方案详情
文
使用J-1500 CD光谱仪评估了3种不同类型VHH抗体的热稳定性和重折叠能力。使用BeStSel程序估计二级结构百分比,以进一步将变性温度与VHH抗体框架的结构变化联系起来。关键词:SARS-CoV-2,冠状病毒,抗体药物,VHH,重折叠,突发性,变性温度,二级结构,圆二色性
方案详情

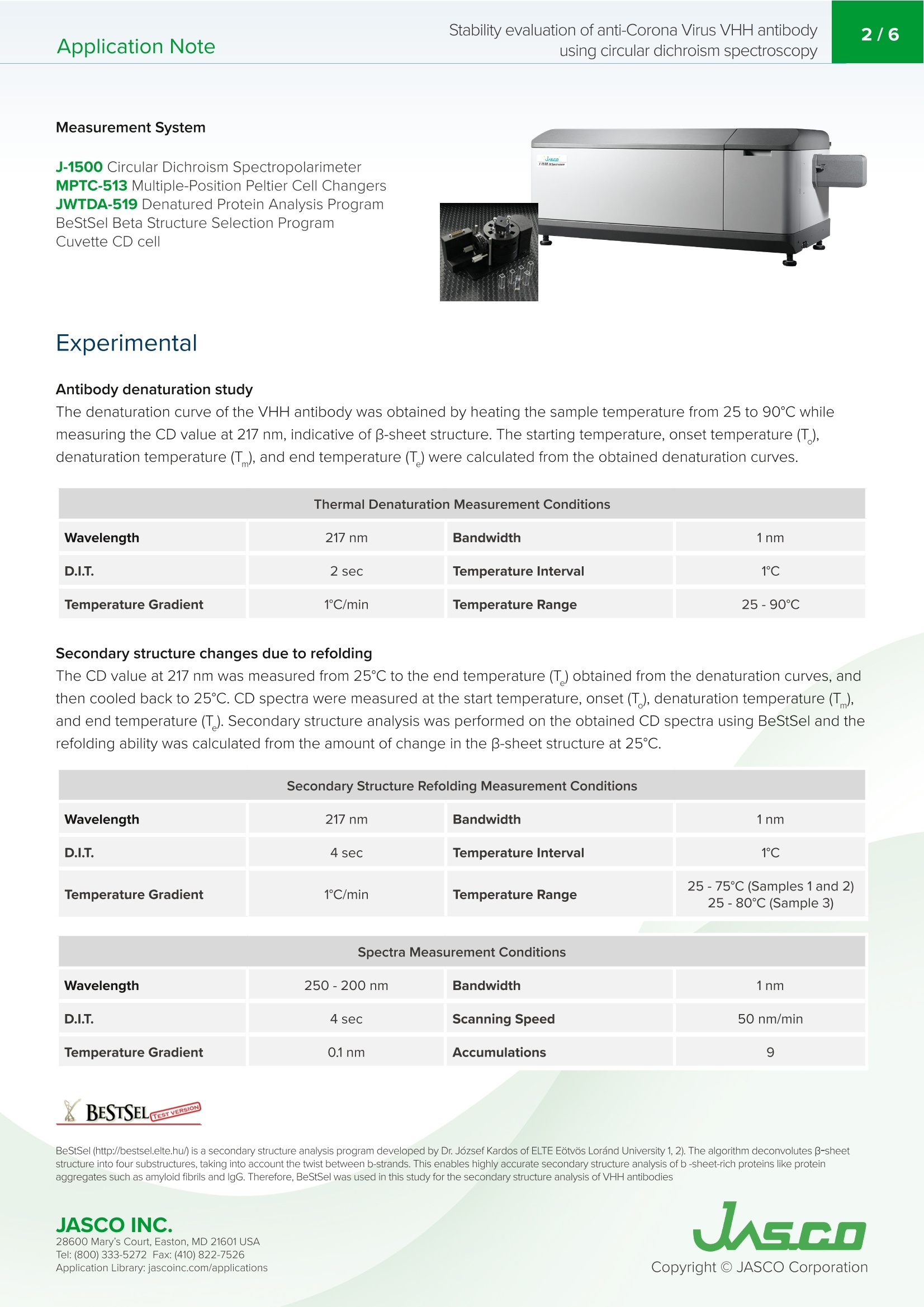
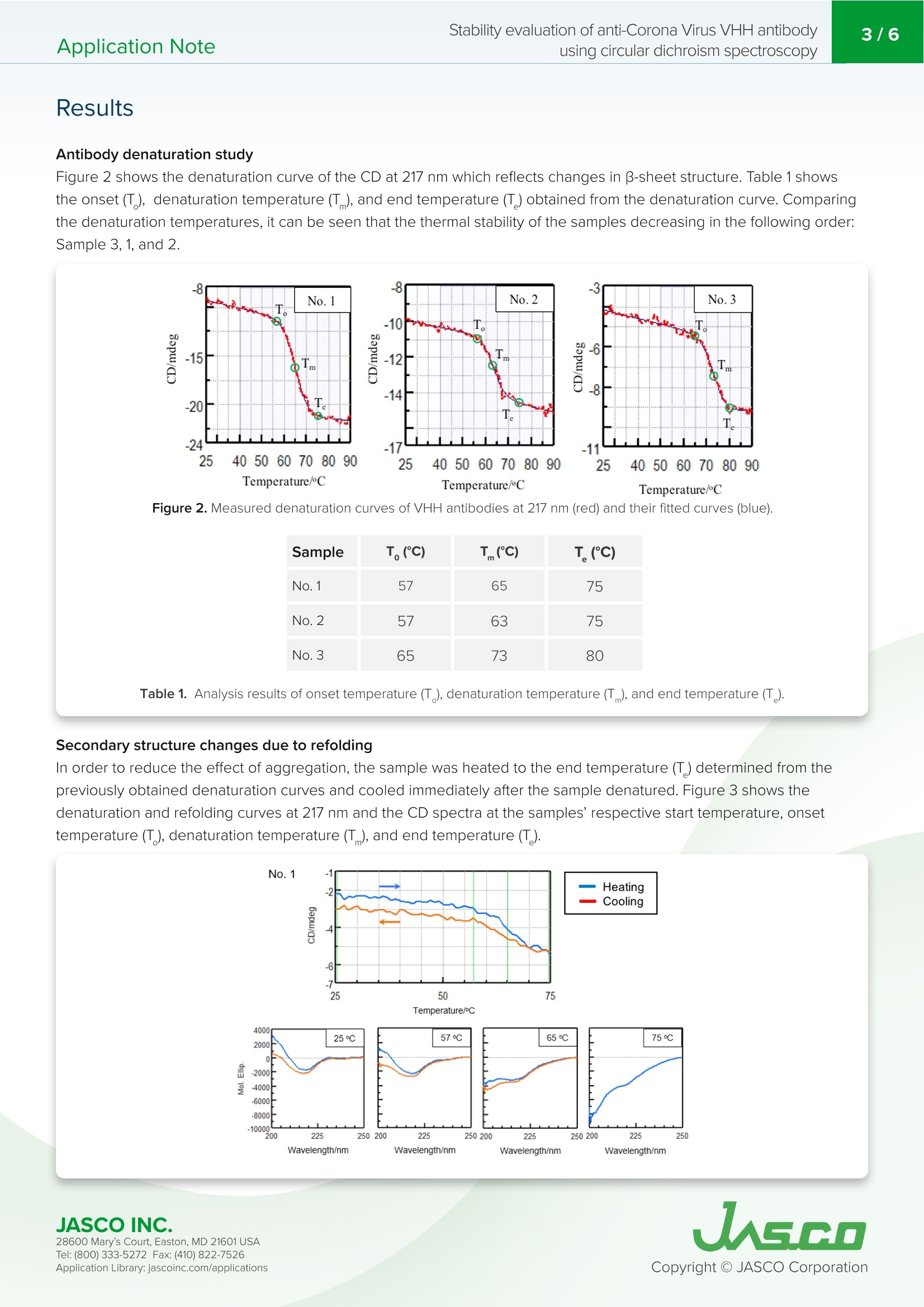
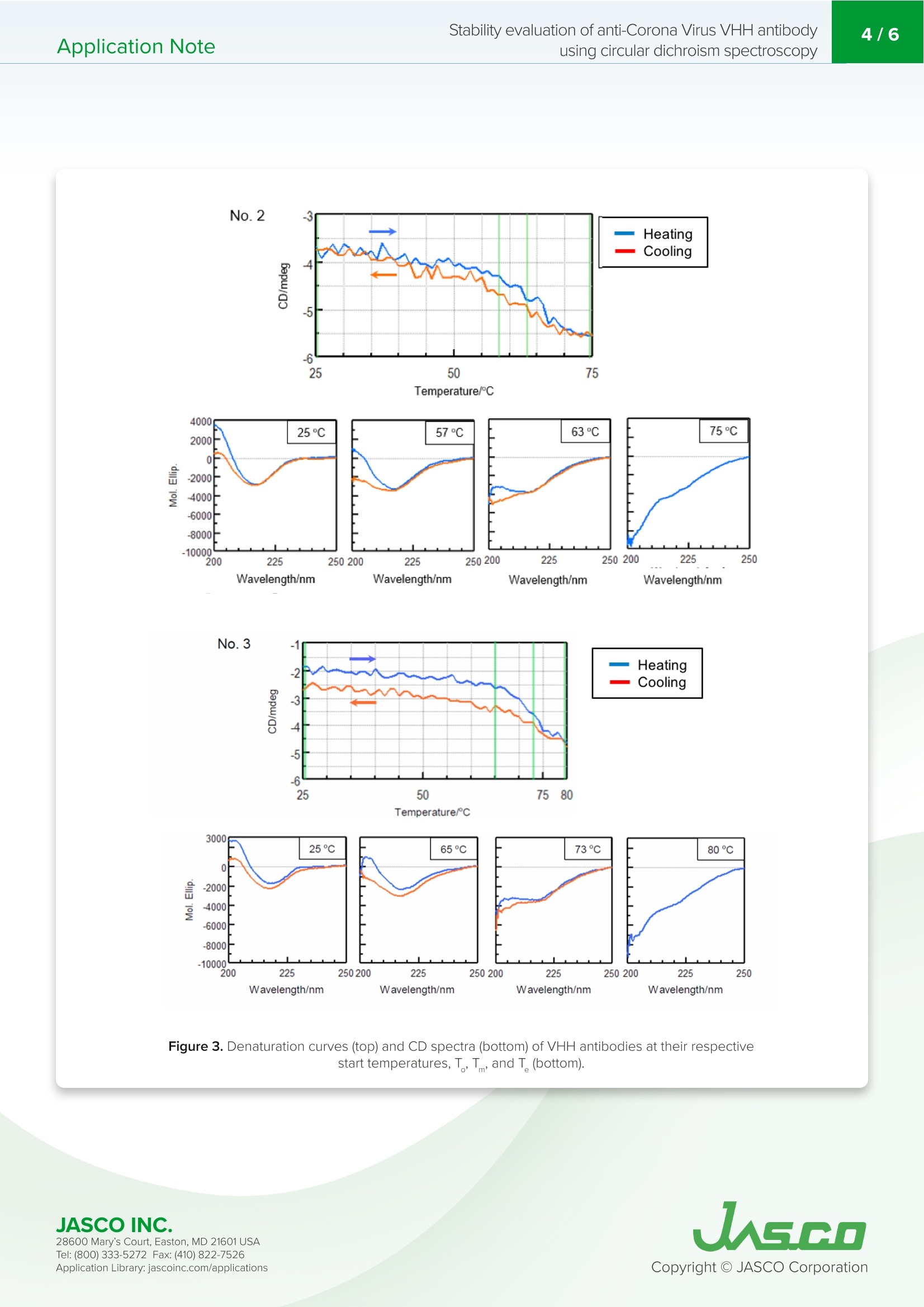
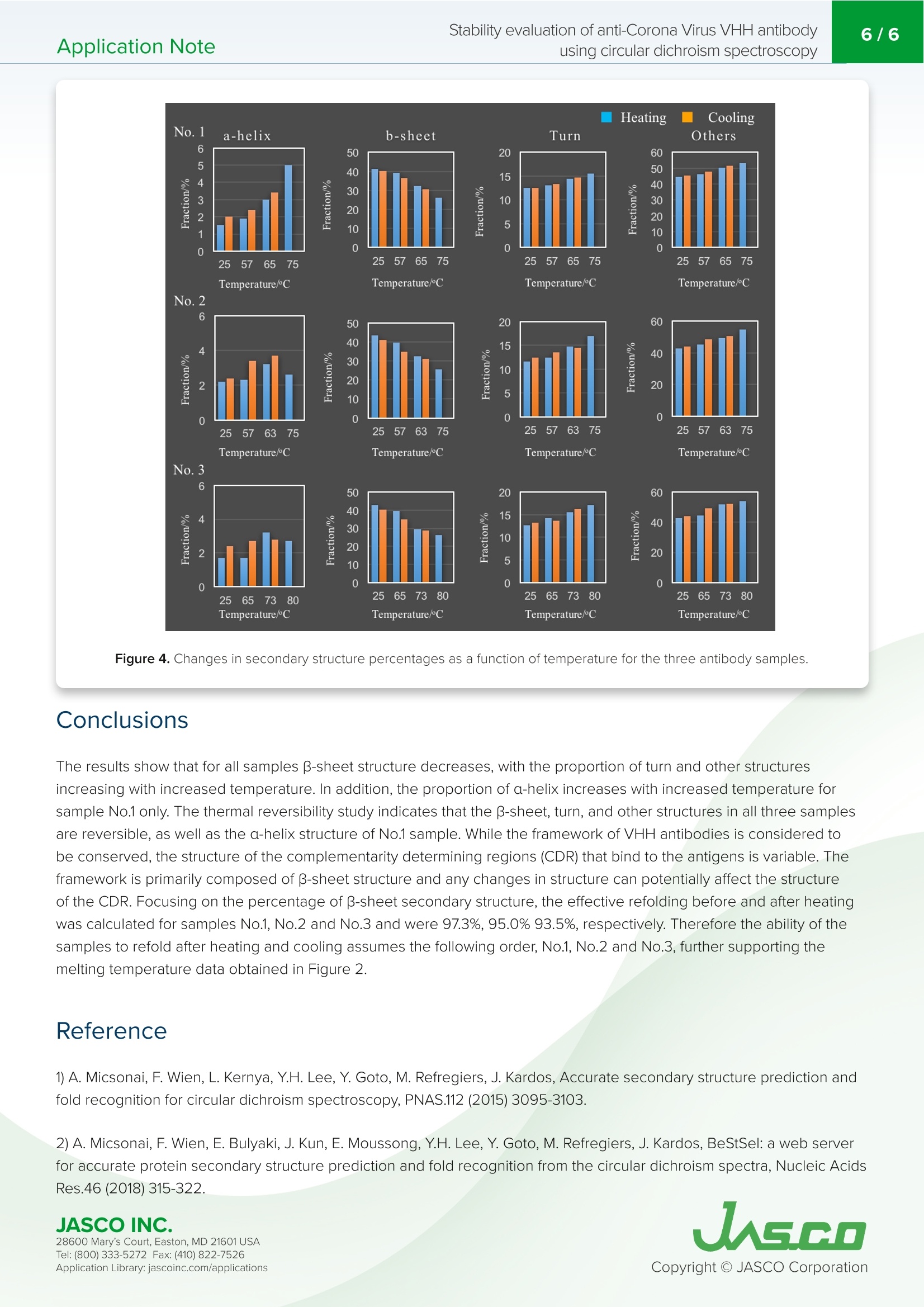
VHH抗体是小的单结构域抗体。它们对热高度稳定,具有高亲和力和结合特异性,低分子量,提高了大规模生产效率,并且可以容易地修饰。由于抗体药物的结构可能会根据配方条件和储存环境而改变并导致其活性损失,因此有必要评估其稳定性以确保质量。特别是,热变性是高度可逆的,这取决于蛋白质,增加了在抗体药物的稳定性评估中确定变性温度和重折叠能力的需要。Application Note200-CD-035 Stability evaluation of anti-Corona Virus VHH antibodyusing circular dichroism spectroscopy2/6 Stability evaluation of anti-Corona Virus VHH antibody using circular dichroism spectroscopy In t rod uc tion The coronavirus S glycoproteins si t on the surface of the virus part i cle (Figure 1) and facilitates r eceptor bind i ng and membrane fusion, which leads to entry of the vi r us into host cel l s. The r efore, the S prote i ns are the main t arget for neutraliz i ng binding and therape u tic drug design VHH ant ib od i es a r e small , single doma in antibodies cor r espondi n g to th e variable r egio n of a heavy chai n camelid a n tibody (Figu r e 1). T h ey are h i gh l y stable with r espect to heat, have h igh affinity and binding speci f icity, a l ow m olecular weight which increases mass production eff i ciency, and can be easily modified. Since the structu r e of a n tibody d r ugs may change a n d cause a loss i n thei r activity depending on t h e formulation cond i tions and storage environme n t , i t is necessary to eva l uate their stabi l ity i n order to ensure quality. In particular, ther m al denaturation is h ighly reversible depend i ng on the protein, i ncreasing the need to determine denaturation temperature and refold in g ability in the stability evaluation of antibody d r ugs. Here, we evaluate t h e thermal stabi li ty and refold in g a b i l ity of 3 d i f f e rent types of VHH a n t i bodi e s u sing a J-1500 CD spect r ometer. T h e secondary st r ucture pe r centages were es t imated using t h e BeStSel program to f ur t he r corre l ate the denaturat i on te m peratures with structural changes to VHH an ti body framework . Figure 1. C oron a vi r u s m o l ec ule s (left ) a n d ca r toon sch em a t ics of t h e sp i k e prot eins a n d VHH ant i b o d y st r u c t u r es . Keywords S A RS -C o V -2,Co ro n a V iru s, A n tib ody dru g s, VHH, R e fo l d in g, On s e t , De n aturat i on temper a tu r e, Seco n d a ry str uc t u r e ,Be s tS el , C i rc u la r D i c h roi s m Measurement System J-1500 Circ u l a r D i c h rois m S p ectropol a r i meter MP T C-513 Mul ti pl e-Positi o n P e lti e r C el l C h anger s JWTDA-519 De n atured P rote in A n a l ysis Prog r am BeS t Se l Be t a St ru c tu re Select i on P r og ra m Cuv ette C D cel l Experimen t al Antibody denaturation study The de n aturat i on curve of t h e VHH ant ib ody was obtained by heating th e s a mple tempe r ature fr om 25 to 90℃ whi l e mea s ur i ng the CD value at 217 nm, i ndica ti ve of B-sheet s t ruct u re. T h e sta r ti n g temperatu r e, onset t emperature (T ),denaturat i on temp er atu r e (T), and end temperature (T ) were calcu l ated f rom t h e o b tai n ed den a tu r atio n curves. Wavelength 217 nm Bandwidth 1nm D.I.T. 2 sec Temperature Interval 1℃ Temperature Gradient 1C/min Temperature Range 25-90℃ Secondary structure changes due to refolding The CD value a t 217 nm was m easured fr om 25℃ to t he end t empera t ur e (T) obtain e d f rom the de n a t urat i on curv e s, a n d then cooled bac k to 25℃. CD s p ectra were measur e d at the star t tempera t ure , ons e t (T ), d e naturat i on tem p erature (T),a n d e n d temperatu r e (T). Seco n dary st r u c ture analysis was performed on the obtained CD spectra u s i n g BeStSel a n d t he r efolding a b i li ty was calcu l a t ed fr om t he amoun t of c h ange i n the B -s h eet st r ucture at 25℃. Secondary Structure Refolding Measurement Conditions Wavelength 217 nm Bandwidth 1nm D.I.T. 4 sec Temperature Interval 1℃ Temperature Gradient 1C/min Temperature Range 25-75℃ (Samples 1 and 2)25-80℃ (Sample 3) Spectra Measurement Conditions Wavelength 250-200nm Bandwidth 1nm D.I.T. 4 sec Scanning Speed 50 nm/min Temperature Gradient 0.1nm Accumulations 9 X BESTSEL Cazverso B eStSel (h tt p ://b estsel .elte.h u/) i s a secondary st r uctu r e a n alysis prog r am deve l oped by Dr . J o zsef K ar do s of E LTE E otvos L o r and U niversity 1,2). The alg o r i t h m deconvolu t es B-s h eet st r uct u re i nto f our subst r uctures, t ak ing i n t o acco u n t t h e t wist betwee n b-s t rands. T h i s enab l es highly accura t e second ar y s t ruc tu r e a n a l ysis o f b -s h e e t -ric h p r otein s l ike p r ote i n agg r eg a t e s suc h as amyloid fi b r i l s a n d l gG. Th e r efore, B e StS e l was u sed in t h is s t u d y f or t h e se c on d ar y struc t u r e a n a l ysis of VH H antibodies JASCO INC. 28600 Mary's C o ur t , E asto n, MD 21601US A T el : (800) 333-5272 F ax: (410) 822-7526A ppl ic a tio n Lib r ary: ja s c o i n c.com /appli c a t i o ns Results Antibody denaturation study Figure 2 shows t h e denaturat i on curve o f the CD at 217 nm w h ich re fl ects changes i n B -s h eet st r ucture. Table 1 shows the onse t (T ), de n aturation temperature (T ), and end tem p erature (T ) obtain e d f rom t he de n aturat i on curve. Comparing the denaturat i on t emperatur e s, it can be s e en t h at the t h ermal stability of the sam p l e s dec r easing in t h e fol l owing ord e r :Sample 3, 1, and 2. -8 No.3 -15 -20 -24 25 Figure 2. Me a sured d en a t ur a tion c ur ve s of VHH a nt i bod i es at 217 nm (re d) a n d t h e ir f i t ted c u r v e s (blu e ). T(C) Sample T。(C) No.1 57 65 75 No.2 57 63 No.3 65 Table 1. An a ly s is res ul ts o f on set t e mpe r at ure (T ), d e n a tur a tion t e mp er a tur e (T ), an d e nd tem p e ratu re (T) Secondary structure changes due to refolding In order to r educe the effect of agg r ega t io n , the s a m p le was heated to t h e e n d tempe r ature (T ) determi n ed f rom the previously o b tai n ed denatura t i o n curves and cooled im med ia tely after t h e sample de n atured. F i gure 3 s h ows the den a turatio n and re f olding curves at 217 nm and th e CD spectra a t t he samples' r espect i ve star t t emperature, o n set temperatu r e (T), denaturat i on temperature (T), a nd e n d temperat u re (T). JASCO INC. W a v el e n gth /n m W a v e l e n g th /n m W a v ele n gt h/n m Wa ve l e n gt h /nm Figure 3. Den a tura t io n cu rv es (t op) a nd C D sp e c t r a (b o t tom ) of V H H an t ibodies at t h e i r res pec tiv e star t temp er atur e s ,T,T ,and T (b ottom). 28600 Mary's C o ur t , E asto n, MD 21601US A T el : (800) 333-5272 F ax: (410) 822-7526A ppl ic a tio n Lib r ary: ja s c o i n c.com /appli c a t i o ns Table 2 and F i gure 4 show the resul t of the secondary structure ana l ysis by us i ng BeStSel . Table 2. Se co n dary s tr uc t ure ana ly s i s r e sults ca l c u la t e d us i n g the BeS t S e l p rogr a m . Number 1 Temperature (℃) a-helical (%) B-sheet (%) Turn (%) Others* (%) 25 1.5 41.3 12.5 44.6 57 1.9 39.1 13.0 46.0 65 3.0 32.1 14.5 50.2 75 5.0 26.3 15.6 53.1 65 3.4 30.6 14.7 51.4 57 2.4 36.4 12.5 45.3 25 2.0 40.2 12.5 45.3 *3,10-helix, I-helix, B-bridge, bend, loop/irregular , i nvis i ble regions of the s t ructure Number 2 Temperature (℃) a-helical (%) B-sheet (%) Turn (%) Others* (%) 25 2.2 43.6 11.6 42.7 57 2.3 39.8 12.4 45.4 65 3.2 32.6 14.8 49.4 75 2.6 25.6 17.0 54.8 65 3.7 31.2 14.5 50.6 57 3.4 34.8 13.5 48.4 25 2.4 41.4 12.4 44.1 Number 3 Temperature (℃) a-helical (%) B-sheet (%) Turn (%) Others*(%) 25 1.7 42.9 12.7 42.6 65 1.7 39.6 14.2 44.4 73 3.2 29.4 15.6 51.8 80 2.7 26.4 17.1 53.8 73 2.8 28.8 16.2 52.2 65 2.7 34.7 13.6 49.0 25 2.4 40.1 13.2 44.2 28600 Mary's C o ur t , E asto n, MD 21601US A A ppl ic a tio n Lib r ary: ja s c o i n c.com /appli c a t i o ns Figure 4. Cha ng es i n s e con dary s t ruc t ur e p ercen ta ge s a s a fun c ti o n of temp er at u r e for t h e t hree an t i b o d y sa m pl e s. Conclu s ions The r es ults show that for all samples B-sheet struct ur e decreas e s, with th e proport i on o f tu rn and ot h er st r uctures i n c r ea s i ng wi th in cr e ased tempe r atur e. In addition, the p ro p o r tion of a -h e li x inc r e ases wit h increased temperature fo r sample No.1 only. T h e t herm a l r e ver s ibility study indica t e s th at t he B-sh ee t , tu rn, and ot h e r st r uc tu r e s i n all th r e e samples are r e v e rsi b le, as well as t h e a-h elix str u ct ure of No.1 sampl e . While the fr amework o f VHH ant i bodies is consider e d to be cons e rv e d, th e st r uctur e of the compleme n tarity det e rmining r e gions (CDR) that b i n d to the ant i gens is var i abl e . T h e framework i s pr i marily composed of B-s h ee t structure and any c h anges in structure ca n pote n t i ally affect t he structu r e of the CDR. Focusing on th e p e r centage o f B-s h eet secondary st r ucture , t he effec t ive refolding be f ore and after heating wa s ca l culat e d for sam p l e s No.1, No.2 and No.3 and were 97.3%, 95.0% 93.5%, r e spectively. Therefore t h e abili t y of t h e samples to r efold af t er h e at i ng and cool i ng assumes t h e following order, No.1, No.2 and No.3, further supporting the melting temperature data obtained in Figu r e 2. R efere n ce 1) A. Micsona i , F. Wie n , L . Ke r nya, Y.H. L e e , Y . Goto, M. Re f r e gier s , J. Kardos, Accura t e secondary structu r e predic t io n and f old r e cogni ti on for circu l ar dic h roism spec t roscopy, PNAS.112 (2015) 3095-3103. 2) A. Micsonai, F. Wien, E. Bulyaki , J. Kun, E. Moussong, Y.H.Lee, Y. Goto, M. Refregiers, J. Ka r dos, BeStSel : a web serve r for acc u r a te protein secondary structure p red i ctio n and fold recognition from the circular dichroism spec tr a, Nucleic Acids Res.46 (2018) 315-322. JASCO INC. 28600 Mary's C o ur t , E asto n, MD 21601US A A ppl ic a tio n Lib r ary: ja s c o i n c.com /appli c a t i o ns
确定

还剩4页未读,是否继续阅读?
佳士科商贸有限公司为您提供《圆二色谱法评价抗冠状病毒VHH抗体的稳定性》,该方案主要用于生物药品原料中抗体稳定性检测,参考标准--,《圆二色谱法评价抗冠状病毒VHH抗体的稳定性》用到的仪器有JASCO圆二色光谱仪CD J-1500
推荐专场
相关方案
更多
该厂商其他方案
更多
















