *Address for correspondence Table l. Chemical composition of industrial Uruguayan and Italian bergamot oils Italian oils Table l. Continued Peak Uruguayan oils Italian oils no. (Compound 1 2 3 Min Max 52 p-caryophyllene 0.502 0.360 0.344 0.225 0.524 0.349 53 trans-o-bergamotene 0.387 0.337 0.312 0.211 0.437 0.299 54 a-humulene* 0.047 0.070 0.063 0.017 0.045 0.027 55 (Z)-B-farnesene* 0.060 0.048 0.040 0.038 0.089 0.057 56 cis-p-santalene* 0.020 0.015 0.018 0.005 0.009 0.007 57 germacrene D 0.100 0.070 0.063 0.039 0.106 0.062 58 bicyclogermacrene 0.053 0.040 0.037 0.023 0.076 0.039 59 (E,E)-o-farnesene t t t t 60 B-bisabolene 0.556 0.472 0.431 0.298 0.648 0.423 61 (E)-nerolidol 0.026 0.021 0.013 0.012 0.036 0.020 62 tetradecanal t t t 0.004 0.010 0.008 63 2,3-dimethyl-3-(4-methyl-3- pentenyl)-2-norbornanol 0.023 0.019 0.012 0.007 0.024 0.013 64 campherenol 0.026 0.020 0.020 0.010 0.025 0.016 65 a-bisabolol 0.031 0.023 0.019 0.011 0.030 0.018 66 nootkatone 0.056 0.037 0.037 0.012 0.068 0.032 Hydrocarbons 56.436 58.610 60.508 42.195 78.258 61.798 Monoterpenes 54.711 57.196 59.200 40.748 76.811 60.505 Sesquiterpenes 1.725 1.414 1.308 0.954 1.894 1.293 Oxygenated compounds 43.277 41.190 39.320 21.540 57.261 38.663 Carbonyl compounds 0.580 0.516 0.510 0.379 1.033 0.827 Alcohols 9.735 9.151 9.062 1.850 20.491 8.331 Esters 32.962 31.523 29.748 17.167 41.484 29.477 * The elution order of these components on 60 m SE-52 column is (Z)-B-farnesene, a-humulene, cis-B-santalene:t= trace Experimental The research was carried out on three industrial bergamot oils produced in July 1995 by “sfumatrice”and four laboratory-prepared oils produced from May to July 1995. The laboratory-prepared oils wereobtained by cold-pressing the fruits applying manual pressure on the rind to release the oil which wascollected on a watch glass, after which it was transferred to a test tube, centrifuged and analyzed.All samples were analyzed by GC using a SE-52 column, 25 m x 0.32 mm for quantitative results, aspreviously reported (6). The component identification of the volatile fraction was carried out by GC/Ms (quadrupole) using SE-52 and Carbowax 20 M capillary columns, 60 m x 0.32 mm (11). The enantiomeric distribution of linalool and linalyl acetate was carried out by GC on a Carlo Erbachromatograph 5160 Mega series, equipped with a Shimadzu data processor C-R3A using the followingexperimental conditions: capillary column, 25 m x 0.25 mm coated with a diethyl,terbutylsilyl-p-cyclodextrin (MEGA, Legnano, Italy); column temperature, 40℃ to 140℃ at 2.5°C/min; injector anddetector temperature, 280℃; carrier gas, hydrogen, 0.70 kg/cm²; split injection; detector, FID.Coumarins and psoralens were analyzed by normal phase HPLC as previously reported (7). Results and Discussion Volatile Fraction: For each sample, the quantitative composition (as a relative percentage of thearea of peaks) for each component, as well as the total amount of hydrocarbons, monoterpenes,sesquiterpenes, carbonyl compounds,alcohols and esters was calculated. These data relative to theindustrial oils are reported in Table I, together with the average composition of the Italian bergamotoils previously analyzed (10). Figure 1 shows the chromatogram of an industrial bergamot oil obtainedby GC using a SE-52 column (25 m). Figure 1. SE-52 chromatogram of an Uruguayan industrial bergamot oil.For the identification of the components, see Table I As can be seen from Table I and Figure 1, 68 components were identified representing an averageof 99.6% of the volatile fraction. The main components were limonene, linalyl acetate, linalool, sabinene +P-pinene, myrcene and y-terpinene which constitute altogether about 94% of the whole oil. The oilswere characterized by a high content of esters (29.7-32.9%) and alcohols (9.0-9.7%) where about98% of these classes of substances was represented by linalyl acetate (28.8-31.8%) and linalool (8.9-9.5%) respectively. Eleven sesquiterpenes were identified, their content ranging from 1.3% to 1.7% in the oils analyzed.Only small amounts of carbonyl compounds were found (0.51-0.58%), with neral and geranal being themain components. A comparison between Uruguayan bergamot oils with Italian bergamot oils revealed that they bothhad a similar composition. In fact the content of each component and substance class for Uruguayanbergamot oil is close to the average values reported for the Italian oils, although the Uruguayan oilsshowed a slightly higher content of oxygenated compounds and a slightly lower content ofmonoterpenes than those of Italian origin. As a result, it is difficult to differentiate between the oils oftwo different origins. The composition of laboratory-prepared Uruguayan bergamot oil and the grouping of componentsinto classes can be seen in Table II. From this data compositional changes that have taken place duringthe production season are shown, with the major differences being the changes in limonene and linaloolcontent. For example, limonene increases from 27.4% in June to 41.4% in July, while linalool decreasesfrom 30.9% in June to 12.6% in July. The linalool content of Uruguayan bergamot oil distinguishes industrial oils from May and Junelaboratory-prepared oils. The oil produced in July showed a composition similar to that of the industrial oils.This pattern can be explained considering that industrial oils are also produced in July. The highercontent of linalool and alcohols in the laboratory-prepared oil produced in July, compared to theindustrial oils, is due to the absence of the recycled water which reduces the amount of the water-solublesubstances in the industrially produced oils. The Enantiomeric Ratio of Linalool and Linalyl Acetate: The chromatogram of an Uruguayanbergamot oil obtained by GC using chiral columns is reported in Figure 2. The value of (+)/(-)-linalooland (+)/(-)-linalyl acetate in the Uruguayan oils analyzed compared with those of Italian bergamot oilscan be seen in Table III. (+)-Linalool is present in trace amounts, not exceeding 0.6% of the total linaloolcontent. (+)-Linalyl acetate is also present in trace amounts; however, this time it is lower than 0.6% of Table ll. Chemical composition of laboratory-prepared Uruguayan bergamot oils Table Il. Continued May 30, 1995 June 6, 1995 June 13, 1995 J July 19, 1995 51 cis-o-bergamotene 0.030 0.030 0.026 0.015 52 p-caryophyllene 0.247 0.269 0.270 0.311 53 trans-o-bergamotene 0.214 0.197 0.297 0.233 54 a-humulene* 0.031 0.035 0.034 0.027 55 (Z)-B-farnesene* 0.035 0.035 0.021 0.028 56 cis-B-santalene* 0.040 0.052 0.040 0.020 57 germacrene D 0.066 0.055 0.062 0.031 58 bicyclogermacrene 0.027 0.029 0.030 0.031 59 (E,E)-a-farnesene t t t t 60 B-bisabolene 0.340 0.312 0.276 0.326 61 (E)-nerolidol 0.017 0.015 0.013 0.015 62 tetradecanal 0.004 0.003 0.004 0.004 63 2,3-dimethyl-3-(4-methyl-3- pentenyl)-2-norbornanol 0.013 0.012 0.012 0.009 64 campherenol 0.013 0.011 0.011 0.014 65 a-bisabolol 0.018 0.012 0.011 0.014 66 nootkatone 0.017 0.020 0.023 0.039 Hydrocarbons 48.493 38.641 42.345 57.088 Monoterpenes 47.452 37.616 41.275 56.066 Sesquiterpenes 1.041 1.025 1.070 1.022 Oxygenated compounds 51.243 61.183 57.467 42.850 Carbonyl compounds 0.698 0.696 0.704 0.725 Alcohols 25.332 31.565 25.266 12.839 Esters 25.205 28.917 31.493 29.286 * The elution order of these components on 60 m SE-52 column is (Z)-B-farnesene, a-humulene, cis-B-santalene: t = trace Table II. Enantiomeric ratio of linalool and linalyl acetate in the bergamot oils analyzed Linalool Linalyl acetate (-) (+) (-) (+) Uruguayan laboratory-prepared oils 99.5-99.8 0.5-0.2 99.6-99.8 04-0.2 Uruguayan industrial oils 99.4-99.6 0.6-0.4 99.5-99.8 0.5-0.2 Italian oils 100.0-99.4 trace-0.6 100.0-99.4 trace-0.6 the total linalyl acetate content. For comparison purposes, the same enantiomeric ratios were found inthe Italian oils. Coumarin and Psorlen Content: Figure 3 shows a HPLC chromatogram of an industrial Uruguayanoil. The coumarins and psoralens found in the industrial and laboratory-prepared oils and the valuesobtained for the Italian oils are shown in Table IV. Bergamottin, 5-geranoxy-7-methoxycoumarin,citropten and bergapten were identified. In the industrial oils, bergamottin and 5-geranoxy-7-methoxycoumarin content falls within the range reported for Italian oils, although that of bergamottinis lower, while that of 5-geranoxy-7-methoxycoumarin is higher than the average values found in Italianoils. However, citropten and bergapten were similar to the Italian minimum values. Laboratory-prepared oils showed a lower content of bergamottin and 5-geranoxy-7-methoxy-coumarin and a higher content of citropten and bergapten than the industrial oils. In conclusion, it canbe stated that from a compositional standpoint Uruguayan bergamot oils could be considered oils ofgood quality. They are similar to Italian bergamot oils, and they can only be distinguished by theircoumarin and psoralen content of the nonvolatile residue. Table IV. Major coumarins and psoralens found in bergamot oils of different origins (mg/100 g) Uruguayan oils Italian oils 2 3 Min Max Industrial oils Bergamottin 1749 1672 1655 1870 1020 2750 5-Geranoxy-7-MeO-coumarin 153 150 153 130 80 220 Citropten 146 144 146 220 140 350 Bergapten 112 111 112 210 110 320 Laboratory-prepared oils May 30, 1995 June 6, 1995 June 13, 1995 July 19, 1995 Bergamottin 1041 973 845 1256 5-Geranoxy-7-MeO-coumarin 74 72 58 104 Citropten 225 230 218 236 Bergapten 129 163 153 103 Acknowledgments The authors thank the Instituto Italo Latino Americano which allowed the collaboration between the Universitiesof Montevideo and Messina and Dr. Dante Irurtia who supplied bergamot oils and fruits. References R. Huet and C. Dupuis, Bergamot essential oil in Africa and Corsica. Fruits, 23, 301-311 (1968) 12R. Huet, Etude comparative de l'buile essentielle de bergamote provenant d'Italie, de Corse et de Cote d'Ivoire.Rivista Ital. EPPOS, 63, 310-313 (1981). 3. M. Koketsu,M. T. Magalhaes,V. C. Wilberg and M.G.R. Donalisio, Oleos Essenciais de frutos citricos cultivadosno Brazil. Bol. Pesqui EMBRAPA Cent. Technol. Agric. Aliment., (7), 21 pp (1983). 4. K.H.C. Baser, Y.Ozek and M. Tutas, Composition of cold pressed bergamot oil from Turkey. J. Essent. Oil Res.,7,341-342(1995). ( 5. . G . Dugo, G. La m onica, A. Cotroneo, A. Tr o zz i , F. Crisp o , G. Li c andro and D. Gioffre, Sulla genuinita delleessenze agrumarie. N o ta XVII. L a composizione del l a frazione volatile dell’essenza d i b e rgamotto calabrese.Essenz. Deriv. Agrum.,57,456-544(1987). ) ( 6. G . D ugo, A. Cotroneo, A. Ve r zera, M. G. Don a to, R. Del D uce , G. Li c an d ro and F. Crispo, Genuin e nesscbaracters of Calabrian Bergamot Essential Oil. Flav. Fragr . J., 6 , 39-5 6 (1991). ) ( 7. L. Mondello, I. Stagno d’Alcontres,R. Del Duce and F. Crispo, On the genuineness ofcitrus essential oils. Part XL. The composition of the coumarins and psoralens of calabrian bergamot essential oils (Citrus bergamia Risso). Flav. Fragr. J., 8 , 17-24(1993). ) ( 8.A. C otroneo, I . Stagno d'Alcontres an d A. Trozzi, On t he g enuineness of citrus essential oils. Part XXXIV. Detection of added reconstituted bergamot oil i n genuine bergamot e s sential o il by b igb r esolution gas cbromatograpby with capillary columns. Fl a v. Fragr. J . , 7 , 15-17 (1992). ) ( 9. L. Mondello, K. D. Bartle, P. Dugo, P. Gans and G. Dugo, Automated LC-GC: a powerful method for essentialoils analysis. Coupled LC-GC-MS (ITD) for bergamot oil analysis. J. Microcol. Se p ., 6, 2 37 - 244 (1994). ) ( 10. A. Verzera, G.Lamonica, L. Mondello, A. Trozzi and G. Dugo, The composition ofbergamot oil. Perfum. Flavor.,21(6), 19-34(1996). ) ( 11. L . Mondello, P . Dugo, A. Basile, G. Dugo and K. D. Bartle, Int e ractive use ofl i near retention indices, on polarand apolar columns, witb a MS-library for reliable identification ofcomplex mixtures. J. Microcol. Sep.,7,581- 591 (1995). )
确定

还剩6页未读,是否继续阅读?
扬州华明仪器设备有限公司为您提供《佛手甘精油中不同国家产地成分对比检测方案 》,该方案主要用于日用化学品/香精香料中不同国家产地成分对比检测,参考标准--,《佛手甘精油中不同国家产地成分对比检测方案 》用到的仪器有
相关方案
更多








