方案详情
文
生物仿制药往往被认为与创新者具有相同的功能和结构,因为它们具有相同的氨基酸序列。然而,抗体在生产过程中受到各种刺激和翻译后修饰,这可能导致功能的显著丧失。其主要因素是热刺激和蛋白酶的破碎。因此,测量由刺激或修饰引起的抗体结构的变化是研究和开发过程中,以及抗体药物质量控制的一个重要步骤。关键词:抗体药物、高阶结构、HOS、二级结构、三级结构、生物仿制药、利妥昔单抗、MabThera®、RIABNITM,、曲妥珠单抗、赫赛汀®、圆二色光谱仪、qHOS,相似性评估
方案详情

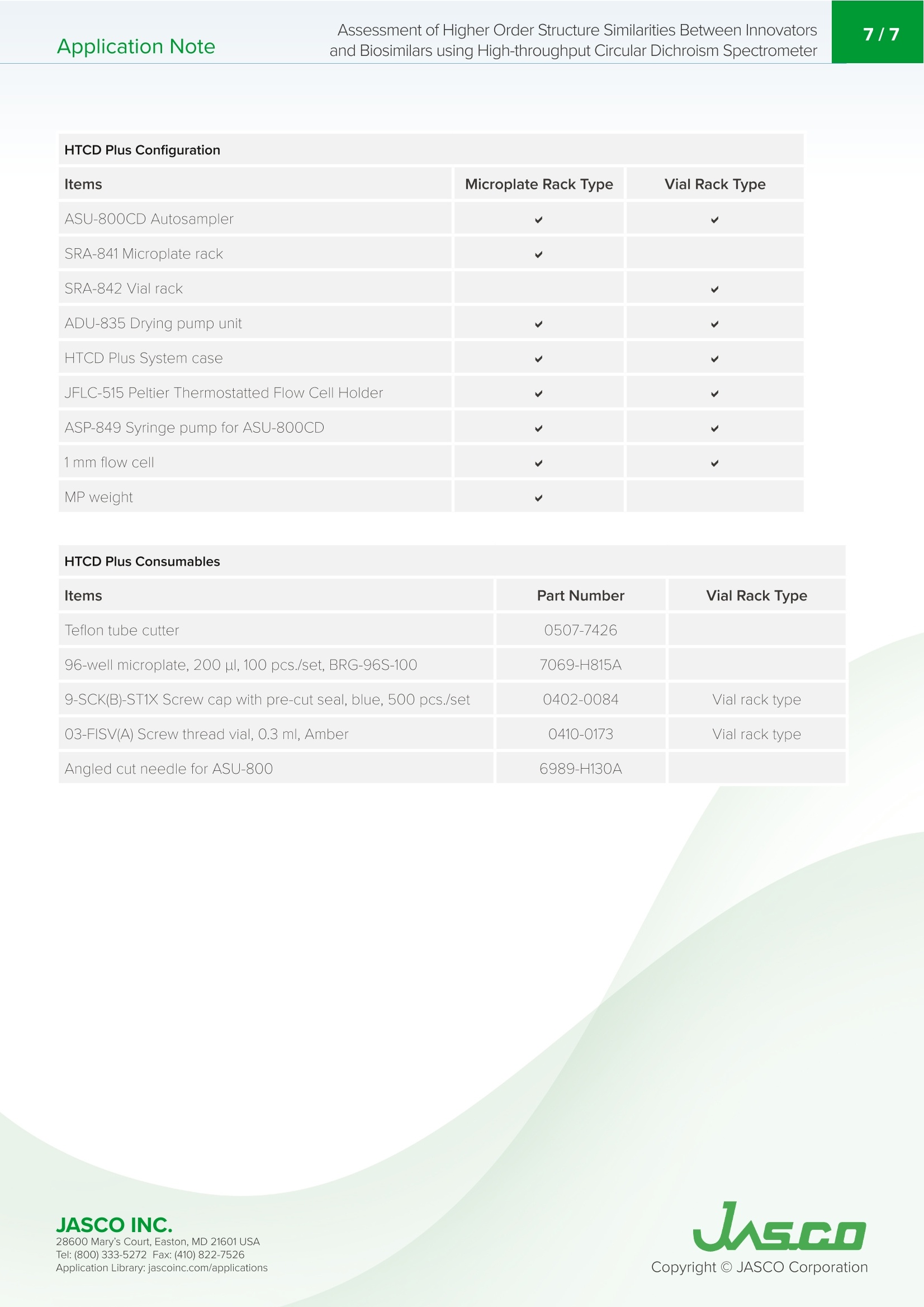
首先,为了证实该系统可以区分具有不同靶点和制剂条件的抗体药物的三级结构,我们确定了利妥昔单抗的创新者MabThera和曲妥珠单抗的创新者赫赛汀三级结构的相似性。MabThera和赫赛汀的近UV/CD光谱的形状显著不同。类似地,根据CD光谱计算的MabThera和赫赛汀之间的平均距离显示出很大的差异。从t检验中获得的p值低于0.05的显著性水平,表明赫赛汀具有与MabThera不同的三级结构。这些结果表明,该系统可以清楚地区分不同抗体药物三级结构的差异。Application Note200-CD-0040 Assessment of Higher Order Structure Similarities Between Innovatorsand Biosimilars using High-throughput Circular Dichroism Spectrometer2/7Application Note Assessment of Higher Order Structure Similarities Between I nnovators and Biosimilars using High-throughput Circular Dichroism Spectrometer T h e ma r ket for thera p eutic antibodies has been d r amat i cally expa n ding ove r the past decade. Antibody d r ugs exhibit therapeu t ic effects suc h as inhibi t ing the growt h of mal i gnant tumo r cells and the activity of immune cells by bi n ding antigens expressed i n target cells with h igh aff in ity and specificity. Such a n tibody drug s are expected to be effective t h erapeutic agents for as yet unmet medica l needs. Figure 1. HTCD Plus hi g h-t hr o u g h pu t CD spec t romete r Ma n y a n tibody drugs are being used wor l dwide, and a l ong with leading a n tibody drugs called i n novators, ma n y b iosimilars, which have the same am i no acid sequence as t h e i n novators but are produced using different expression cells, a r e also beginning to be used. Biosimilars tend to be t h ough t of as h aving the sa m e funct i on a n d structure as the innovators because they have the same amino ac i d sequence. However , antibodies are subjected to various stimuli and post -t ranslational mod i ficat i ons during t h e p roduct i on process, wh i ch may resu l t i n a signif i ca n t loss of function. The mai n factors are thermal s t imulation a n d f r agmentation by proteases. Therefore , measuring changes in ant i body structure d u e to st i mu l at i on or modif i ca t ion is a n essential step i n the r esearch a n d development p rocess, and for quality con t r ol of antibody drugs. T h e ICH Q5E" guid e lin e f or quality control of biological p roducts states that the comparability of the hig h er order s t ruc t ure (H OS) of an tib odies befo r e and after a change in the manu f a c turing process shou l d be objectively evaluated. Global guidanc e 2,3) f rom t h e F DA4 and EMA 5 also states that H OS compariso n s s h oul d be per f ormed and secondary struc t ure composit i on rat i os f or innovators and b iosimi l ars should b e evaluated . Circular dich r oism (CD) spectroscopy is widely used to evaluate ant i body q u ali t y, as it is a s i m p le way to obta in i nformation on t h e HOS of ant i bodies. To i mplement the a b ove gu i delines, there is a need f or a method t h at can object i vely and sensi t ively evaluate the similarity of CD spectra.T h e JASCO [qHOS] p rogram, which was developed to meet t h ese requirements, is capable of detecting significant d i fferences in spectra wit h high sensi t ivity, and perfor min g stati s tical tests. H e re we r e port t h e r e sul t s of a n HOS similar i ty ass e ssmen t of RIABNIT M (a biosimilar to MabThera/Rituxan @, t h e i n novator of Rituximab), and H e rce p t in @ (the i nnovator of Trast u zumab), using the JASCO HTCD Plus high-t h roughput CD spec tr omete r (F i g. 1) and th e [q H OS] p rogram. K e ywords A n tibody d r ugs, Hi g h e r o r der st r uc tu re, HOS, Secon d ary stru c ture , T er t iary structure, Biosim i la r s, R itu x imab ,M a b The r a @,R IABNI TM, Tras t uzumab , Herce p t in@, Circ u l ar dichroi sm s p e c tr omet er, q HOS, Si mil a r ity a s s e ss men t System The HTCD Plus high-throughput circular dichroism spectrometer is cap abl e of co n tin u o u sly me a s uri ng u p t o 192sa m pl es (with tw o 96-wel l pl a t es , u p to 120 sa m ple s w h en u si n g via l s ) i n a fu l l y automa t ed man n er, in a dditio n to c l ean i n g an d d r ying samples, an d i s i dea l f or application s that req u i r e pr e c ise meas u r eme n t o f mu ltipl e sam pl es, su ch as antibod y d ru g s im il a rity assess m e n ts. The [qHOS] program c onve r t s th e s pe ctra l d i ffere n ce i n to a p a r a met e r c all ed th e “Eu c li d e an d is t a n c e". By weightin g th e E u c l i dea n d i stance t o a cc o u nt fo r n o ise an d spe c tr a l v a ri a ti o n , d iff ere n ces b e t w ee n spe c tra c an be d etec t ed w i th h igh sen si tivity (“w e i g h te d Eucl i dean d is t a n ce "). The [q H OS ] prog ra m allo w s stat i stical sign ifi ca n ce tes tin g to determine wh eth e r th e di f f eren c es b e t w ee n s p e ctra are d u e t o n o i s e o r sampl e prepara ti o n e rr o r s , o r ar e in f a c t c hem i c a l l y meanin gful d iff ere nc e s (Fi g . 2). Th ree t y pes of stati s t i cal tes t s can b e sele ct ed a cco rd in g to th e pu rp os e (T able 1). Table 1. Procedure for each statistical test Assay Features Student's t-test This method tests for significant differences in spectral distances, taking into account the variancein standard sample spectral distances. Data for multiple standard samples and a single unknown sample are used. This test is used forquality assessment of manufactured antibody drugs. Welch's t-test This method tests for significant differences in spectral distances, taking into account thevariances in both standard and sample spectral distances. Data for multiple standards and unknown samples are used.This test is used for comparing the HOS of innovators and biosimilars, the HOS for antibody drugs before and after manufacturing process changes, and for lot inspections. Equivalence test (TOST) This method tests for significant differences in spectral distances, taking into accountthe variances in both standard and sample spectral distances. In this method, it can beset a range (equivalence margin) in which the distance between the spectrum of each standard sample and the average spectrum of standard samples, and the distancebetween the spectrum of each unknown sample and the average spectrum of unknownsamples, are equivalent respectively. Data for multiple standards and unknown samples are used. 自 Tests can be performed based on the FDA guidance for similarity assessment.b) Samples Rituximab Trastuzumab MabThera (Innovator) Herceptin (Innovator) RIABNI (Biosimilar)Both samples were prepared at a concentration of 10 mg/mL Powder was dissolved in H,O to a concentration of 10 mg/mL Addi t ive: Sodium citrate dihydrate 7.4 mg/mL , Sodium chloride 9.0 mg/mL, Sodium hydroxide 9.0 mg /mL, Polysorbate 80 0.7mg/mL Addit i ves: Trehalose hydrate 4.7 mg/mL , L-histidine hydrochloride hydrate 0.11 mg /mL, L-hist i dine 7.4x102 mg/mL , Polysorbate 2.1x10mg/mL JASCO INC. 28600 Mary's C o ur t , E asto n, MD 21601US A T el : (800) 333-5272 F ax: (410) 822-7526A ppl ic a tio n Lib r ary: ja s c o i n c.com /appli c a t i o ns 28600 Mary's C o ur t , E asto n, MD 21601US A T el : (800) 333-5272 F ax: (410) 822-7526 A ppl ic a tio n Lib r ary: ja s c o i n c.com /appli c a t i o ns Measurement Conditions Far-UV/CD spectrum Near-UV/CD spectra Bandwidth 1.0nm 1.0nm D.I.T. 2 sec 4 sec Accumulations 4 times 4 times Sample Concentrations 0.5 mg/mL 10 mg/mL Scanning Speed 50 nm/min 20 nm/min Data Interval 0.1nm 0.1nm Optical Path Length 0.2 mm 0.5mm Number of Samples 9 9 Analysis Conditions Distance Calculation Method Euclidean distance Concentration Correction Absorbance (far-UV: Abs,200near-UV: 0.5 Abs.280) Smoothing Savitzky-Golay Weighting Noise Statistical Testing Welch's t-test Significance Level 0.05 Re su l t s F i r st, to confirm that t his system ca n d i scriminate betwee n the tertiary structures of an tib ody d r ugs with dif f erent ta r gets and formulation cond i t i ons, we determ in ed the similarity of the te r tiary st r uctures of MabT h era, the i nnovator of Rituximab and Hercepti n , the in n ova t or of Trastuzumab (Fig.3). The s h apes of the n ear-UV/CD spectra of MabThera and Hercept i n di f fer s i g n ificantly (F i g . 3a). S i m il ar l y, the average dista n ces betwee n MabT h era and Hercept i n ca l culated from the CD spectra show a l arge d i fference (F i g. 3b). The p-value obtai n ed from the t-test i s below the significance l evel of 0.05,indicat i ng that Hercepti n has a different tert i ary structure to MabThera. These results show that the system can clea r ly dist i nguish differences in the tert i ary structu r e of different antibody drugs. JASCO INC. 28600 Mary's C o ur t , E asto n, MD 21601US A T el : (800) 333-5272 F ax: (410) 822-7526 A ppl ic a tio n Lib r ary: ja s c o i n c.com /appli c a t i o ns Assessm e nt of Higher Order Structure Simi l a r ities Between I n novators a n d Biosimila r s using Hig h-thro u ghput C i rcular Dic h roism Sp e ctro m eter b) Distance an d te s t r e s ults f or M abTh e r a (re d) a nd Hercept i n (b l u e ). Next, we determined the simila r ity of the ter t iary a n d seconda r y structures of RIABNI and MabThera (Fig.4). The results for the tertiary str u cture simi l a r ity o b tained f r om near-UV/CD spectra a r e shown i n Figures 4a and 4b, and the resu l ts for t h e seconda r y structure similar i ty obtai n ed from far-UV/CD spectra a r e shown i n Figures 4c and 4d . For both t h e near- a n d far -UV results, the CD s p ec tr um of t h e b ios i mi l ar RIABNI i s i n excel l ent ag r eement wi th that of th e in novator MabTh e ra , and t h e calculat e d distance is v e ry small . Th e r e sults of t h e t -t e st showed that t h e p-valu e was l a r ger than the significance l e v e l of 0.05, objec t iv el y co n firming t h at RIABNI ha s the same ter ti a r y and secondary structures as MabT h era. Figure 4. Results of HOS similarity assessment for Rituximab i nnovator (r ed) and biosimilar (blue)a) and c) mea n sp e ctra of MabTh e r a (r ed), RIABN I (b lue), standard dev i atio n for Mab T hera (gray).b) an d d) dista n ces a n d tes t r esu l t s for M ab Thera (red ) a n d RIABNI (blue). C onclusion Using the H T CD Plu s h i g h-th rou g h put C D s p ectrometer a n d the [qHOS ] program, w e f ou n d sign if ican t diffe r e n ces bet w een t he ter ti a r y stru c tu r es of Ma b T h era and Herce p t in , two an tib od y d ru gs wit h diffe r en t t a r get s and for mu la t ion cond iti o n s. O n the ot h er h and, RI A B NI , a biosimil a r o f MabThera, wa s d e t e r mined to h a ve the s a me te rt i a ry and seco n dary st r uc tu res a s M ab T h er a b a sed on st a t i st i c al t estin g . U si n g th i s s ystem , i t is p ossible to n o t o n ly de t e r mi n e t h e sim il ari t y b e t w een t he HOS of a bi os i mi lar a n d an in n ov a tor , bu t a l so to a ccu ra te l y a n d easi l y evalu a te w h ether th e HOS of th e ant i bod y d r ug i s ch a n ge d by p os t -tr a n sl a ti o n al m o d i f i c ati o n o r ex t e rn al stress. Ref e re nc es 1) In tern a t i o n al C o nf eren c e o n Ha r monisa ti o n (I CH) of Tec h ni c al R eq u ireme nt s fo r R eg i stra ti o n o f Ph armaceu ti ca l s f o r Hum a n U s e. I CH A U THO R I ZE D MA NU FAC TU RING CHANGES FOR THERAPEU TI C MA B S 833 H a r mo n ised Trip a r tit e Gu idel in e C om pa r ab ility o f Bi o t echn ol og i ca l /Bi o l og i ca l Prod u ct s Su bject t o C h an g e s i n t h ei r Manu f actur i n g Pr ocess Q5E .2004 2) De p a rtm e n t of Bi o t e chno l og y , G o v e rn men t o f I nd ia. G ui d e l i nes o n s i mi l a r bi o l og ic s : R e g u lat o r y r eq u i r e m e n t s fo r m ark e tin g a u tho ri za tio n i n In di a . 2012 3) C en t er fo r D r u g E valu ati o n of Chin a Nati o n a l Me d i c al Pr o d uc t Ad mi n is tr a t i o n. Te c h ni c al g u i de l in es for d e v e l o pm e n t a n d e v al u a tion o f bios i milar s . 2015 4) U S Food an d D r u g Ad mi n is trat i o n. D e velopme n t of Thera p eutic Pr o tein B io s imil ars: C o mparativ e A n a l ytical A ss e s smen t a n d Ot h er Q u a lit y-R e l ate d Con s i d e r ation s . 2019 5) E u r o p ea n M e d i c in es Agency. Gui d e l ine on s imi lar bio l o g ical m e d i c in al pr oduc t s con t a inin g b iot e c h n o l ogy-d e r i ved protein s as act i v e subs t a n ce : quality i s su es (r evi si o n 1). 2014 6)Y. Tso n g , X. Dong, and M. She n , D evelopment of statis ti cal me th ods for a n a l y ti ca l sim il ar it y a s sessmen t , J. B ioph a r m .Stat. 2017 Sys t em C onf i gu r a t ion Model Description Part Number Main Unit J-1500-450ST Spectrometer "^).*2) 7000-J006A Options N, gas flow meter 7069-J025A FLM-525 HTCD Plus (Microplate rack type)*3) 0.2 mm flow cell 7069-J186A 0.5 mm flow cell 7069-J415A Program JWQHOS-531 qHOS Higher order structure similarityevaluation software for Spectra Manager 2/2.5 4880-J111A *1) J-1500-150ST (P/N: 7000-J005A) also can be used instead of J-1500-450ST. In this case, a water circulator f or cool i ng the light source is not necessary. *2) A water circulator for cooling t h e light source, a nitrogen gas cylinder, and a regulator for the cylinder are required separately. *3) In addition to the microplate rack type, the vial rack type is also available. The configurat i on and consumables are shown in the tables below. The consumables are included in the standard configuration. JASCO INC. A ppl ic a tio n Lib r ary: ja s c o i n c.com /appli c a t i o ns Items Microplate Rack Type Vial Rack Type ASU-800CD Autosampler √ √ SRA-841 Microplate rack SRA-842 Vial rack √ ADU-835 Drying pump unit √ √ HTCD Plus System case JFLC-515 Peltier Thermostatted Flow Cell Holder √ ASP-849 Syringe pump for ASU-800CD 1 mm flow cell √ √ MP weight √ HTCD Plus Consumables Items Part Number Vial Rack Type Teflon tube cutter 0507-7426 96-well microplate, 200 ul, 100 pcs./set, BRG-96S-100 7069-H815A 9-SCK(B)-ST1X Screw cap with pre-cut seal, blue, 500 pcs./set 0402-0084 Vial rack type 03-FISV(A) Screw thread vial, 0.3 ml,Amber 0410-0173 Vial rack type Angled cut needle for ASU-800 6989-H130A JASCO INC. 28600 Mary's C o ur t , E asto n, MD 21601US A T el : (800) 333-5272 F ax: (410) 822-7526A ppl ic a tio n Lib r ary: ja s c o i n c.com /appli c a t i o ns
确定






还剩5页未读,是否继续阅读?
佳士科商贸有限公司为您提供《利用高通量圆二色度光谱仪评估创新者与生物仿制药之间的高阶结构相似性》,该方案主要用于生物药品原料中含量测定检测,参考标准--,《利用高通量圆二色度光谱仪评估创新者与生物仿制药之间的高阶结构相似性》用到的仪器有JASCO圆二色光谱仪CD J-1500
推荐专场
该厂商其他方案
更多
















