方案详情
文
Vaccination prevents millions of deaths from infectious diseases per year; however, there are still millions of people who die from these diseases due to weak thermostability demanding cold chains, long-term stability issues and distribution challenges. All these limitations can be overcome by creating a dry product through lyophilization. With the urgency to develop a coronavirus vaccine, it is a crucial time to discuss the process of freeze-drying vaccines.
方案详情

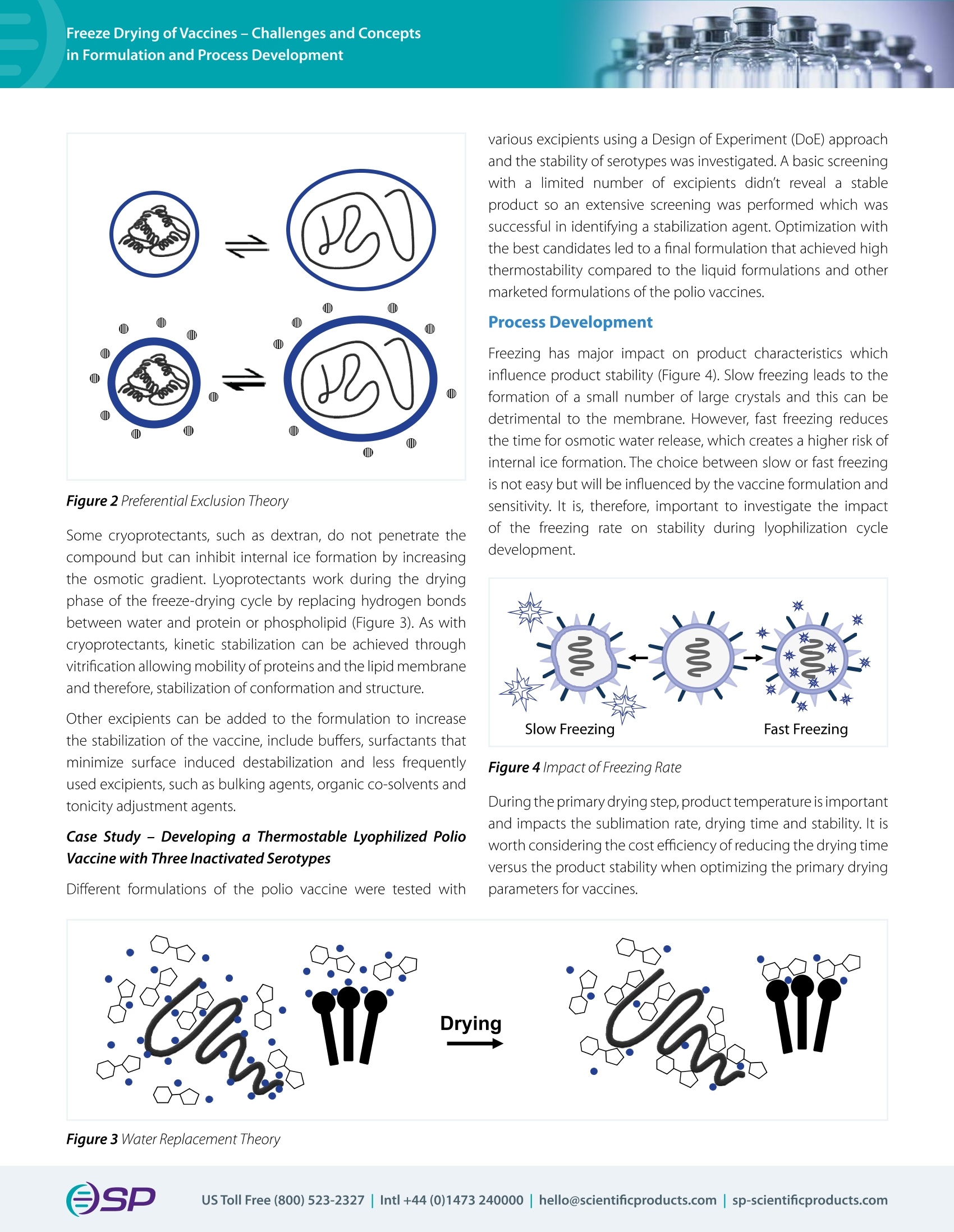
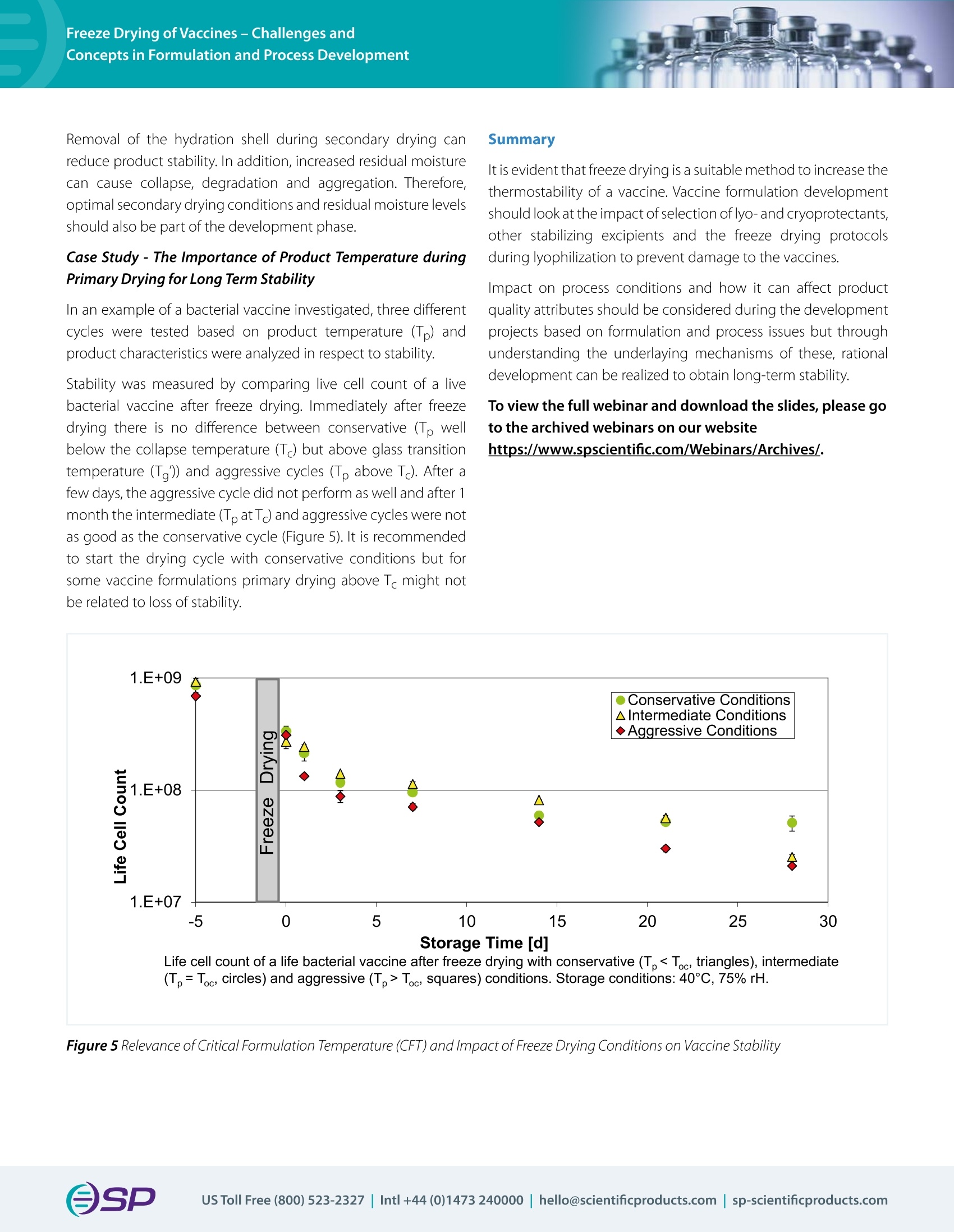
Vaccination prevents millions of deaths from infectious diseases per year; however, there are still millions of people who die from these diseases due to weak thermostability demanding cold chains, long-term stability issues and distribution challenges. All these limitations can be overcome by creating a dry product through lyophilization. With the urgency to develop a coronavirus vaccine, it is a crucial time to discuss the process of freeze-drying vaccines.Recently, Ms. Julia Kosan, a PhD candidate from Friedrich Alexander University, Erlangen, Germany presented a webinar that discussed the suitability of freeze-drying vaccines to increase their thermostability. This tech note summarizes the webinar and includes a selection of questions from the Q&A session.W E B I N A R T E C H N O T EFreeze Drying of Vaccines – Challenges and Concepts in Formulation and Process Development OSPVirTis Presenter: Julia Kosan Ph D candidate, Friedrich Alexander University, Erlangen, Germany - 7th May 2020 Co-Author: PD Dr. Henning Gieseler, CSO of Gilyos GmbH Vaccination prevents millions of deaths from infectious diseases per year; however, there are still millions of people who die from thesediseasesduetoweakthermostabilitydemandingcold chains,long-termstabilityissuesanddistributionchallenges. All these limitations can be overcome by creating a dry product through lyophilization. With the urgency to develop a coronavirus vaccine, it is a crucial time to discuss the process of freeze-drying vaccines. Recently, Ms. Julia Kosan, a PhD candidate from Friedrich Alexander University, Erlangen, Germany presented a webinar that discussed the suitability of freeze-drying vaccines to increase their thermostability. This tech note summarizes the webinar and includes a selection of questions from the Q&A session. Impact of Lyophilization on Vaccines There are many types of vaccines all of which trigger specifci immune responses.Severalcoronavirusvaccinesunderdevelopmentare basedonrecombinantvectorvaccinewhichusescoronavirus genomic material packaged into a viral vector. Freeze-drying a vaccine provides a signifciant advantage but there are several obstacles that need to be overcome. Complex formulations, especially vaccines that are made up of several antigens or multiple strains can result in challenging critical formulation temperatures and complicated freeze-drying processes. Freezing and drying can stress the vaccine whereby the extent of this process sensitivity will vary between difefrent vaccines. Internal ice formation and direct damage to a component of the vaccine e.g. the lipid membrane, proteins or nucleic acids can act as stress factors. During freezing, intraviral ice crystals can form which will increase the volume of the product and may damage the lipid bilayer (Figure 1). The ice also creates new interfaces between liquid and ice and increases the risk of surface induced aggregation. Figure 1 Stress Factors During Freeze Drying In the primary drying step of the lyophilization cycle, drying above the critical formulation temperature results in increased mobility of the amorphous phase which allows protein interactions and can increase membrane permeability. As part of the secondary drying stage where the hydration shell of each is removed, protein aggregation and inactivation can occur, and in the case of phospholipids, the change in thermotropic phase transition can also increase membrane permeability. Secondary drying directly afefcts residual moisture levels which can impact long term stability. Required Characteristics of Vaccine Formulations Optimally, vaccines need to be stable in both liquid state for at least 24 hours and dry state for long term storage. To achieve this, vaccines need to be developed with appropriate formulation and processes. Stabilizers (cryo- or lyo-protectants) play a huge role in developing a stable vaccine formulation. During freezing, the amorphous cryoprotectants, such as saccharides and sugar alcohols thermodynamically stabilize through preferential exclusion of the cryoprotectant and hydration of the protein (Figure 2). They also provide kinetic stabilization through vitrifciation which decelerates aggregation of proteins and lipid membrane. Figure 2 Preferential Exclusion Theory Some cryoprotectants, such as dextran, do not penetrate the compound but can inhibit internal ice formation by increasing the osmotic gradient. Lyoprotectants work during the drying phase of the freeze-drying cycle by replacing hydrogen bonds between water and protein or phospholipid (Figure 3). As with cryoprotectants, kinetic stabilization can be achieved through vitrifciation allowing mobility of proteins and the lipid membrane and therefore, stabilization of conformation and structure. Other excipients can be added to the formulation to increase the stabilization of the vaccine, include bufefrs, surfactants that minimizesurfaceinduceddestabilizationandlessfrequently used excipients, such as bulking agents, organic co-solvents and tonicity adjustment agents. CaseStudy–DevelopingaThermostableLyophilizedPolio Vaccine with Three Inactivated Serotypes Difefrentformulationsofthepoliovaccineweretestedwith various excipients using a Design of Experiment (DoE) approach and the stability of serotypes was investigated. A basic screening with a limited numberofexcipientsdidn’t reveal a stable product so an extensive screening was performed which was successful in identifying a stabilization agent. Optimization with the best candidates led to a final formulation that achieved high thermostability compared to the liquid formulations and other marketed formulations of the polio vaccines. Process Development Freezinghasmajorimpactonproductcharacteristicswhich influence product stability (Figure 4). Slow freezing leads to the formation of a small number of large crystals and this can be detrimental to the membrane. However, fast freezing reduces the time for osmotic water release, which creates a higher risk of internal ice formation. The choice between slow or fast freezing is not easy but will be influenced by the vaccine formulation and sensitivity. It is, therefore, important to investigate the impact of the freezing rate on stability during lyophilization cycle development. Figure 4 Impact of Freezing Rate During the primary drying step, product temperature is important and impacts the sublimation rate, drying time and stability. It is worth considering the cost efcfieincy of reducing the drying time versus the product stability when optimizing the primary drying parameters for vaccines. Figure 3 Water Replacement Theory Removalofthehydrationshellduringsecondarydryingcan reduce product stability. In addition, increased residual moisture cancausecollapse,degradationandaggregation.Therefore, optimal secondary drying conditions and residual moisture levels should also be part of the development phase. Case Study - The Importance of Product Temperature during Primary Drying for Long Term Stability In an example of a bacterial vaccine investigated, three difefrent cyclesweretestedbasedonproducttemperature(Tp)and product characteristics were analyzed in respect to stability. Stability was measured by comparing live cell count of a live bacterial vaccine after freeze drying. Immediately after freeze dryingthereisnodifefrencebetweenconservative(Tpwell below the collapse temperature (Tc) but above glass transition temperature (Tg’)) and aggressive cycles (Tp above Tc). After a few days, the aggressive cycle did not perform as well and after 1month the intermediate (Tp at Tc) and aggressive cycles were not as good as the conservative cycle (Figure 5). It is recommended to start the drying cycle with conservative conditions but for some vaccine formulations primary drying above Tc might not be related to loss of stability. Summary It is evident that freeze drying is a suitable method to increase the thermostability of a vaccine. Vaccine formulation development should look at the impact of selection of lyo- and cryoprotectants, otherstabilizingexcipients andthefreezedryingprotocols during lyophilization to prevent damage to the vaccines. Impact on process conditions and how it can afefct product quality attributes should be considered during the development projects based on formulation and process issues but through understanding the underlaying mechanisms of these, rational development can be realized to obtain long-term stability. To view the full webinar and download the slides, please go to the archived webinars on our website https://www.spscientifci.com/Webinars/Archives/. (Tp = Toc, circles) and aggressive (Tp > Toc, squares) conditions. Storage conditions: 40°C, 75% rH.
确定

还剩1页未读,是否继续阅读?
德祥科技有限公司为您提供《疫苗的冷冻干燥-在配方和工艺开发中的挑战和概念》,该方案主要用于其他中冷冻干燥疫苗检测,参考标准--,《疫苗的冷冻干燥-在配方和工艺开发中的挑战和概念》用到的仪器有SP台式冻干机Virtis AdVantage Pro、Biopharma Technology冻干显微镜LyoStat5、Biopharma Micropress冻干饼强度测试仪
推荐专场
相关方案
更多
该厂商其他方案
更多