方案详情
文
在本申请说明中,分子量测量是基于欧洲药典方法进行的,用于使用ChromNAV的低分子质量肝素分子量计算程序分析平均分子量和分子量分布。
关键词:低分子肝素, Parnaparin sodium, Dalteparin sodium,欧洲药典,分子量测定,紫外检测器,折射率检测器,低分子肝素分子量计算程序
方案详情

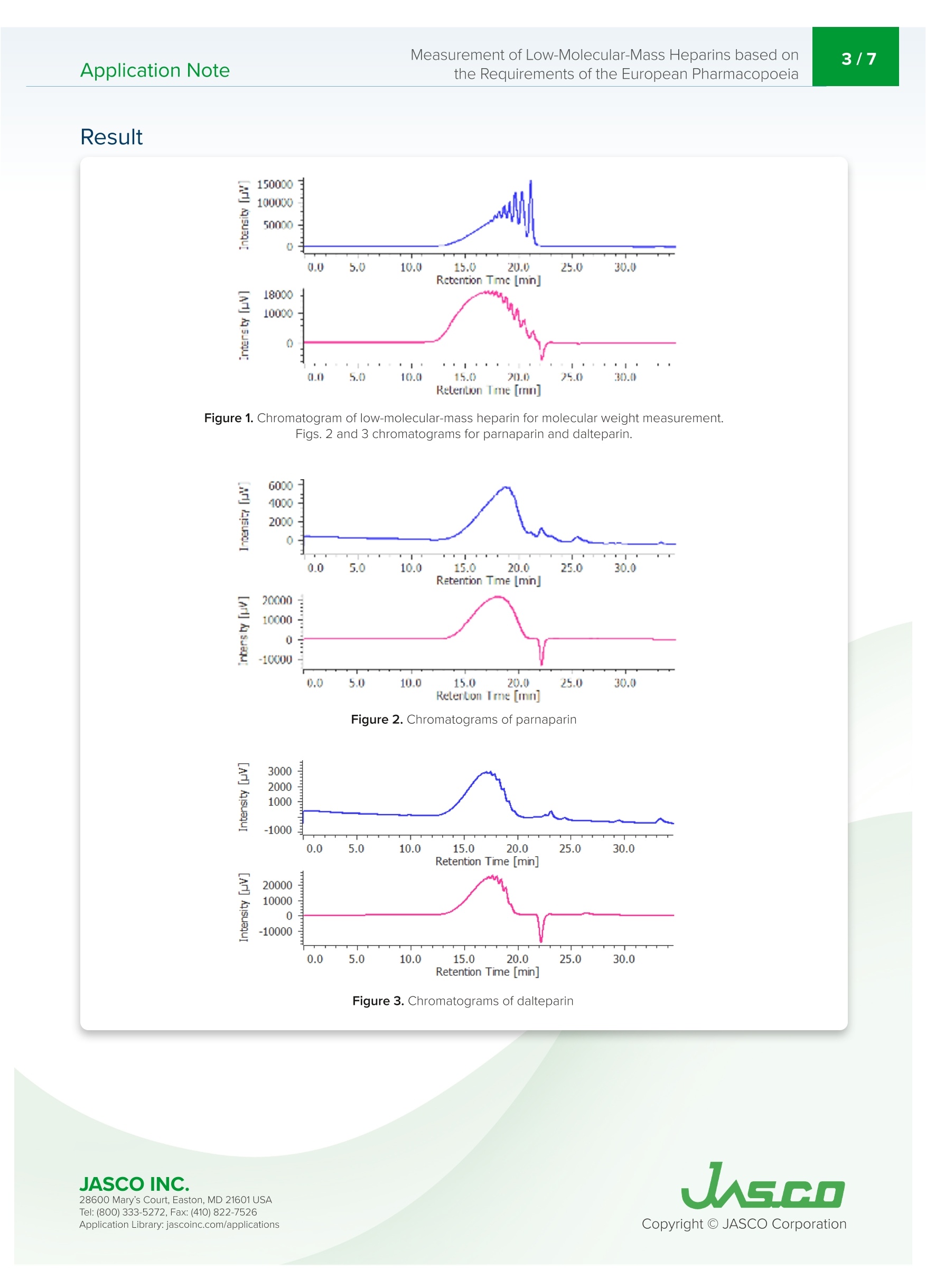
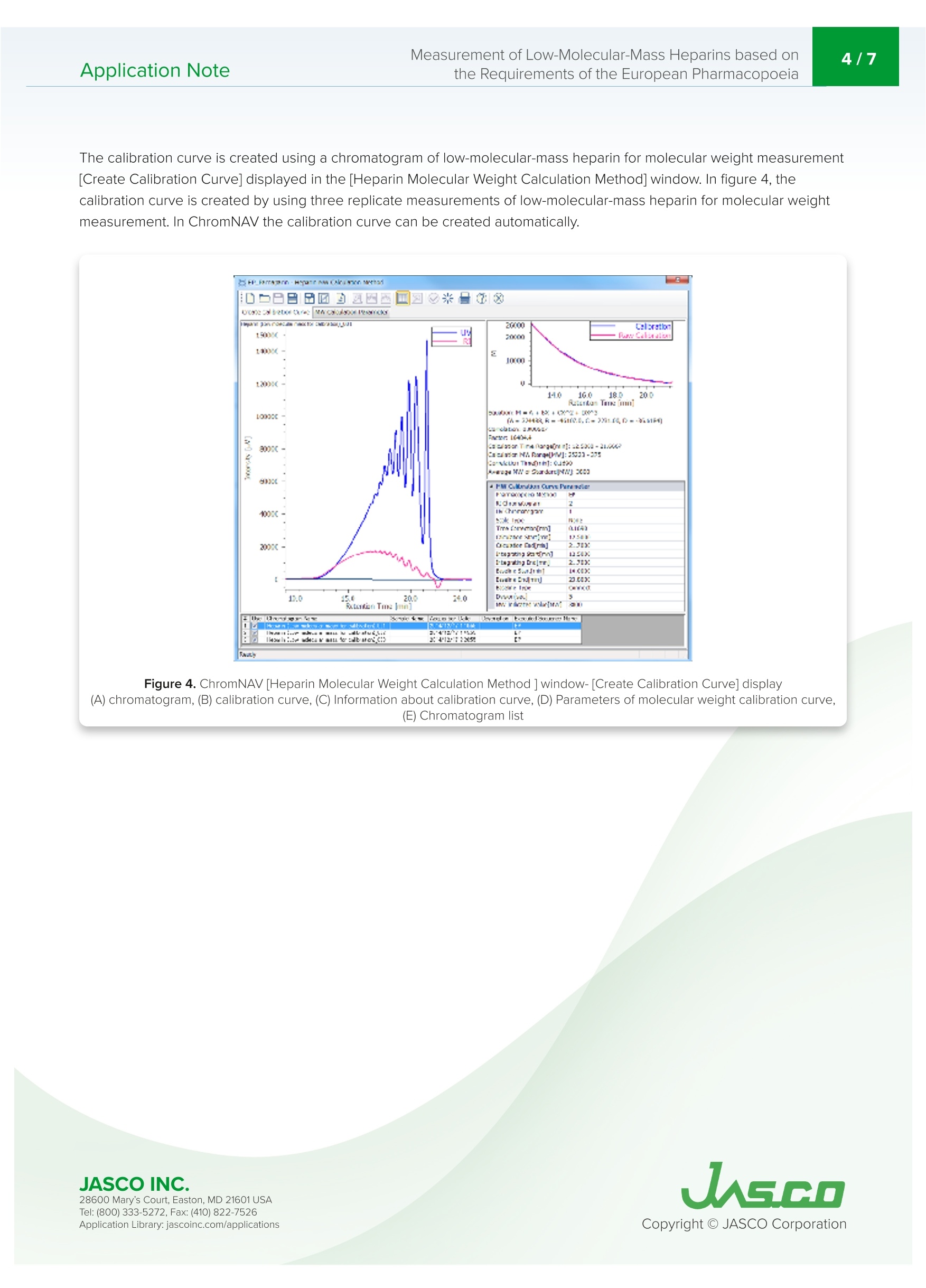
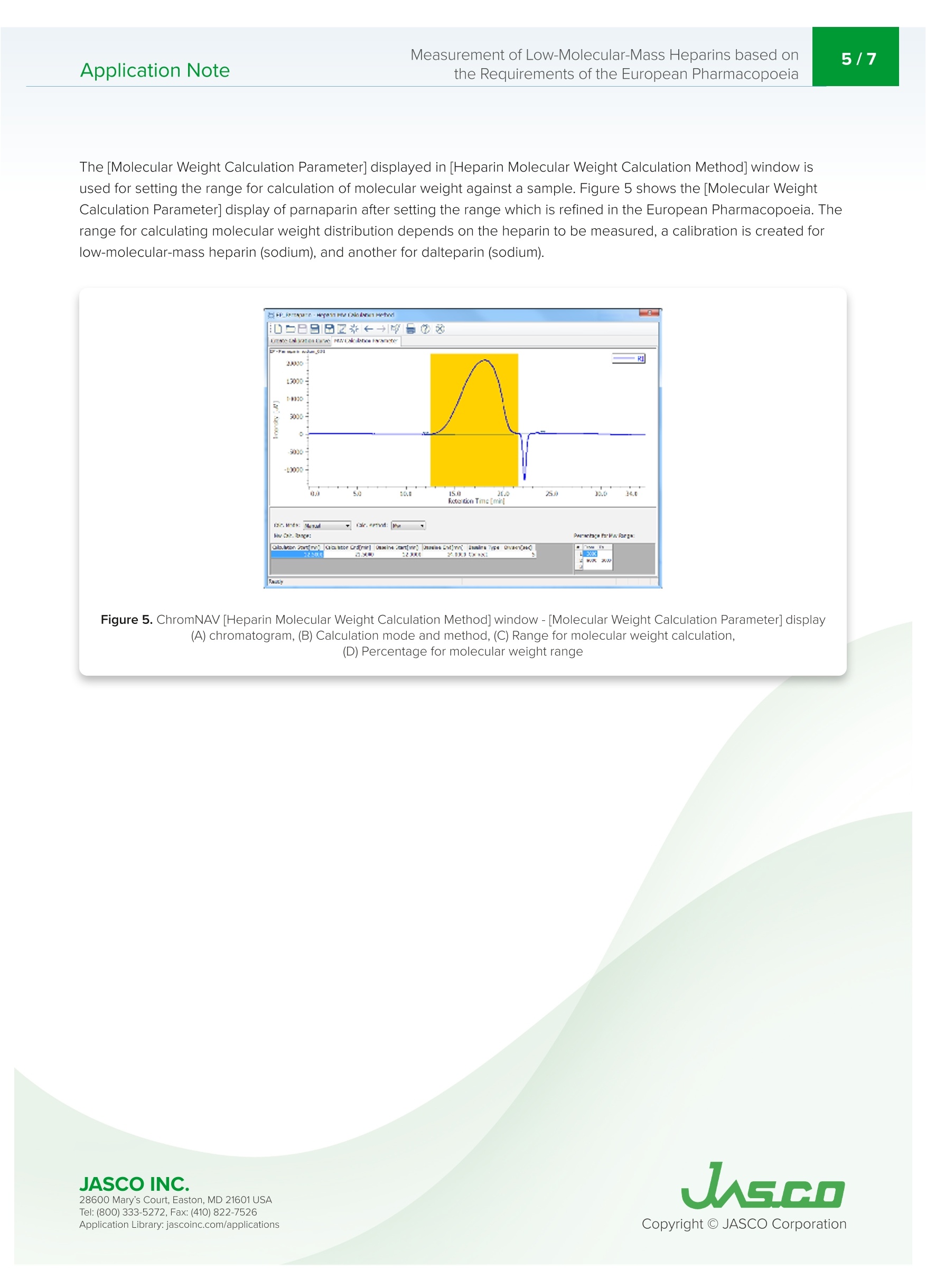
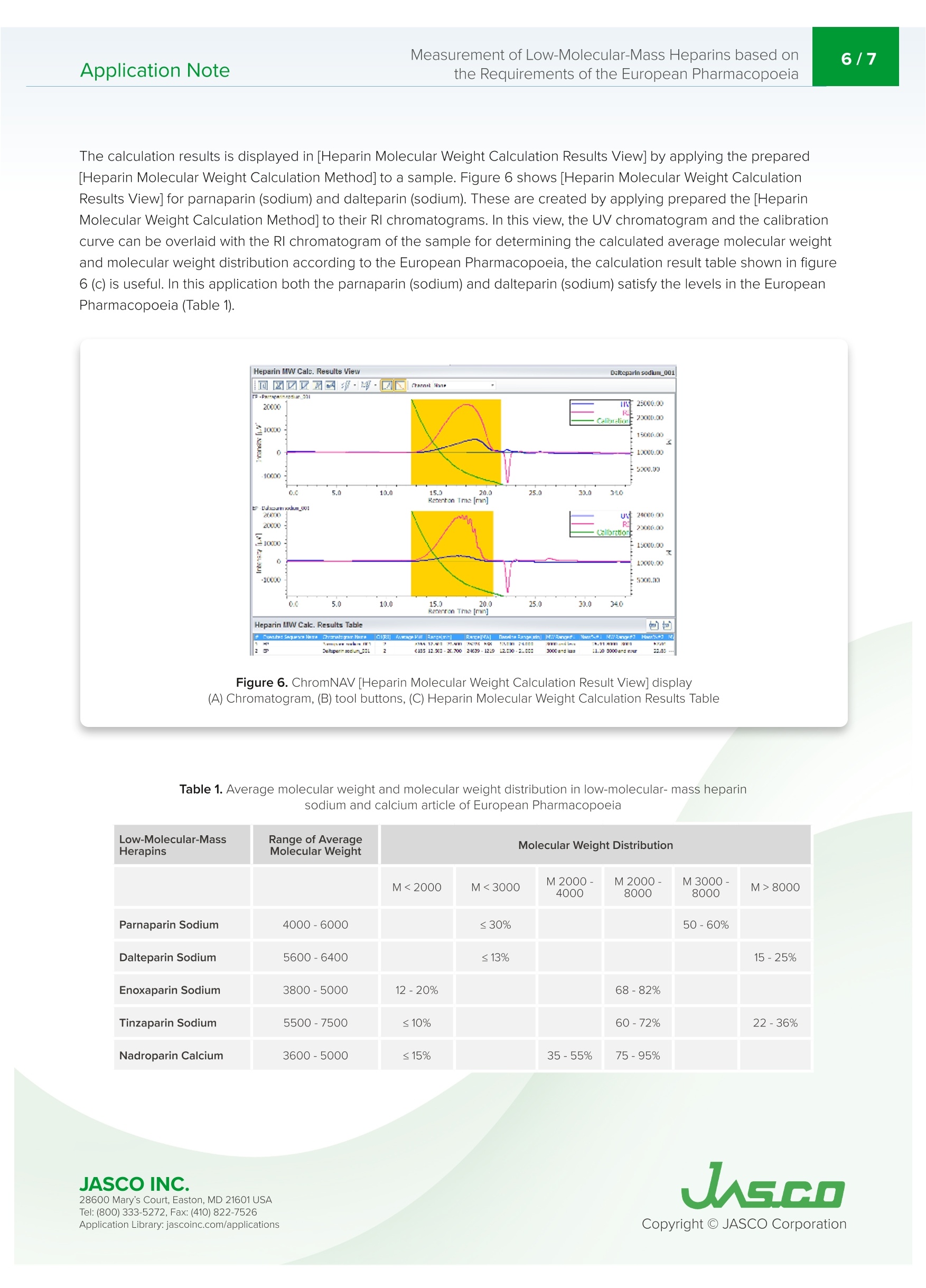
肝素是从猪小肠中获得的粘多糖,在制药领域被广泛用作治疗和预防血栓栓塞、治疗弥散性血管内凝血综合征的抗凝血药物,以及用作使用体外血液循环设备(如血液透析和人工心/肺)的抗凝血剂。低分子肝素是通过酶处理和化学处理普通肝素生产的。根据分解方法和分子量分布,低分子肝素可分为帕那肝素、达肝素、依诺肝素等。欧洲药典第八版(EP),分子量测量方法在低分子量肝素的医药产品文章中定义,并且平均分子量和分子量分布也在低分子质量肝素(钠)和低分子质肝素(钙)的医药文章中定义。带UV-Vis探测器和折射率探测器的SEC是定义的测量方法。使用该方法,通过使用低分子量肝素作为参考样品来创建使用低分子质量肝素的分子量校准曲线。在使用SEC的常见分子量测量方法中,根据保留体积和分子量创建校准曲线。这种常见的方法使用具有已知分子量的低聚物和聚合物作为标准。然而,分子量必须通过标准化RI和UV的系数和强度比来计算,以创建校准曲线。这种分析通常过于复杂,无法使用普通的商业SEC软件。在本申请说明中并证明,分子量测量是基于欧洲药典方法进行的,用于使用ChromNAV的低分子质量肝素分子量计算程序分析平均分子量和分子量分布。Application Note747014H(1/6) Measurement of Low-Molecular-Mass Heparins based on2/7Application Notethe Requirements of the European Pharmacopoeia Measurement of Low-Molecular-Mass Heparins base d on the Requirements of the European Pharmacopoeia I n trod u c t ion Hepari n s are a m ucopolysacc h a ri de obtai n ed f r om po r c i ne smal l i ntestine and i s widely used in the p h armace ut ical f ield as an a n t i clotti n g drug for t r e atm en t and pr e vent i on o f t hromboembol i sm , tr e at m ent of dissemi n at e d i ntravascular coagulation syndrome, and as an ant i -blood c l otti n g agent for u sing extracorporeal blood ci r cu l a t ing apparat u s suc h as blood dialysis a nd artif i cial heart/lung. Low-molecular -m ass h eparin i s produced by enzyma t ic treatme n t and che m ical treatment of unf r act i o n ated heparin. Low-molec ul ar-mass h ep arin i s c l assi f i e d as parnapar in, dal te p arin, e noxaparin, and so on by a d e compos i t i o n met h od and mo l ecular weight distr i bu ti on . LC-4000 Series H P LC System The Europe a n Pharmacopoei a 8t h Edition (EP), molecular we i ght measurement method i s def i ned i n a p h armace u t ical prod u cts article for low-m olecula r -mass heparin a n d t h e average m ol e cular we i ght a nd molecu l a r weight dist ri but i o n a r e also d e fined i n the p h ar maceut i cal ar t ic le f or low-molecular -mass h e p ar i n (sodium) and low-molec ul ar-mass hepari n (ca l c i um). SEC with a UV-Vis detector a n d Refractive Index detector are the defined measureme n t met h od . Us i ng t his m ethod, a mo l ec u lar weight cal i brat i o n curve us i ng l ow-molecular-ma s s hepari n i s crea t ed by using low-mo l ecular -mass hepar in as a re f e r e n ce sample. In common molecular weight measurement methods u sing SEC, a cal i b r ation curve i s c r ea t ed f r om t h e r etention volume and molecula r weight. T hi s common m e t h od us e s oligo m ers and po l ymers wit h k nown mo l ec ul ar weight as t he standards. However, molec ul ar we i g h ts have to be ca l culated by standardizi n g coef fi cient and i nte n s i ty rat i o of Rl and UV to create the cal i bration curve.This analysis is ty p ical l y too compl i cated for n or m a l comme r cial SEC software to be used . I n this ap p licat i o n note, mo l ec u lar weight m easure m ent i s made based o n the European Pharmacopoeia method for t h e a n alysis of average mo l ec u lar weight and mol e cu l ar we i ght di s tribution u sing th e ChromNAV Low-Molecular-Mass Hepar in Mo l ecula r Weigh t Calculation prog r am for C h ro m NAV. Keywords Low-molecular-mass h epar i n, Pa rn apar i n sodium , Daltepa rin sodium, European Pharmacopoeia, Molecular weight measurement, UV detector, Refractive I ndex d e tector, L ow-Molecular-Mass Heparin Molecular weight calculation program Ap p l i cat i on L i b ra r y: jasco i nc.co m /ap p licat i ons Exp er imen t al Equipment Pump PU-4185 Pump Option Degasser Autosampler AS-4050 Autosampler Option TC Unit Column Oven CO-4060 Detector UV-4075, RI-4030 Conditions Column TSKgel G2000SWXL (7.8 mm I.D. x300 mm L, 5 um) Eluent Aqueous solution of 0.2 M anhydrous sodium sulfate(adjusted to pH 5.0 with 0.05 M sulfuric acid) Flow Rate 0.5 mL/min Column Temp 30℃ Wavelength 234nm Injection Volume 25mL Standard 20 mg of low-molecular-mass heparin for calibration CRS*in 2 mL of the eluent*number average relative molecular mass: 3,800 Sample 20 mg of Parnaparin sodium CRS in 2 mL of the eluent20 mg of Dalteparin sodium CRS in 2 mL of the eluent 20 m g o f Da ltepa r i n s o d i u m C R S in 2 mL of t h e elu e nt S O ,N a n =1 to 21,R =H or SON a ,R'=SOsNaor CO-CH n =3 to 20,R =H or SOsN a ,R'=SOsNaor CO-CH3 R2=H and R3=CO,NaorR2=CO,Naand R3=H R2=H and R3=CO,NaorR2=CO,Naand R3=H P a r n a p a r in S od i u m D al te par in S odi u m 28600 M ary's C ou r t, East on , MD 21601 USA Te l : (800) 333-5272, F ax:(410) 822-7526 Figure 1. C h ro m a tog r am of low -m o l ecular -m a ss h e pa rin f or m o l e cu l ar we i g ht me a su r e m e n t .Fi g s . 2 a nd 3 ch roma to gr a m s f o r par n a par in a n d d a l tep ar in . Figure 2. C h ro m a t o g ra m s of p arn a pa ri n Figure 3. Ch r o mat ogram s o f da l te par i n Th e cal i bration curve i s c r eat e d u sing a c h romatogram of low-molecular -mass hep arin for molec ul ar weight meas u rement [Create Calibration Curve] displayed i n the [Hepar in Mo l ecular We i ght Calculation Method] window. I n f i gure 4, t h e calibrat i on curve is c r eated b y usi n g three r epl i cate meas u rements of l ow-molecular-mass h epar i n for m olecular weigh t meas u r e ment. In ChromNAV t he ca l i b rat i on curve can b e c r ea t e d automatically. Figure 4. C hro mNAV [H e par in Mo lecu l a r W e ig h t C a l c u lati on Me t hod ] w i ndow -[Cre a t e C al ib ra tio n C u rv e ] d is pl a y (A ) c h r o m at o gr am , (B) c a li bra t i o n cu rve, (C) I nf o rm a t i o n a b o ut c a l ibra t i o n c ur v e, (D ) P a ram et e rs of m o lecu la r w eig h t c a l ib ratio n cur ve,(E) C h ro m a to g ra m li s t The [Molecular Weight Calculat i o n Pa r amete r] displayed in [Heparin Molecular We i ght Calculation Method ] window is used for setti n g t he range for calculation of m olecular weight agai n st a sa m ple. Figure 5 shows t h e [Molecular We i ght Calculation Parameter ] dis p la y of par n apa ri n after s e tting t h e r ange which is r ef i ned i n t h e E u ropean Pharmacopoeia . T h e r ange f or calculating molecular we i ght distribution d e pends on the h epar i n to b e me a sured, a calibratio n i s created for l ow-molec u lar-mass hepar i n (sodi u m ), and anot h e r fo r daltepa r in (sodium ). Figure 5. Ch ro m N AV [H ep ar i n M o lecu lar W e ig h t C a l c u la tio n Method ] wi n dow-[Mo lecu l a r W ei gh t C a lcu la t io n P a ra m e t e r ] d i spla y (A ) ch ro m a t o gra m , (B ) Ca l c ula t io n m o de a n d meth od, (C ) Ra n ge f o r m o le c u l a r we i gh t ca l cula t io n ,(D) P e rc en t ag e f o r m o l ec u la r we ig ht r an g e 28600 M ary's C ou r t, East on , MD 21601 USA Te l : (800) 333-5272, F ax:(410) 822-7526 The calculation r esults is displayed in [Heparin Molecula r Weig h t Calcu l at i on Res ul ts View] by applyi n g t h e p r epared [H epari n Mo l ecu l ar Weigh t Calculation Method] to a sample. F i gu r e 6 s h ows [H epar i n Molecular Weight Ca l culat i on Results View] for p arnapar in (sod i u m) and daltepar in (sodiu m ). T h ese are created b y a pp l y i n g prepared the [He p arin Molecular We i ght Calculatio n Met h od ] to their RI c hr omatogra m s. In t h is view, the UV c h ro m atogram a n d the cal i b ration c u rve can be over l aid wi t h t h e Rl c h ro m atogram of th e samp l e fo r d e t e r mini n g the ca l c u lat e d average mo le cular weight and molecular we i g h t distribu t ion a c cord i ng to the Europea n Pharmacopoe i a, t h e calculation r es u l t t a b l e s h own in fi gure 6 (C) is useful. In t hi s appl i cat i on both the par n apar i n (sodium ) and daltepar i n (sodium ) sa t isfy t h e l eve l s in the E uropean Pharmacopo e ia (Tab l e 1). Figure 6. C h ro m N AV [H e p ar in Mo l ec u l ar W e i gh t Calc ul at io n R e su l t Vi e w] d is pl ay (A) Ch rom a togram , (B) t o o l butto n s , (C ) H ep ar in Mo l e cu la r W e ig h t C alcu l a ti o n R es u l t s T a b l e Table 1. Averag e mo l e cu la r weig ht a nd m o l e cu la r weig ht d is t r i b u t i o n i n l o w -m o lecu la r- m ass he p a ri n s o d iu m and c a lc iu m ar t ic l e o f Eu ro p ean P h arm ac opo eia Refe r ences 1. Acupuncture, Thrombus, He m ostat i c, 19 (2),187-190 (2008). 2. Europ e a n Pha r macopoeia 8.0 vol u me l l,"H eparins, l ow-mo l ecular-mass mo n og r a p h 0828", (2014), Co u nci l of Europe. 3. Europe a n Phar m acopoeia 8.0 volume I l,“Parnapari n sodium mo n ograph 1252", (2014), Cou n cil of Europe. 4. European Pharmacopoeia 8.0 volume I l,"Parnapar in sodi u m monograph 1252", (2014), Counci l of Europe. 5. European Pharmacopo ei a 8.0 vol u me ll,"Dalt e par in sodi u m mo n ogr a ph 1195”, (2014), Counci l of Europe. 6. European Phar m acopoeia 8.1 supp l eme nt ,“E n oxapa r in sod i um monograp h 1097", (2014), Council of Europe. 7. Europea n Pha r macopoe i a 8.0 volume ll,"Tinzaparin sodium mo n ograph 1271,(2014), Counci l of Europe. 8. European Pha r macopoe i a 8.0 vol u me ll,"Nadropar in calcium monograph 1134",(2014), Cou n c il of Europe. Code Model Description WWA3 PU-4185 Semi-Micro Pump C477 DG-4000-04 4-Channel Analytical Degasser Unit RR95 AS-4050 Autosampler C502 TC-4000-1 Sample Temperature Control Unit RRA1 CO-4060 Column Oven WW88 UV-4075 UV/Vis Detector WW94 RI-4030 Refractive Index Detector R354 RI-4030 Bottle Stand TSK gel G2000SWxl (7.8 mml.D. x300 mmL, 5 um) C006x4 RI-4030 LC-Net CG cable x 4 ChromNAV V2 ChromNAV V2+PC C294 Low-Molecular-Mass Heparins Calculation Program 078C HPLC Start Up Kit for LC-4000 089C Maintenance Tool Kit I n this a n alysi s , p l e ase u se t he pu m p c le a n ing mec hani sm du e to a high salt concentrat i on mobi l e phas e . F r esh ultrapure water is required for th e cl e a ni ng mechanism before each measurement.
确定



还剩5页未读,是否继续阅读?
佳士科商贸有限公司为您提供《根据欧洲药典要求测量低分子量肝素》,该方案主要用于生物药品药物研发中分子量测量/分布、低分子肝素检测,参考标准--,《根据欧洲药典要求测量低分子量肝素》用到的仪器有JASCO高效色谱仪LC-4000
推荐专场
相关方案
更多
该厂商其他方案
更多