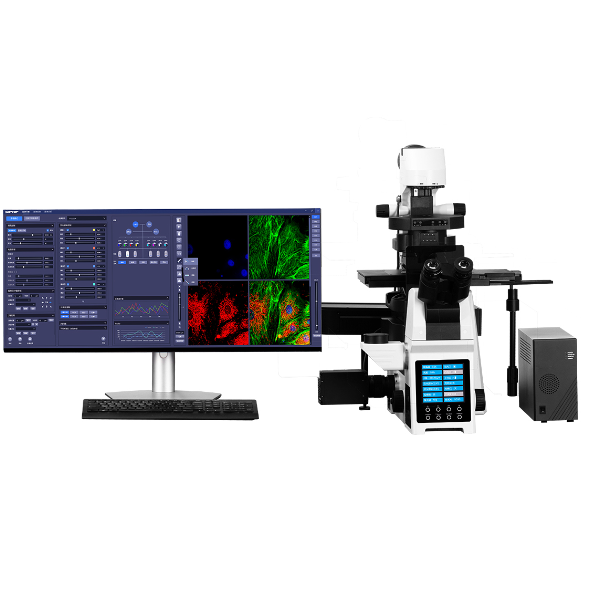
方案详情
文
超分辨率显微镜
方案详情

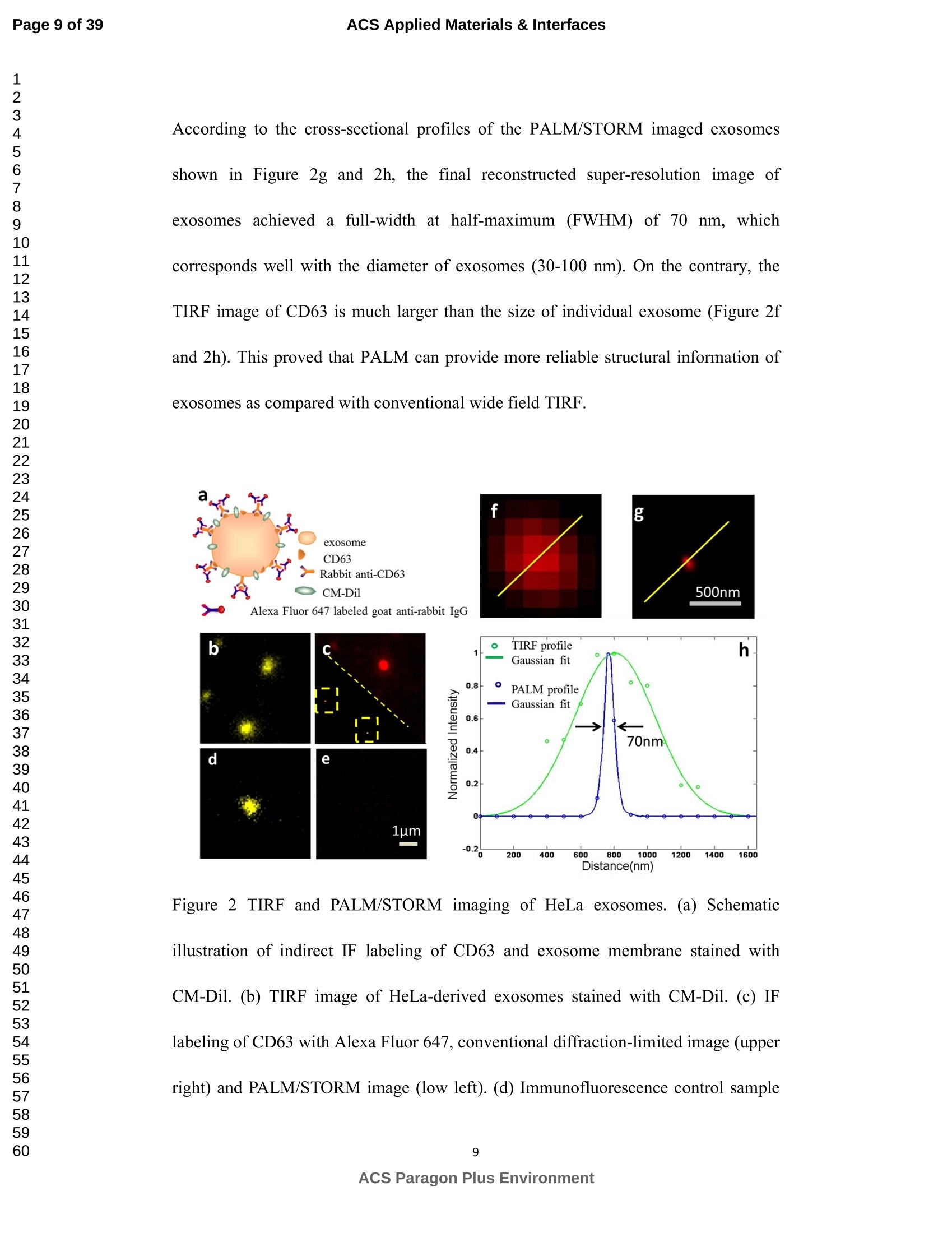
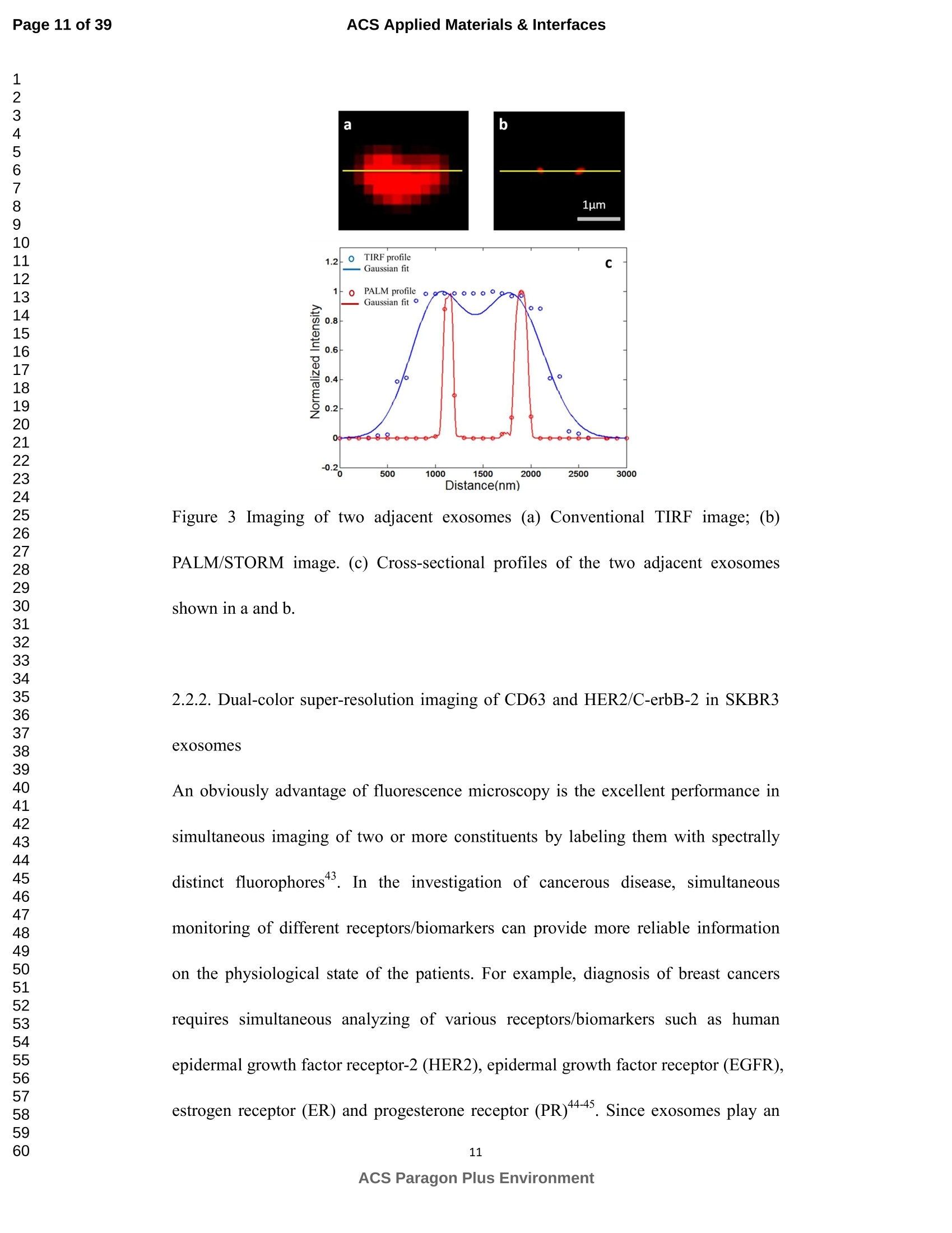
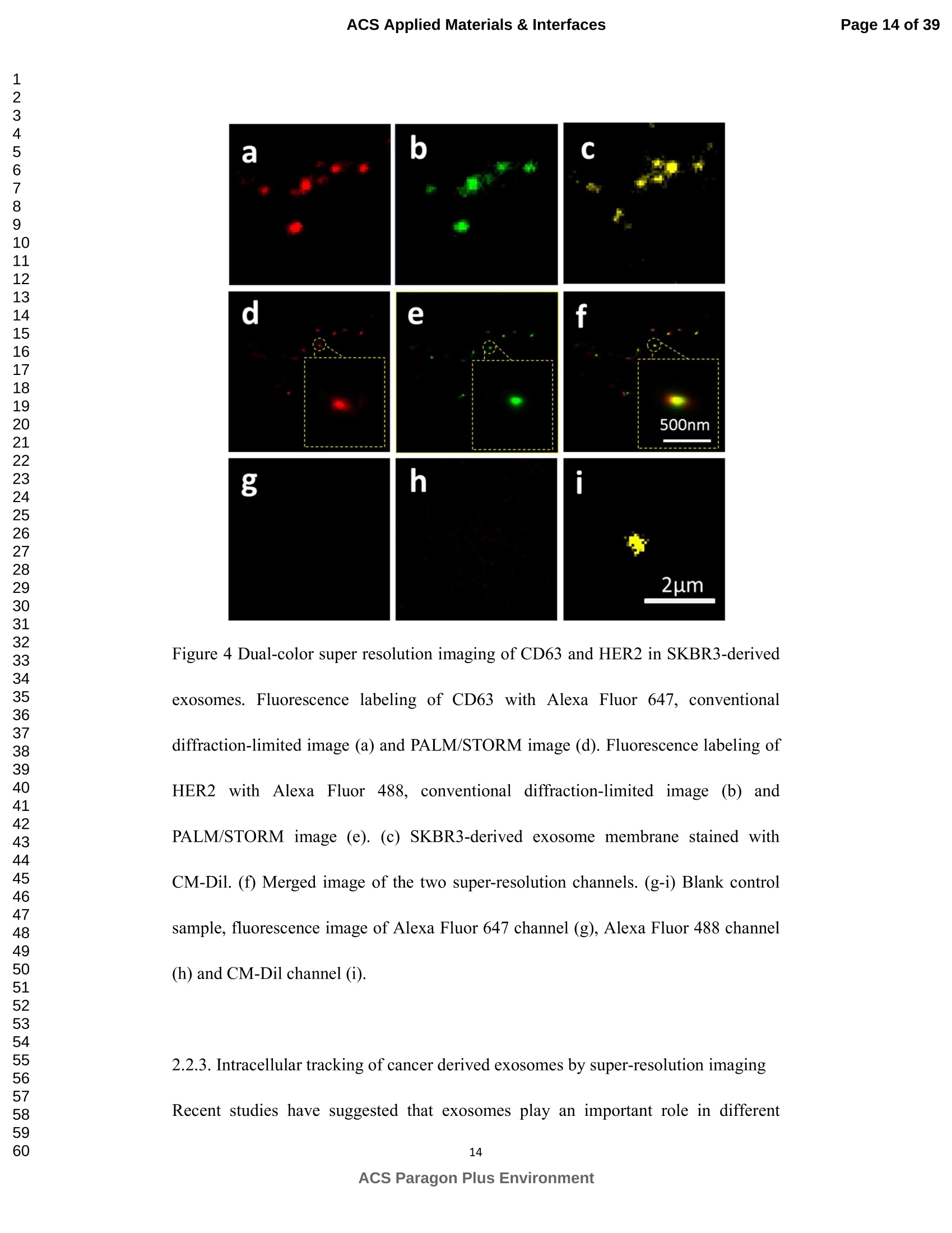
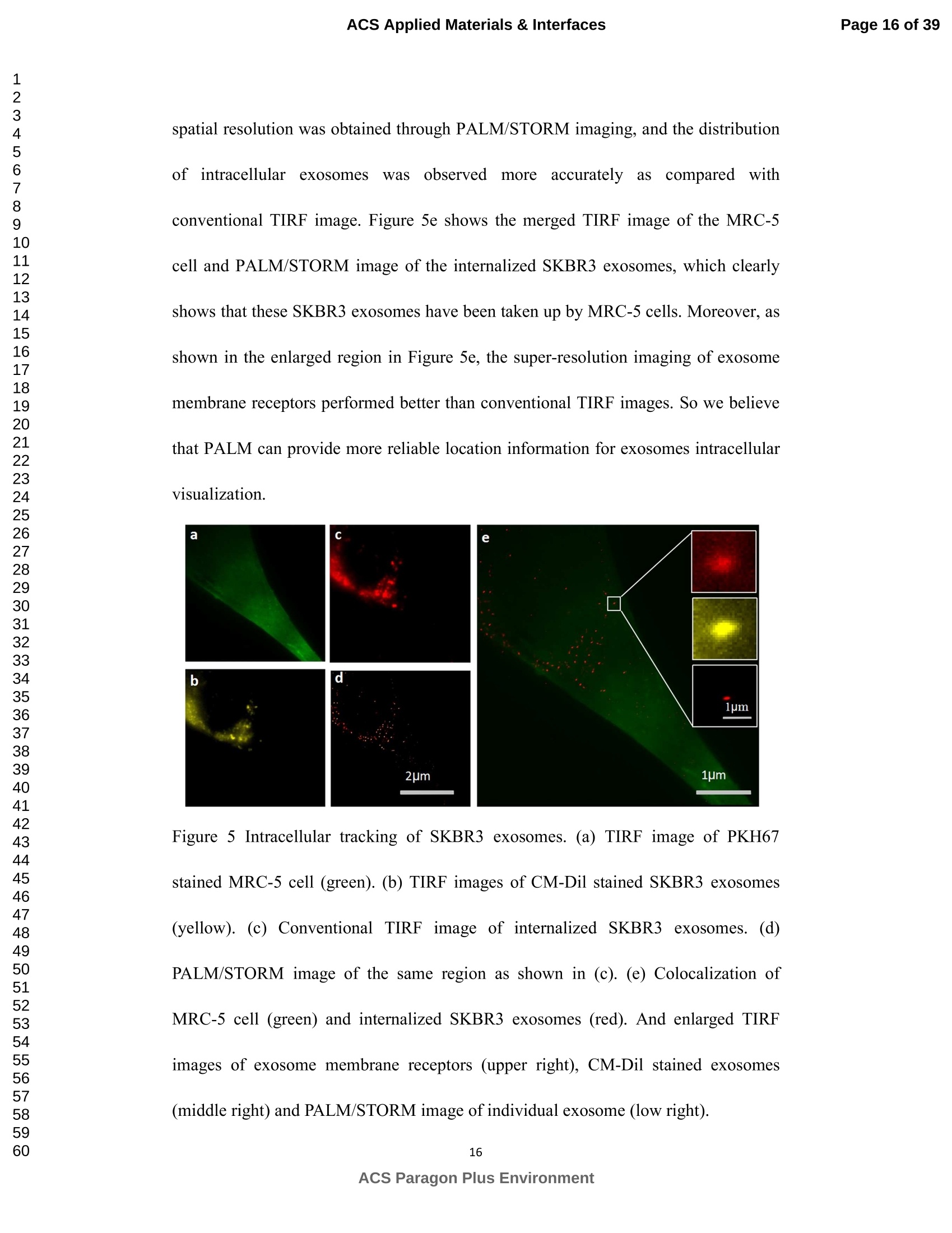
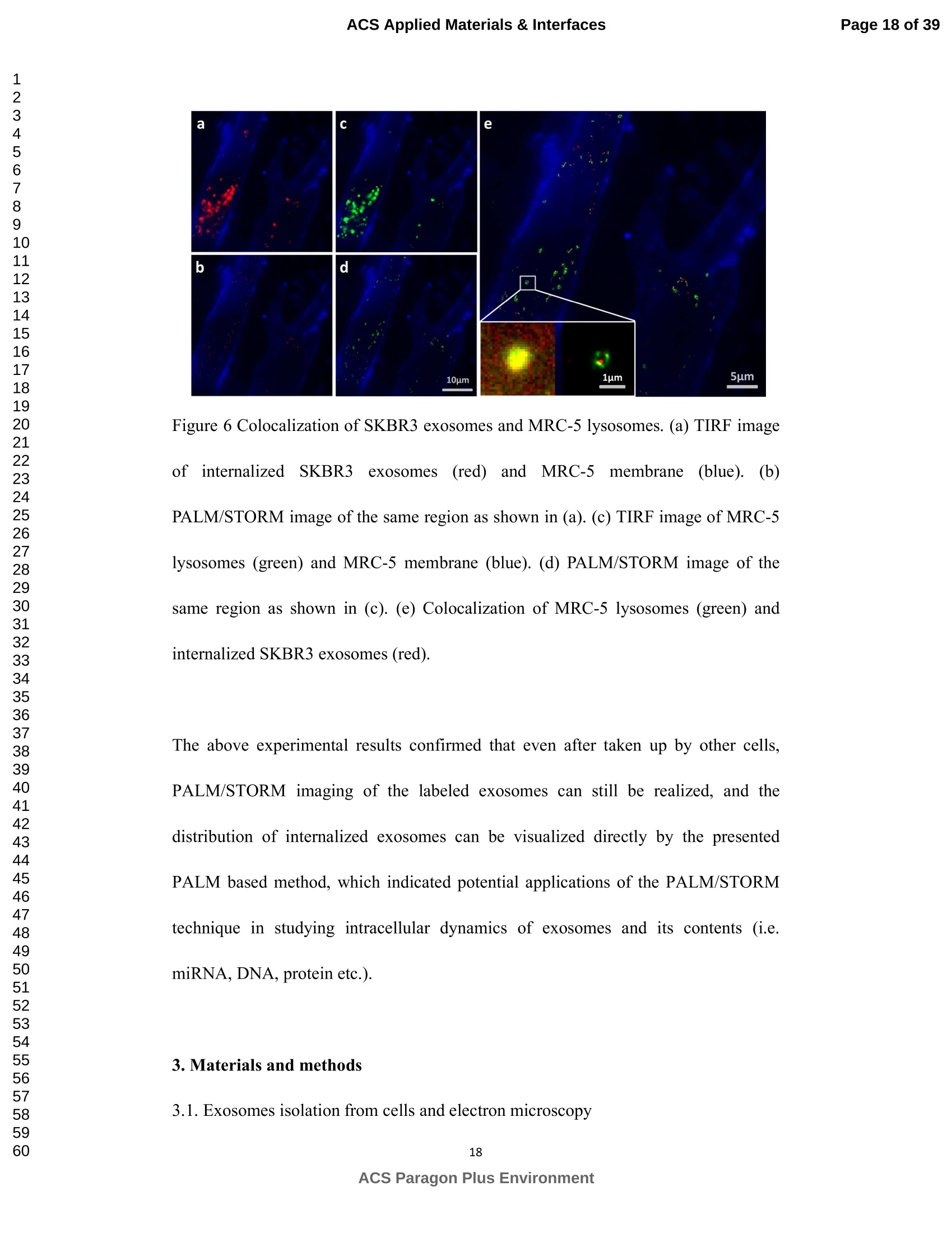
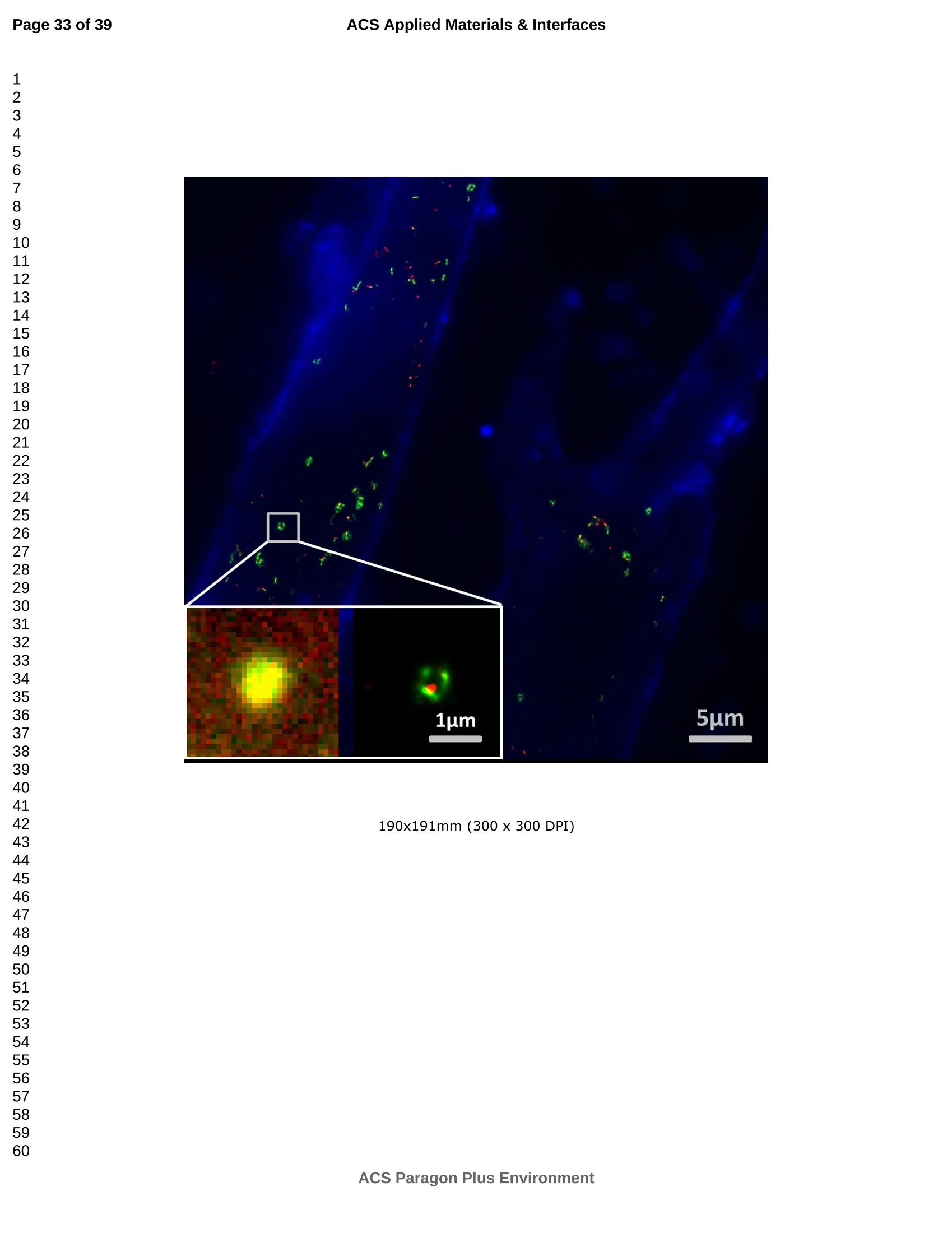
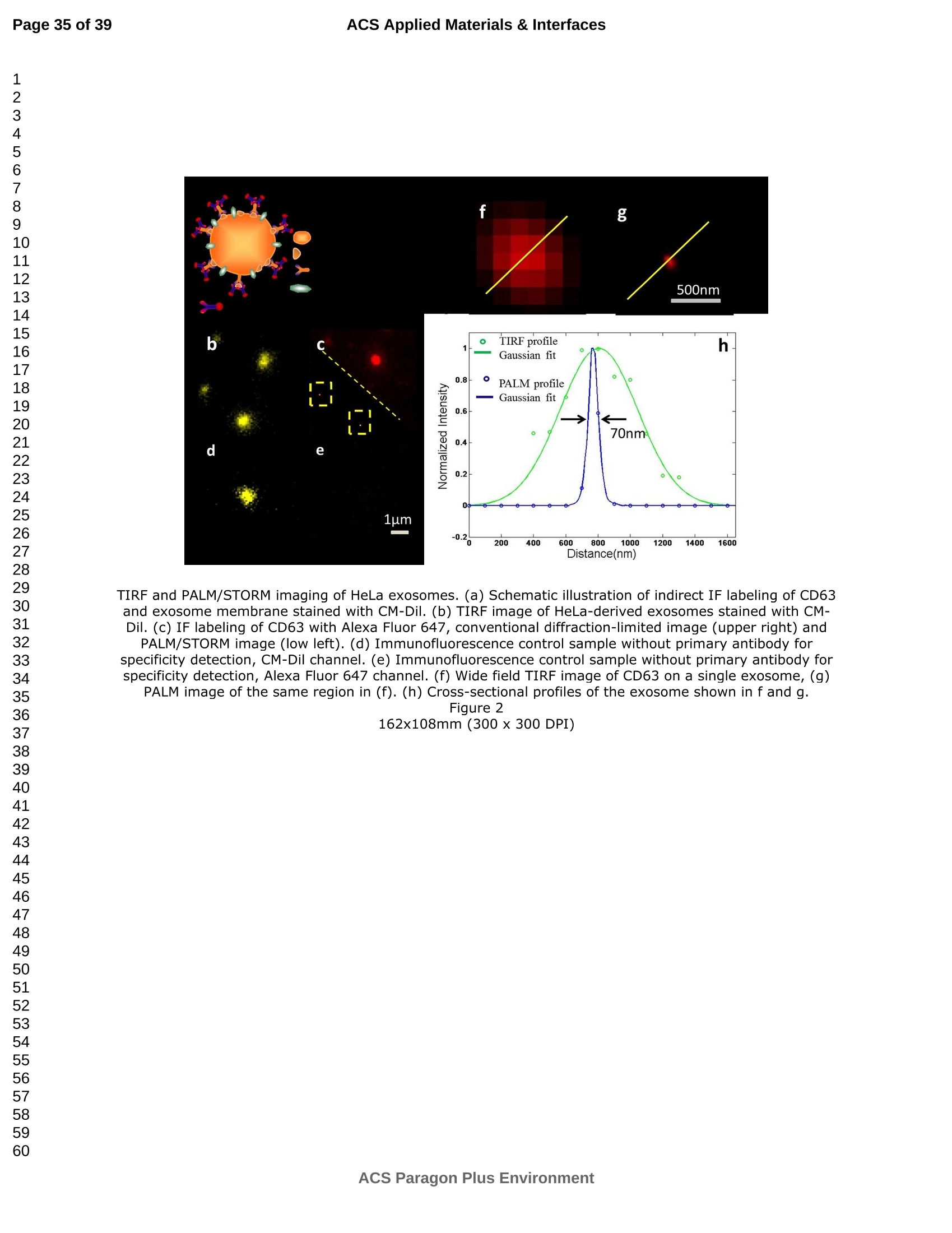
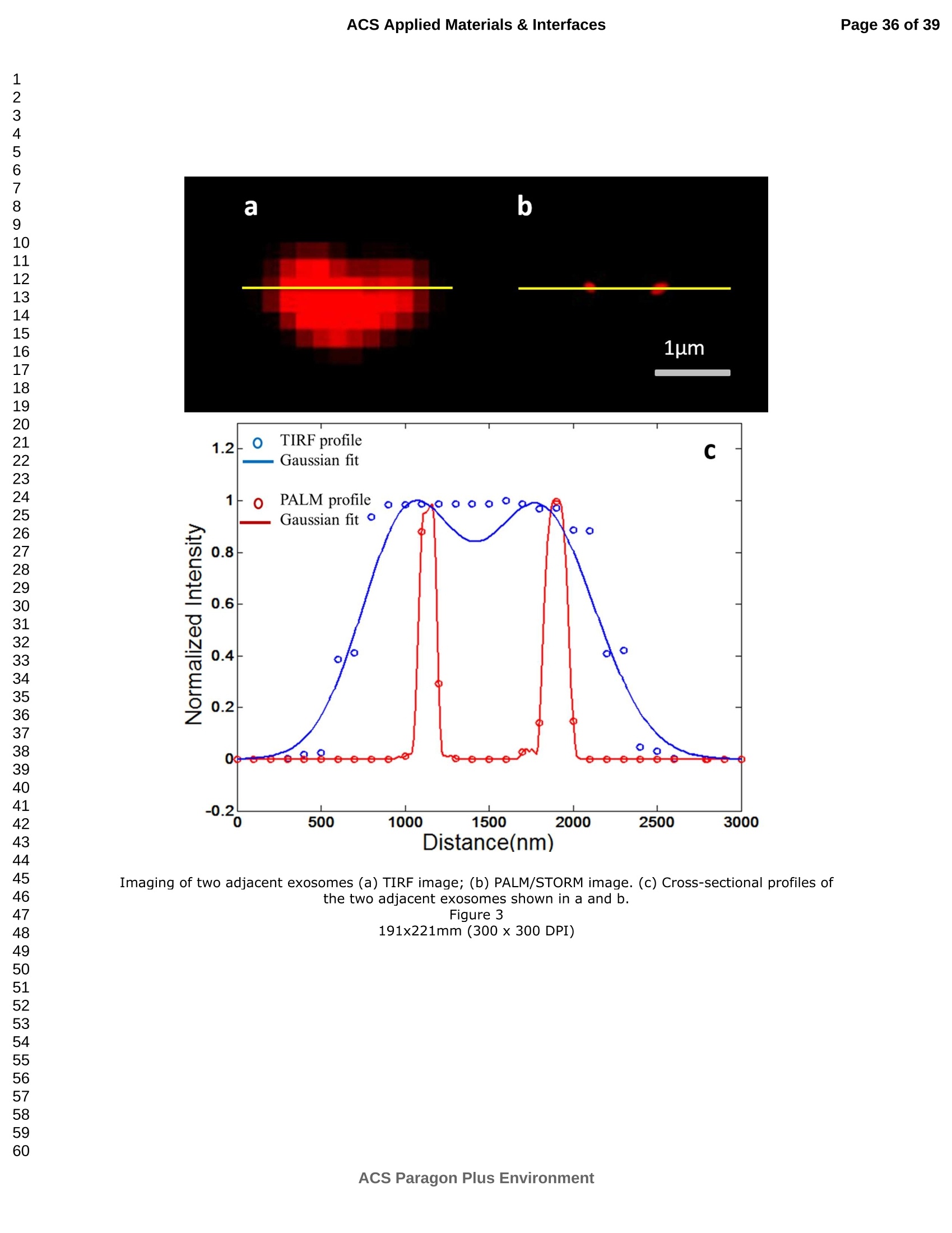
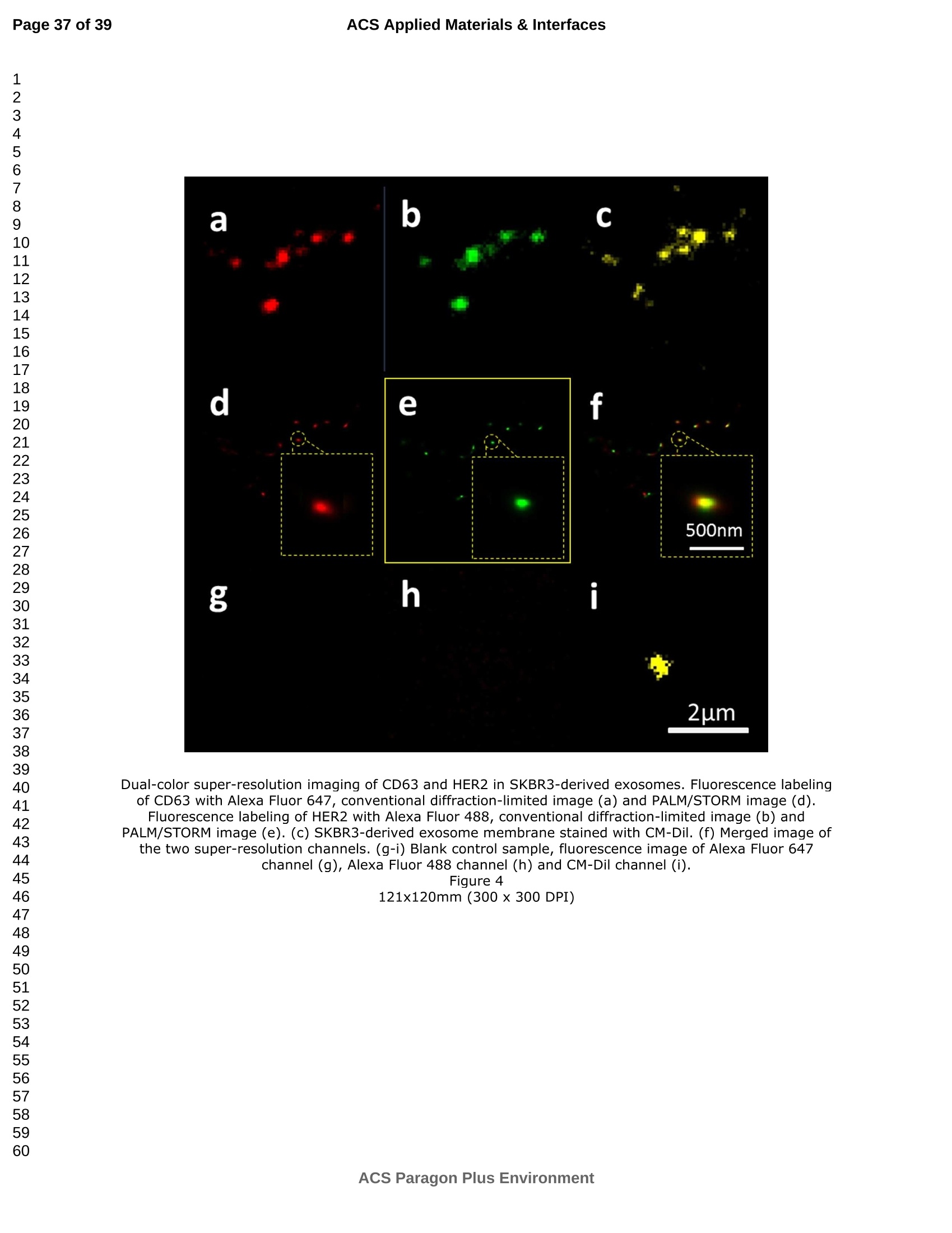
外泌体是由细胞分泌的富含蛋白质的小膜囊泡。考虑到外泌体在不同生理活动中的重要作用以及在诊断药物递送方面的潜在价值,研究者们在体外追踪和内容物分析方面投入了大量的精力。近年来,各种超分辨率显微镜的出现为外来体的研究提供了强有力的工具。在这里,研究者展示了基于单分子定位的超分辨率成像技术(PALM/STORM)在癌症来源的外泌体的成像和跟踪中的应用。在实验中,首先,从肿瘤细胞的培养基中提取癌症来源的外泌体。然后,外泌体膜受体用光可切换探针标记,其允许通过光激活定位显微镜(PALM)或随机光学重建显微镜(STORM)对这些膜受体进行超分辨率成像。通过使用人乳腺癌细胞来源的外泌体,研究者展示了外泌体膜上的两种膜受体的同时双色PALM/STORM成像。此外,外泌体的成功标记和成像使得可以观察癌症来源的外泌体与正常细胞之间的相互作用。同时,研究者利用PALM/STORM成像实现了癌源性外泌体和溶酶体在受体细胞中的共定位。由于外泌体在细胞间通讯中起着至关重要的作用,研究者预计所提出的基于PALM/STORM的外泌体成像和跟踪在研究外泌体介导的癌症转移机制中具有巨大的潜力。#01研究介绍外泌体是直径为30-100 nm的小细胞外囊泡(EV)。外泌体由多种培养的细胞在多泡体(MVB)与细胞膜融合时分泌。由于它们的来源,外泌体富含蛋白质的子集,包括四跨膜蛋白家族的成员(CD 9、CD 63和CD 81)、内体运输蛋白的成员(ESCRT相关蛋白/阿利克斯)和热休克蛋白Hsp 60、Hsp 70和Hsp 702。这些小囊泡代表了细胞间通讯和物质交换(例如胞质蛋白质、脂质和核酸的递送)的重要方式,而没有直接的细胞间接触,并且对于一些实际临床应用(例如诊断和药物递送)是潜在的。几个世纪以来,光学显微镜极大地促进了我们对分子和细胞生物学的理解以及对外泌体的研究。与其他成像技术相比,荧光显微镜的突出优势是其与样品的兼容性,这确保了非侵入性成像和高生化特异性。然而,对于常规光学显微镜来说,几百纳米的中等分辨率限制了外来体的准确定位和成像,因为它们的尺寸相对较小(〈100 nm)。近年来,一系列新颖的光学超分辨成像技术克服了光学衍射极限。具体地,通过单分子定位显微镜已经实现了20 nm至50 nm的横向成像分辨率,这有利于可视化超小细胞结构。典型的光学超分辨成像技术包括近场扫描光学显微镜(NSOM)、受激发射损耗(STED)显微镜 、光激活定位显微镜(PALM)、随机光学重建显微镜(STORM)等,其中PALM和STORM共享类似的工作原理。首先,进行具有光可切换性质的单个荧光团的顺序成像和定位。然后,可以从这些激活的荧光团的位置重建具有亚衍射极限分辨率的图像。到目前为止,许多具有不同光开关原理的荧光团已被用于基于单分子定位的超分辨率显微镜,包括有机染料,荧光蛋白,和量子点。如今超分辨率显微镜已被用于亚细胞结构的定性或定量分析。随着多色、3D和活细胞成像的进步,这项技术在构建纳米尺度的细胞结构图和提供以前未解决的生物结构和过程细节方面具有巨大潜力。在不同的超分辨率成像技术中,PALM/STORM可以提供最高的分辨率(20-50 nm),这很好地适合外泌体的大小。在此,研究者展示了PALM/STORM技术在癌症来源的外泌体的成像和追踪中的应用。第一次,外泌体描绘和跟踪细胞内的超分辨率显微镜。#02结果和讨论(节选)2.1.外泌体的分离和TEM分析在实验中,使用两种癌细胞系,即人乳腺癌细胞(SKBR 3细胞)和人宫颈癌细胞(HeLa细胞)。由于其小尺寸,外泌体纯化曾经是具有挑战性的。到目前为止,已经开发了用于从细胞培养上清液、尿液或其他生物流体纯化外来体的可靠且方便的方法,包括超速离心、超滤、在抗体偶联的磁珠上捕获、和基于聚合物的沉淀。对于特定的应用,具有不同隔离结果的每种方法都有其优点和缺点。外泌体的纯化基本上通过基于聚合物的沉淀完成。考虑到它们的小尺寸(30至IOOnm),外泌体可以用电子显微镜表征,并且提取的外泌体在图1中可视化。TEM结果证实,从细胞培养基中成功提取了外泌体。图1 从HeLa细胞(a)和SKBR3细胞(b)分离的外来体的透射电子显微镜(TEM)图像2.2基于PALM/STORM的癌症来源的外来体的超分辨率成像2.2.1.HeLa外泌体中CD63的超分辨率成像单个HeLa衍生的外来体的成像结果示于图2中。在膜染料CM-Dil的帮助下,研究者可以鉴定外泌体的存在。图2b示出了通过用561 nm激光激发CM-Dil的外来体的TIRF图像。在定位外泌体后,进行这些外泌体的PALM/STORM成像,结果示于图2c中。图2c的左下图是从15000帧重建的CD 63的PALM/STORM图像。而图2c的右上图是如图2b所示的相同区域的宽场TIRF图像。显然,与TIRF图像相比,PALM/STORM图像提供了高得多的空间分辨率。从免疫荧光样品中获得了特异性的进一步证据。通过用PBS替换兔抗CD 63抗体并保持其他程序和反应条件不变来制备对照样品。对照样品的结果示于图2d和2 e中。CM-Dil通道证实了外来体的定位,然而,观察到Alexa Fluor 647的荧光可忽略不计(图2 e)。这充分证明了在没有一抗的情况下,Alexa Fluor 647标记的二抗不能与外来体膜蛋白反应。结果,在图2c中观察到的Alexa Fluor 647的荧光真正来源于CD 63,并且IF反应显示出良好的特异性。根据图2g和2 h中所示的PALM/STORM成像的外来体的横截面图,外泌体的最终重建超分辨率图像达到70 nm的半峰全宽(FWHM),这与外泌体的直径(30-100 nm)很好地对应。相反,CD 63的TIRF图像远大于单个外来体的大小(图2f和2 h)。这证明与常规宽场TIRF相比,PALM可以提供更可靠的外泌体结构信息。图2 HeLa外来体的TIRF和PALM/STORM成像(a)用CM-Dil染色的CD 63和外来体膜的间接IF标记的示意图。(b)用CM-Dil染色的HeLa衍生的外来体的TIRF图像。(c)用Alexa Fluor 647标记CD 63,常规衍射限制图像(右上)和PALM/STORM图像(左下)。(d)不含特异性检测用一抗的免疫荧光对照样品,CM-Dil通道。(e)不含特异性检测用一抗的免疫荧光对照样品,Alexa Fluor 647通道。(f)单个外泌体上CD 63的宽场TIRF图像。(g)(f)中相同区域的PALM图像。(h)f和g中示出了外泌体的横截面轮廓。传统显微镜的分辨率约为几百纳米,大约是亚细胞区室的大小,因此不足以确定许多细胞内细胞器的纳米级位置和数量。外泌体的PALM/STORM成像的优越性可以通过对两个相邻的外泌体进行成像来更好地证明。如图3所示,呈现了两个紧密堆积的外来体上的CD 63的TIRF和PALM/STORM图像。根据两个外泌体的截面轮廓,由于衍射极限,常规TIRF显微镜无法识别这两个紧密堆积的外泌体。而PALM/STORM图像清楚地显示了两个外泌体的存在定位。因此,由于能够分辨超出细胞器水平的亚细胞结构,PALM/STORM已经满足了外来体的纳米级定位精度,即使它们彼此相邻。图3两个相邻外来体的成像(a)常规TIRF图像;(B)PALM/STORM图像2.2.2.SKBR 3外泌体中CD 63和HER 2/C-erbB-2的双色超分辨成像荧光显微镜的一个明显优势是通过用光谱上不同的荧光团标记两种或更多种成分同时成像的优异性能。在癌症疾病的研究中,同时监测不同的受体/生物标志物可以提供关于患者的生理状态的更可靠的信息。例如,乳腺癌的诊断需要同时分析各种受体/生物标志物,例如人表皮生长因子受体-2(HER 2)、表皮生长因子受体(EGFR)、雌激素受体(ER)和孕酮受体(PR)。由于外泌体在肿瘤转移中起重要作用,因此外泌体上不同受体的同时成像可以为研究外泌体介导的癌症转移提供信息。因此,证明了外泌体上两种受体的同时双色PALM/STORM成像。为了实现双色PALM/STORM成像19,除Alexa Fluor 647外,还使用了Alexa Fluor 488。先前的研究表明,Alexa Fluor 488也适用于PALM/STORM成像。此外,它的荧光信号可以很好地与Alexa Fluor 647区分开来,这可以大大防止双色成像中的串扰。来源于SKBR 3细胞的外泌体在此用作模型外泌体,并且待成像的靶受体是CD 63和HER 2(人表皮生长因子受体-2)。由C-erbB-2/HER 2阳性肿瘤细胞系SKBR 3释放的外来体表现出HER 2/C-erbB-2过表达。此处,如材料和方法部分中所述,CD 63用Alexa Fluor 647 IF标记,HER 2用Alexa Fluor 488 IF标记。为了构成有效标记的额外证据,我们使用共聚焦显微镜的波长扫描功能来描绘Alexa Fluor 488、Alexa Fluor 647和CM-Dil标记的外来体的荧光发射强度。与理论分析一致,两种染料的荧光信号可以很好地区分,这防止了多色成像中的串扰。外泌体膜用CM-Dil染色。此处还添加空白对照以证明IF的特异性。双色PALM/STORM成像结果见图4。红色通道是CD 63,绿色通道是HER 2,黄色通道是外来体膜。可以看出,Alexa Fluor 488和Alexa Fluor 647的荧光信号都可以在SKBR 3外来体上检测到(图4a、d和b、e)。虽然在空白对照中没有检测到对应于这些荧光团的荧光(图4g和h),但证实IF标记具有优异的特异性并且双色标记是成功的。与图4d和4 e中所示的PALM/STORM图像相比,图4a和4 b中所示的宽场TIRF图像表现出不令人满意的分辨率。图4f中所示的合并的双色PALM/STORM图像表明CD 63和HER 2都在SKBR 3外来体上表达。上述实验结果表明,使用PALM/SORM和IF技术可以实现外泌体上不同蛋白质的同时双色超分辨率成像。图4 SKBR 3衍生的外来体中CD 63和HER 2的双色超分辨率成像用Alexa Fluor 647对CD 63进行荧光标记,常规衍射受限图像(a)和PALM/STORM图像(d)。用Alexa Fluor 488对HER 2进行荧光标记,常规衍射受限图像(b)和PALM/STORM图像(e)。(c)用CM-Dil染色的SKBR 3衍生的外泌体膜。(f)两个超分辨率通道的合并图像。(g-i)空白对照样品,Alexa Fluor 647通道(g)、Alexa Fluor 488通道(h)和CM-Dil通道(i)的荧光图像。通过超分辨率成像细胞内追踪癌症来源的外来体最近的研究表明外来体在不同的生理和病理过程中起重要作用,例如免疫应答的调节、肿瘤转移和炎症。由于外泌体的功能取决于它们与受体细胞相互作用的能力,因此研究已经非常重视外泌体摄取的机制。在遇到它们的目的地时,外泌体可以与受体细胞的膜融合,然后将内容物直接释放到细胞质中或通过内吞途径有效地内化。最近的报告表明,肿瘤细胞可以释放液(富含蛋白质和rna)的一个子集为pre-metastatic利基preparation瀑特异性细胞。调查的细胞间tumor-derived液囊和正常细胞之间的通信是一个液的主要工作理解角色在威胁生命的癌症转移。所以我们用人类胚胎肺成纤维细胞(MRC-5)作为模型正常细胞观察的细胞吸收SKBR3液由正常细胞,可以更好的展示超分辨率成像技术的潜在应用液相关的生物过程的研究。在实验中,CD63 SKBR3液如果贴上Alexa萤石647,和外来体膜沾CM-Dil在材料和方法部分描述。然后他们被MRC-5培养细胞。MRC-5细胞的膜与PKH67染色。孵化后1小时,对这些MRC-5细胞进行TIRF和PALM/STORM成像。在图5中,研究者意识到内化的colocalization SKBR3液和MRC-5细胞。如图5 c和d,获得了更高的空间分辨率成像,通过手掌/风暴更准确地观察和细胞内液的分布与传统相比TIRF形象。图5 e显示了合并TIRF MRC-5单元的图像和棕榈/风暴的形象内化SKBR3液,这清楚地表明,这些SKBR3液已经被MRC-5细胞。此外,如扩大区域如图5所示,执行的外来体膜受体超分辨率成像比传统TIRF图像。所以研究者相信,棕榈液胞内可以提供更可靠的位置信息可视化。图5 SKBR 3外泌体的细胞内追踪(a)PKH 67染色的MRC-5细胞的TIRF图像(绿色)。(b)CM-Dil染色的SKBR 3外来体的TIRF图像(黄色)。(c)内化的SKBR 3外来体的常规TIRF图像。(d)如(c)中所示的相同区域的PALM/STORM图像。(e)MRC-5细胞(绿色)和内化的SKBR 3外来体(红色)的共定位。以及外泌体膜受体的放大的TIRF图像(右上)、CM-Dil染色的外泌体(中右)和单个外泌体的PALM/STORM图像(右下)。先前的研究表明细胞摄取外来体依赖于内吞作用和吞噬作用,一旦内吞,大量外来体随后被转运到溶酶体中进行降解。然而,常规荧光显微镜的中等分辨率阻碍了对外来体的溶酶体运输的直接监测。在这里,使用PALM/STORM成像,研究者进一步实现了MRC-5细胞的内化SKBR 3外泌体和溶酶体的共定位。类似地,用Alexa Fluor 647 IF标记SKBR 3外来体,并用LysoTracker Red标记MRC-5的溶酶体。MRC-5的膜用PKH 67染色。由于两个原因,选择LysoTracker Red作为溶酶体染料。首先,它对溶酶体的酸性膜具有高度选择性。其次,它可以通过561 nm照明成像和关闭,并通过405 nm光重新激活,这使得它适合于PLAM/STORM成像。实验结果如图6所示。观察到外泌体和溶酶体的良好共定位,这意味着大部分外泌体定位在溶酶体内。此外,如图6 e中放大的双色PALM/STORM图像所示,在溶酶体内部清楚地看到外来体.所获得的超分辨率图像为以下结论提供了更有力的证据:在与受体细胞结合后,外泌体将被转运到溶酶体中以进一步降解。图6 SKBR 3外来体和MRC-5溶酶体的共定位(a)内化的SKBR 3外泌体(红色)和MRC-5膜(蓝色)的TIRF图像。(b)如(a)中所示的相同区域的PALM/STORM图像。(c)MRC-5溶酶体(绿色)和MRC-5膜(蓝色)的TIRF图像。(d)如(c)中所示的相同区域的PALM/STORM图像。(e)MRC-5溶酶体(绿色)和内化的SKBR 3外来体(红色)的共定位。上述实验结果证实,即使在被其他细胞摄取后,标记的外泌体的PALM/STORM成像仍然可以实现,并且内化的外泌体的分布可以通过所提出的基于PALM的方法直接可视化,这表明PALM/STORM技术在研究外泌体及其内容物(即miRNA、DNA、蛋白质等)的细胞内动力学中的潜在应用。#03结论使用IF和PALM/STORM技术实现癌症来源的外来体的基于单分子定位的超分辨率成像。与传统的视场显微镜相比,PALM/STORM为观察外来体提供了更好的空间分辨率,其尺度可达纳米级。此外,还呈现了癌症来源的外泌体的同时双色PALM成像。通过与传统的宽视野显微镜相比,很明显,使用PALM/STORM成像技术,研究者可以更精确地可视化掺入的外泌体的细胞内位置。因此,PALM/STORM是一种新的强大的方法,用于细胞内跟踪外来体和其他纳米结构,具有优异的空间分辨率。此外,凭借这些优异的特性,我们有充分的理由相信,超分辨率成像将非常适用于后续的外泌体和外泌体相关生理过程的研究。力显智能现已发布的超高分辨率显微成像系统 iSTORM,成功实现了光学显微镜对衍射极限的突破,使得在20纳米的分辨率尺度上从事生物大分子的单分子定位与计数、亚细胞及大分子复合物结构解析、生物大分子生物动力学等的研究成为现实,从而给生命科学、医学等领域带来重大突破。ACSAPPLIEDMATERIALS&INTERFACES ACS Applied Materials & InterfacesPage 1 of 39 Article Imaging and Intracellular T r acking of Cancer Derived Exosomes U si n g Si n gle Molecule L oca l ization Based Super Resolution Microscope Chen Chen, Shenfei Zong, Zhuyuan Wang, Ju Lu, Dan Zhu, Yizhi Zhang, and Yiping Cui ACS Appl. Mater. Interfaces, Just Accepted Manuscript· DOI : 10.1021/acsami .6b09442·Publication Date (Web): 12 Sep 2016Downloaded from http://pubs.acs.org on September 13, 2016 Just Accepted “Just Accepted” manuscripts have been peer-reviewed and accepted for publication. They are posted online prior to technical editing, formatting for publication and author proofing. The American Chemical Society provides “Just Accepted"as a free service to the research community to expedite the dissemination of scientific material as soon as possible after acceptance. “Just Accepted” manuscripts appear in full in PDF format accompanied by an HTML abstract.“Just Accepted”manuscripts have been fully peer reviewed, but should not be considered the official version of record. They are accessible to all readers and citable by the Digital Object Identifier (DOI@). “Just Accepted” is an optional service offered to authors. Therefore, the “Just Accepted" Web site may not include all articles that will be published in the journal. After a manuscript is technically edited and formatted, it will be removed from the“Just Accepted” Web site and published as an ASAP article. Note that technical editing may introduce minor changes to the manuscript text and/or graphics which could affect content, and all legal disclaimersa nd ethical guidelines that apply to the journal pertain. ACS cannot be held responsible for errors or consequences arising from the use of information contained in these “Just Accepted” manuscripts. ACS Applied Mater i als & Interfaces i s published by t he Amer i can Chemical Society.1155 Sixteenth Street N.W., Washington, DC 20036 Published by American Chemical Society. Copyr i ght C American Chemical Society. However, no copyright claim is made to original U.S. Government works, or works produced by employees of any Commonwealth realm Crown government in the course of their duties. Imaging and Intracellular Tracking of Cancer Derived Exosomes Using Single Molecule Localization Based Super Resolution Microscope Chen Chen, Shenfei Zong, Zhuyuan Wang, Ju Lu, Dan Zhu, Yizhi Zhang, Yiping Cui*Advanced Photonics Center, Southeast University, Nanjing 210096, China *cyp@seu.edu.cn Abstract Exosomes are small membrane vesicles secreted by cells and enriched with plenty of proteins. Considering their significant roles i n different physical activit i es and potential value for diagnostic drug delivery, researchers have put great efforts in in-vitro tracking and contents analysis of exosomes. Recently, the emergence of di f ferent kinds of super-resolution microscopy provides powerful tools for exosome study. Here, we demonstrate the application of single molecule localization based super resolution i maging technique (PALM/STORM) i n t he imaging and tracking of cancer-derived exosomes. In t he experiment, f irst, cancer-derived exosomes are extracted from the culture media of tumor cells. Then the exosome membrane receptors are labeled with photoswitchable probes, which allow super resolution imaging of these membrane receptors via photoactivated localization microscopy (PALM) or stochastic optical reconstruction microscopy (STORM). By using human breast cancer cell derived exosomes,, we demonstrated simultaneous dual-color PALM/STORM imaging of two kinds of membrane receptors on the exosome membrane. Moreover, the successful labeling and i maging of exosomes make i t possible to observe the interaction between cancer-derived exosomes and normal cells. Meanwhile, we realized the colocalization of cancer-derived exosomes and lysosomes in recipient cells with PALM/STORM imaging. Since exosomes play a vital role i n intercellular communications, we anticipate that the presented PALM/STORM based imaging and tracking of exosomes holds a great potential in the investigation of the mechanism of exosome mediated cancer metastasis. Keywords: exosomes, membrane receptors, super-resolution imaging,PALM/STORM, intracel l ular t racking 1. Introduction Exosomes are small extracellular vesicles (EVs) with a diameter of 30-100 nm.Exosomes are secreted by a variety of cultured cells upon fusion of the multivesicular bodies (MVB) with the cell membrane . Because of their origin, exosomes are enriched with a subset of proteins including members of the t etraspanin f amily (CD9,CD63 and CD81), members of the endosomal trafficking proteins (ESCRT-related proteins/Alix) and heat-shock proteins Hsp60, Hsp70 and Hsp702.5. These small vesicles represent an important manner for the i ntercellular communication and exchanging of substances (such as delivery of cytosolic proteins, lipids and nucleic acids) without direct cell-to-cell contact3. 6-9and are potential for some practical clinical application (e.g. diagnosis and drug delivery)10-11. For centuries, optical microscopy has greatly facilitated our understanding of molecular and cellular biology as well as the i nvestigation of exosomes'’. Compared with other imaging t echniques, the outstanding advantage of fluorescence microscopy i s its compatibility with samples, which ensures noninvasive imaging and high biochemical specificityl3. However, the moderate resolution of several hundred nanometers for conventional optical microscopy limitss the accurate localization and i maging of exosomes considering their relatively small size (<100 nm). Recently, the optical diffraction limit has been overcome by a series of novel optical super resolution imaging techniques. Specifically, lateral imaging resolutions of 20 nm to 50 nm have been achieved by single molecule localizat i on microscopy,which i i :stbeneficial for visualizing ultra-small cellular structures. The typical optical super resolution imaging techniques include near-field scanning optical microscopy (NSOM), stimulated emission depletion (STED) microscopy15-16, photoactivated localization mic SC (PALM)17-18, stochastic optical r econstruction microscopy (STORM)4 and so on.Among these, PALM and d STORM share 2a similar working principle. First,sequentially imaging and localization of single fluorophores with photoswitchable nature are conducted. Then images with sub-diffraction-limit resolution can be reconstructed from the positions of these activated f luorophores 19-20. So far, many f l uorophores with different photoswitchable principles have been exploited for single molecule localization based super resolution microscopy including organic dyes17,21-22,fluorescent proteinsl3, 18, 23and quantum dots24-26. Nowadays, super r esolution microscopy has been applied for qualitative or quantitative analysis of subcellular structures27-28. This technology, with the advances i n multi-color, 3D, and living-cell imaging, holds a great potential in building a map of cellular st r ucture at the nanometer scale and providing previously unresolved details of biological structures and processes29. Among different super resolution imaging techniques,PALM/STORM can prov i de the highest resolution (20-50 nm), which well suits the size of exosomes. Herein, we demonstrated the application of PALM/STORM technique in the imaging and tracking of cancer derived exosomes. For the first t ime, exosomes were depicted and t racked intracellularly by super resolution microscopy. We f irst extracted cancer derived exosomes from the conditioned cell culture media, and subsequently labeled the exosome membrane receptors via indirect immune fluorescent label i ng (IF). IF is the most widely used method to detect endogenous proteins. Directly l abeling and imaging of the exosome membrane receptor can be realized with a fluorophore tagged primary antibody. However, usually, primary antibody itself is already quite expensive and d fluorophore tagged primary antibodycosts muchh more. On the contrary,secondary antibodies are more common and f luorophore tagged secondary antibodies are much cheaper. Besides , secondary ant i bodies in indirect immunof l uorescence can also act as signal amplifiers, which can significantly i mprove the l abeling density30-31Consequently y ,, i i r ndirect immunofluorescence labeling usingbothprimary and secondary antibodies is usually used i n super resolution imaging. As a r esult, in t he experiment, the exosome membrane receptors were first labeled with a primary antibody, followed by magnification with a secondary antibody coupled with small organic dyes. The employed organic dyes (i .e. Alexa Fluor 488 and Alexa Fluor 647), which can be switched reversibly for hundreds of cycles between on and off states, are suitable to target intracellular structures using PALM/STORM based super resolution imaging l 4, 19,33. PALM/STORM techniques require t hat t he employed fluorophores can "blink” so as to realize single molecule based localization. Previous studies have proven that Alexa Fluor 488 and Alexa Fluor 647 well meet t he requirements of PALM/STORM1.Moreover, these photoswitchable f luorescent dyes possess a quite small size (around 2 nm), which i s benef i cial for achieving high labeling densities14,33.The experimental r esults showed that simultaneous dual-color PALM/STORM i maging of the target exosome membrane proteins can be r eal i zed.Finally, the interaction between cancer derived exosomes and normal cells are also investigated and the accumulation of exosomes in lysosomes are directly observed by the P presented PALM/STORM basedmethod.Compared with conventional microscopy, PALM/STORM provides bet t er spatial resolution for observing and tracking exosomes at the nanometer scale, which can provide more unobserved details of exosome structures and dynamic processes. 2. Results and discussion 2.1. Isolation of exosomes and TEM analysis In the experiments, two cancer cell l ines were used, i.e. human breast cancer cells (SKBR3 cel l s) and human cervical cancer cells (HeLa cells). Because of its small size, exosome puri f ication was once challenging. So far, dependable and convenient methods have been developed for exosome puri f ication from cell culture supernatant,urine or other biological fluids, including ultracentrifugation', ultrafiltration35,capture on antibody-coupled magneticbeads34. 36-37and polymer-based prec i pitat i on38-400. Each method with different isolation outcomes has its advantage and weakness for a particular application. Herein, we adopted a mature exosome collection kit provided by System Biosciences (SBI) to isolate exosomes from conditioned cell culture media. Exosome collection was performed according t o manufacturer’s instructions. The puri f ication of exosomes was basically finished through polymer-based precipitation. Given their small size (30 to 100 nm), exosomes can be characterized with an electron microscopy and the extracted exosomes are visualized in Figure 1. The TEM results confirmed that exosomes are successfully extracted from the cel l culture media. Figure 1 Transmission electron microscopy (TEM) images of exosomes i solated from HeLa cells (a) and SKBR3 cells (b). Enriched exosomes were casted onto carbon coated copper mesh and stained by phosphotungstic acid. 2.2 PALM/STORM based super resolution i maging of cancer derived exosomes 2.2.1. Super-resolution i maging of CD63 in HeLa exosomes To prove that PALM/STORM can be employed in super resolution imaging of exosomes, we first utilized CD63 as the model target protein to be imaged, because CD63is a C common surface marker found on exosomes.We utilized i ndirect immunofluorescence labeling (IF), a form of fluorescent antibody technique used to detect i mmune complexes and microorganisms"l , to realize PALM/STORM based super resolution imaging of CD63. Indirect IF uses two antibodies. The primary antibody i s unconjugated and a fluorophore-conjugated secondary antibody directed against the primary antibody i s used for detection". And the fluorescent dye conjugated to secondary ant i body must be suitable for PALM/STORM imaging. We chose the dye Alexa Fluor 647 to l abel CD63, which exhibits a high photon yield and can be switched reversibly between on and off state with high efficiency and span the visible wavelength range. Figure 2a shows t he schematic illustration of the IF experiment. CD63 on the exosome membrane was first labeled with Rabbit anti-CD63,followed by immune recognition with Alexa Fluor 647 labeled goat anti-Rabbit IgG.In addition, the exosome membrane was stained with 1,1'-dioctadecyl-3,3,3',3'-tet r amethylindocarbocyanineperchlorate (CM-Dil) to further confirm the existence of exosomes. CM-Dil is a fluorescent dye which can diffuse within lipid membranes and are often used as a membrane tracer2. PALM/STORM i maging experiments were performed with a Zeiss Elyra P.1microscope equipped with 405 nm, 488 nm, 561 nm and 642 nm l aser. CD63 was imaged using PALM/STORM technique while exosome membrane was imaged via total internal reflection (TIRF) technique. Detailed experimental procedures can be found in the Materials and Methods section11, 19. The imaging results of individual HeLa derived exosomes are shown in Figure 2.With the help of membrane dye CM-Dil, we can identify the presence of exosomes.Figure 2b shows the TIRF image of exosomes by exciting CM-Dil with 561 nm laser.After locat i ng the exosomes, PALM/STORM imaging of these exosomes s were performed and the results are presented in Figure 2c. The bottom l eft panel of F i gure 2c is the PALM/STORM image of CD63 reconstructed from 15000 f rames. While the top right panel of Figure 2c i s wide field T IRF image of the same region as shown in Figure 2b. Obviously, the PALM/STORM image offers a much higher spatial resolution as compared with the TIRF i mage. Further evidence for specificity was obtained from the i mmunofluorescence sample. The control sample was prepared by replacing the rabbit anti-CD63 antibody with PBS and keeping other procedures and reaction conditions unchanged. The results of t he control sample are shown in Figure 2d and 2e. The CM-Di l channel confirmed the localization of exosomes, however,negligible fluorescence of Alexa Fluor 647 was observed (Figure 2e). This well proved that without primary antibody, Alexa Fluor 647 labeled secondary antibody cannotreact with he exosomes membrane e proteins. As a result t ,, the observed fluorescence of Alexa Fluor 647 in Figure 2c was t ruly originated from CD63 and the IF reaction showed a fine specificity. According to the cross-sectional profiles of the PALM/STORM imaged exosomes shown in Figure 2g and 2h, the final reconstructed super-resolution i mage of exosomes S achieved a full-width at half-maximum (FWHM) of 70 nm, which corresponds well with the diameter of exosomes (30-100 nm). On the contrary, the TIRF image of CD63 is much larger than the size of i ndividual exosome (Figure 2f and 2h). This proved that PALM can provide more reliable structural information of exosomes as compared with conventiona l wide field TIRF. Figure 2 TIRF and PALM/STORM i maging of HeLa exosomes.(a) Schematic illustration of indirect IF labeling of CD63 and exosome membrane stained with CM-Dil. (b) TIRF image of HeLa-derived exosomes stained with CM-Dil. (c) IF labeling of CD63 with Alexa Fluor 647, conventional di f fraction-l i mited i mage (upper right) and PALM/STORM image (low left). (d) Immunofluorescence cont r ol sample without primary antibody for specificity detection,CM-Dil channel.(e)Immunofluorescencee control sample e withou i t t primary antibodlyy ffor speci f icity detection, Alexa Fluor 647 channel . (f) Wide field TIRF image of CD63 on a single exosome, (g) PALM image of the same region i n (f ). (h) Cross-sectional profiles of the exosome shown in fand g. The resolution of traditional microscopy, which is about several hundred nanometers,is approximately the size of subcellular compartments and as a result, is i nsuff i cient for determining the nanoscale position and quant i ty of many i ntracellular organelles'2.And the superiority of PALM/STORM imaging of the exosomes can be better demonstrated by imaging two adjacent exosomes. As shown in Figure 3, TIRF and PALM/STORM images of CD63 on two closely packed exosomes are presented.According to the cross-sectional profiles of the t wo exosomes, conventional TIRF microscope cannot identify these two closed packed exosomes due to the di f fraction limit. While, while the PALM/STORM image clearly shows t he presenee-localization of the two exosomes. Therefore, with the ability to resolve subcellular structures beyond the organelle level, PALM/STORM has met the nanoscale localization accuracy of exosomes even if they are adjacent to each other. Figure 3 Imaging of two adjacent exosomes (a) Conventional TIRF i mage; (b)PALM/STORM image. (c) Cross-sectional profiles of the two adjacent exosomes shown in a and b. 2.2.2. Dual-color super-resolution i maging of CD63 and HER2/C-erbB-2 in SKBR3exosomes An obviously advantage of fluorescence microscopy is the excellent performance in simultaneous imaging of two or more constituents by labeling them with spectrally distinct fluorophores43. In the investigation of cancerous disease, simultaneous monitoring of different receptors/biomarkers can provide more reliable i nformation on the physiological state of the patients. For example, diagnosis of breast cancers requires simultaneous analyzing of various receptors/biomarkers such as human epidermal growth f actor receptor-2 (HER2), epidermal growth factor receptor (EGFR),estrogen receptor (ER) and progesterone receptor (PR)44-45. Since exosomes play an important role in tumor metastasis, simultaneous imaging of di f ferent receptors on exosomes can be informative for the i nvestigation of exosome mediated cancer metastasis. Hence , simultaneous dual-color PALM/STORM imaging of two kinds of receptors on exosomes was demonstrated. To realize dual color PALM/STORM imaging1, Alexa Fluor 488 was also used here in addition to Alexa Fluor 647.Previous study has shown that Alexa Fluor 488 is also suitable for PALM/STORM imaging. Besides, its f luorescence signal can be well distinguished from Alexa Fluor 647, which can greatly prevent crosstalk in dual color imaging. Exosomes derived from SKBR3 cells were employed as the model exosome here, and the target receptors to be imaged are CD633 and HER2 (human epidermal growth factor receptor-2). Exosomes released by the C-erbB-2/HER2-postive tumor cell l ine SKBR3 exhibit HER2/C-erbB-2 overexpression46-47. Here, CD63 was I F labeled with Alexa Fluor 647 and HER2 was IF labeled with Alexa Fluor 488 as described i n the Materials and Methods section. To constitute an addi t ional evidence for ef f icient labeling, we used t he wavelength scanning function of confocal microscopy to depict the fluorescence emission i ntensity of Alexa Fluor 488, Alexa Fluor 647 and CM-Dil labeled exosomes (Figure S1). Consistent with t heoretical analysis, the fluorescence signal of two dyes can be well distinguished which prevents crosstalk i n multi-color imaging. And the exosome membrane was stained with CM-Dil. A blank control was also added here to prove t he specificity of I F. Dual color PALM/STORM imaging results were presented in Figure 4. The red channel is CD63, green channel i s HER2 and yellow channel i s exosome membrane.As can be seen, fluorescence signals of Alexa Fluor 488 and Alexa Fluor 647 can both be detected on the SKBR3 exosomes (Figure 4 a, d and b, e ). While no fluorescence corresponding to these fluorophores was detected in the blank control (Figure 4g and h), confirming t hat IF l abeling has an excellent specificity and dual color labeling was successful. The wide field TIRF images shown in Figure 4a and 4b exhibit unsatisfactory resolutions as compared with the PALM/STORM images shown in Figure 4d and 4e. The merged dual color PALM/STORM i mage shown i n Figure 4f indicated that CD63 and HER2 were both expressed on SKBR3 exosome ie s s .. This corresponds well with the fact that CD63 is a common protein found on almost all the cell exosomes and HER2 is overexpressed on SKBR3 exosomes2,5,46-47. The above experimental results demonstrates that simultaneous dual color super resolut i on imaging of different proteins on exosomes can be realized using PALM/SORM and IF techniques. Figure 4 Dual-color super resolution i maging of CD63 and HER2 in SKBR3-derived exosomes. Fluorescence labeling of CD63 with Alexa Fluor 647, conventional diffraction-limited i mage (a) and PALM/STORM i mage (d). Fluorescence label i ng of HER2 with Alexa FFluor 488,, conventional diffraction-limited 1i image ((b)and PALM/STORM image (e). (c) SKBR3-derived exosome membrane stained with CM-Dil. (f) Merged i mage of the two super-resolution channels. (g-i ) Blank control sample, fluorescence image of Alexa Fluor 647 channel (g), Alexa Fluor 488 channel (h) and CM-Dil channel (i). 2.2.3. Intracel l ular tracking of cancer derived exosomes by super-resolution imaging Recent studies have suggested that exosomes play an important role in different physiologicall and pathologicalprocesses, ffor example regulat i on of immune responses48, t umor metastasis, and i nflammation10,49-5500. Since functions of exosomes depend on their abil i ty to interact with recipient cells, studies have put much emphasis on the mechanisms of exosome uptake. Upon encountering with their destinat i ons,exosomes may fuse with recipient cel l s’membrane and then releassee conentst cytoplasm directly or be i nternalized efficiently through endocytosis pathway°. Recent reports have showed that tumor cells can release exosomes (which are enriched W with asubset of proteins and RNAs)to organ-specific cellsfor pre-metastatic niche preparation1-52. I nvestigation of the i ntercellular communication between tumor-derived exosomes and normal cells i s a primary work to understand the e roles of exosomes in 1life-threatening cancer r metastasis. So We used human embryonic l ung fibroblast (MRC-5) as the model normal cells to observe the cellular uptake of SKBR3 exosomes by normal cells, which can better demonstrate the potential application of super resolut i on imaging technique i n the study of exosomes related biological procedures. In the experiment, CD63on n:SKBR3exosomes were IF labeled with Alexa Fluor 647, and the exosome membrane was stained with CM-Dil as described in the Materials and Methods section. Then they were co-cultured with MRC-5 cells. The membrane of MRC-5 cells was stained with PKH67. After incubation for 1 hour, these MRC-5 cells were subjected to TIRF and PALM/STORM imaging3. In Figure 5, we realized the colocalization of internalized SKBR3 exosomes and MRC-5 cell. As shown in Figure 5c and d, a much higher spatial resolution was obtained through PALM/STORM i maging, and the distribution of intracellular exosomes was observed more accurately as compared with conventional TIRF image. Figure 5e shows the merged TIRF image of the MRC-5cell and PALM/STORM i mage of the internalized SKBR3 exosomes, which clearly shows that these SKBR3 exosomes have been taken up by MRC-5 cells. Moreover, as shown in the enlarged region i n Figure 5e, the super-resolution i maging of exosome membrane receptors performed better t han conventional TIRF images. So we believe that PALM can provide more reliable location i nformation for exosomes intracellular visualization. Figure 5 Intracellular tracking of SKBR3 exosomes. (a) T I RF i mage of PKH67stained MRC-5 cell (green). (b) TIRF images of CM-Dil stained SKBR3 exosomes (yellow). (c) Conventional1TIRF image of internalized SKBR3exosomes.(d)PALM/STORM image of the same region as shown in (c). (e) Colocalization of MRC-5 cell (green) and internalized SKBR3 exosomes (red). And enlarged TIRF images of exosome membrane receptors (upper right), CM-Dil stained exosomes (middle right) and PALM/STORM image of i ndividual exosome (l ow right). Previous studies have shown that cellular uptaken of exosomes i s dependent on endocytosisand phagocytosis,a and once endocytosed, considerable amount of exosomes are subsequently transported into lysosomes for degradation . However,direct monitoring of t he l ysosomal l trafficking of exosomes is hindered by moderate resolution of conventional f luorescence microscopy54-577. Here, using PA LM/STORM imaging,, we further realized t he colocalization of i nternalized SKBR3 exosomes and lysosomes of MRC-5 cells. Similarly, SKBR3 exosomes were IF labeled with Alexa Fluor 647 and lysosomes of MRC-5 were labeled with LysoTracker Red. Membrane of MRC-5 was stained with PKH 67. LysoTracker Red is selected as the lysosomal dye due to two reasons. First, it is highly selective for the acidic membrane of lysosomes. Second, it can be i maged and switched off by 561 nm illuminationand reactivated d by y440055nm light,W which makes S:it S suitable for PLAM/STORM i maging58. The experimental results are shown i n Figure 6.Well colocalization of the exosomes and lysosomes was observed, which means a large portion of exosomes were localized i nside lysosomes. Moreover, as shown i n the enlarged dual color PALM/STORM image in Figure 6e, exosomes were clearly visualized in the interior of lysosomes. The acquired super resolution image provides more powerful evidence for the conclusion that after binding to recipient cells, exosomes will be transported into lysosomes for f urther degradation54. Figure 6 Colocalization of SKBR3 exosomes and MRC-5 lysosomes. (a) TIRF image of f i internalized s SKBR3 exosomes (red) andMRC-5membrane (blue).(b)PALM/STORM image of the same region as shown i n (a). (c) TIRF i mage of MRC-5lysosomes (green) and MRC-5 membrane (blue). (d) PALM/STORM image of the same region as shown in (c). (e) Colocalization of MRC-5 lysosomes (green) and internalized SKBR3 exosomes (red). The above experimental results confirmed that even after taken up by other cells,PALM/STORM imaging of the labeled exosomes can still be realized, and the distribution of i nternalized exosomes can be visualized directly by the presented PALM based method, which indicated potential applicat i ons of t he PALM/STORM technique in studying intracellular dynamics of exosomes and its contents s d (i .e.miRNA, DNA, protein etc.). 3. Materials and methods 3.1. Exosomes isolation from cells and electron microscopy Exosomes were extracted from supernatant of cells using ExoQuick-TC Exosomes Precipitation Solution (System Biosciences, Mountain View, CA, U.S.A.). First,human cervical cancer cells (HeLa) and human cancer cells (SKBR3) purchased from China Type Culture Collection were seeded i nto a cel l culture f lask (Corning, 25 cm²)and cultured until 70% confluence. Then 5 mL of the conditioned culture media was col l ected and centrifuged at 3000xg for 15 minutes to remove cel l s and cell debris.The supernatant was mixed with 1 mL of ExoQuick-TC Exosomes Precipitation Solution and then refrigerating overnight. The mixed solution was centrifuged at 10000xg for 20 minutes. Al l traces of fluid were removed by aspiration and the remaining exosome pellet was resuspended i n 100 uL PBS and stored at -75℃. The number of exosomes was counted using a TIRF microscope (Zeiss Elyra P.1) by staining the exosome membranes with CM-Dil. And the calculated concent r ation of exosomes in the original s tock solution is 5.46x10 /uL for HeLa and 4.88×107/uL f or SKBR3 exosomes. Specimens for TEM measurement were prepared by placing extracted exosomes onto carbon coated grids and absorbed for 10 minutes before drying. After t hat, excess liquid was sucked away from the edge of the copper mesh with filter paper and stained for contrast using 2% phosphotungstic acid for 10 minutes. Then images were taken by transmission electron microscopy. 3.2. Fluorescent l abelling of exosomes membrane receptors To immunof l uorescence label CD63 of HeLa-derived exosomes, 4 uL of HeLa exosome specimens was diluted i n 400 pL of PBS buffer and then mixed with 1 uL of primary antibody (1 mg/mL rabbit anti-CD63, bs-1523R, Beijing Biosynthesis Biotechnology Company) for 2 hours at room temperature. For the blank control, the primary ant i body was replaced by PBS buffer. After t hat, the reaction solution was puri f ied by ultrafiltration (50 Kda) at 6000 rpm for 20 minutes. The precipitation was resuspended in PBS. Next, 1 pL of secondary ant i body (2mg/mL Alexa Fluor 647labeled Goat anti-Rabbit IgG, ab150079, Abcam) was added together with 0.5 uL of CM-Di l (KGMP002, KeyGen BioTECH). The mixture was vigorously stirred for 1hour at room temperature and then purified again by ultrafiltration (50 Kda) at 6000rpm for 20 minutes to remove excess reagents. Final l y, the precipitat i on was resuspended in 400 uL of PBS for super-resolution i maging. To simultaneously label CD63 and HER2 of exosomes secreted from SKBR3 cells,the IF experiment followed a similar procedure as d described above. The only difference is that for the primary antibodies, 1 pL rabbit ant i -CD63 and 6 uL mouse anti-HER2 (MAB-0198,Maxim) were used, while for the secondary antibodies, 0.5uL of Alexa Fluor 647 labeled goat anti-rabbit IgG and 0.5 uL of Alexa Fluor 488labeled goat anti-mouse IgG were used. 3.3. Super-resolution imaging of exosome membrane receptors Exosomes at sui t able concentrations were fixed in chambered slides by electrostatic adsorption. First, the Lab-Tek Chambered Coverglass (NO.155409) was i mmersed i n a i n n aqueous s solution of 0.1% polyethylenimine (PEI) for 15 minutes to ensure adequate positive charge assembled. Next, the as-prepared sample was added and absorbed for 15 minutes. The imaging buffers used was phosphate buffer saline with 136 mM β-mercaptoethanol (BME) at pH 8.0, and an oxygen scavenger system (5%glucose,0.5mg/mL glucose oxidase and 40 ug/mL catalase)to reduce photobleaching’. The imaging experiments Wwere performed with a Zeiss Elyra P.1 super-resolution microscope equipped with a 100xoi l -i mmersion objective (Alexa Fluor 647 channel: excitation (EX) 642 nm (150mW), emission (EM) 655-690nm;Alexa Fluor 488 channel: EX 488 nm (100mW), EM 495-575nm; CM-Di l channel:EX 561 nm (100mW), EM 570-650nm). After sample preparation, the chamber slide containing samples and buffers was mounted onto the microscope and stabilized for 20 minutes. For CD63 imaging, the sample was continuously excited by a 642 nm laser. Meanwhile, a 405 nm activation l aser was also continuously applied to activate the Alexa Fluor 647 from dark state. For dual-color super resolution i maging of CD63and HER2, Alexa Fluor 488 was excited with 488 nm laser and Alexa Fluor 647 was excited with 642 nm laser, respectively. The exposure time of each frame was 50 ms.The super resolution images of each channel were reconstructed from 15000 frames and analyzed with t he Zeiss Elyra P.1 super-resolution imaging sof t ware. 3.4. Visualizing intracellular exosomes by super-resolut i on imaging Human embryonic l ung f ibroblast (MRC-5) purchased f rom China Type Culture Collection were cultured in MEM under standard cell culture environment (5% CO2,37oC) for 48 h. To stain the membrane of MRC-5 cells, we removed the culture media and washed the cells once using culture media without serum. After that, 100uL of Di l uent C (PKH67 Fluorescent Cell linker kits, MINI67, sigma-aldrich) was added to the cell pellet with gent l e pipetting to i nsure complete dispersion. Next, 0.5uL of PKH67 diluted in 100 pL of Diluent C was r apidly added, and immediately mixed with the sample by pipetting. Cells with dyes were i ncubated for 4 minutes with periodic mixing at room temperature and then the staining was stopped by adding 200 pL of serum for 2 minutes to prevent binding of excess dye. After washing away the serum, 400 uL of the culture media was added for continuous culture. To realize the co-culture of SKBR3 exosomes and MRC-5 cells, 10 uL of SKBR3exosomes IF labeled with Alexa Fluor 647 was added into the culture media of MRC-5 cel l s. Then excess exosomes were washed away by culture media 1 hour later.After that, these MRC-5 cells were fixed with 4% paraformaldehyde (KeyGen BioTECH) for 20 minutes and finally 200 uL of t he imaging buffer was added for PALM/STORM imaging. Moreover, for colocal i zation of exosomes and lysosomes,lysosomes were labeled with 0.2 pL of LysoTracker (LysoTracker@Red DND-99,L7528, Thermo Fisher Scientific) diluted in 200 uL culture media for 20 minutes before cel l fixing. 4. Conclusion Single molecule localization based super resolution imaging of cancer derived exosomes is realized using IF and PALM/STORM techniques. As compared with conventional 1wield field 11microscopy, PALM/STORMprovides better spatial resolution for observing exosomes at scales down to the nanometric level. Besides,simultaneous dual-color PALM imaging of the cancer derived exosomes i s also presented. By comparison with t raditional wide field microscope, it is obvious that with PALM/STORM imaging technique,,we e can more e precisely visualize the intracellular positions of the incorporated exosomes. As a result, PALM/STORM i s a new powerful method for intracellular tracking of exosomes and other nanosized structures with an excellent spatial resolution. Moreover, with these excellent features,we have abundant reasons to believe that super-resolution imaging will be quite applicable for subsequent research on exosomes and exosome related physiological procedures. Acknowledgements This work was supported by the National Key Basic Research Program of China (No.2015CB352002)),, the Natural1SScience Foundationn of Jiangsu u Province (No.BK20150623), the Natural Science Foundation of China (NSFC) (No. 61535003,61505027),the Excellent Youth Foundation of Jiangsu Province (BK20140023), the Innovation Fund of School of Electronic Scienc c e e a a nd Engineering Southeast University (No.2242015KD002), the Science Foundation for The Excellent Youth ACS Applied Materials & Interfaces Scholars of Southeast University and the Fundamental Research Funds for the Central Universities. References 1. Fevrier, B.;; Raposo, G., Exosomes:Endosomal-Derived 1Vesicles Shipping Extracellular Messages. Curr Opin. Cell Biol. 2004,16(4), 415-421. 2. LaCava, J.; Houseley, J.; Saveanu, C.; Petfalski, E.; Thompson, E.; Jacquier, A.;Tollervey, D., RNA Degradation by the Exosome is Promoted by a Nuclear Polyade-nylation Complex. Cell 2005, 121(5),713-724. 3. Lai, C. P. K.; Breakefield, X. O., Role of Exosomes/Microvesicles i n the Nervous System and Use in Emerging Therapies. Front. Physiol. 2012, 3. 4. Melo, S. A.; Luecke, L. B.; Kahlert, C.; Fernandez, A. F.; Gammon, S. T.; Kaye,J.; LeBleu, V. S.; Mittendorf, E. A.; Weitz, J.; Rahbari, N.; Reissfelder, C.; Pilarsky, C.;Fraga, M. F; Piwnica-Worms, D.; Kal l uri, R., Glypican-1 Identi f ies Cancer Exosomes and Detects Early Pancreatic Cancer. Nature 2015,523 (7559), 177-182. 5. Simons, M.; Raposo, G., Exosomes- Vesicular Carriers for Intercellular Communication. Curr Opin. Cell Biol. 2009,21(4),575-581. 65..Colombo, M.; Moita , C .; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.;Manel, N.; Moita, L. F.; Thery, C.; Raposo, G., Analysis of ESCRT Functions in Exosome Biogenesis, Composition and Secretion Highlights the Heterogeneity of Extracellular Vesicles. J. Cell Sci. 2013, 126 (24),5553-5565. 7.Lai, C. P; Kim, E. Y .; Badr, C. E.; Weissleder, R.; Mempel, T. R.; Tannous, B. A.; Breakefield, x. O., Visualization and Tracking of Tumour Extracellular Vesicle Delivery and RNA Translation Using Mult i plexed Reporters. Nat. Commun. 2015,6. 8. Preker, P.; Nielsen, J.; Kammler, S.; Lykke-Andersen, S.; Christensen, M. S.;Mapendano, C. K.; Schierup, M. H.; Jensen, T. H., RNA Exosome Depletion Reveals Transcription Upstream of Active Human Promoters. Science 2008, 322 (5909), 1851-1854. 9.Thery, C.; Zitvogel, L.; Amigorena, S., Exosomes: Composition, Biogenesis and Function. Nat. Rev Immunol.2002,2(8),569-579. 10. Lakkaraju, A.; Rodriguez-Boulan, E., Itinerant Exosomes: Emerging Roles in Cell and Tissue Polarity. Trends Cell Biol. 2008, 18(5),199-209. 11. Vlassov, A. V; Magdaleno, S.; Setterquist, R.; Conrad, R., Exosomes: Current Knowledge of Their Composition, Biological Functions, and Diagnostic and Therapeutic Potentials. Biochim. Biophys. Acta. 2012,1820(7),940-948. 12. Huang, B.; Babcock, H.; Zhuang, X. W., Breaking the Diffraction Barrier:Super-Resolution Imaging of Cells. Cell 2010, 143(7),1047-1058. 13. Fernandez-Suarez, M.; Ting, A. Y., Fluorescent Probes for Super-Resolution Imaging in Living Cells. Nat. Rev. Mol. Cell Biol. 2008,9(12), 929-943. 14. Rust, M. J.; Bates, M.; Zhuang, X. W., Sub-Diffraction-Limit I maging by Stochastic Optical Reconstruction Microscopy (STORM). Nat. Methods 2006,3(10),793-795. 15. Hell, S. W., Toward Fluorescence Nanoscopy. Nat. Biotechnol. 2003, 21 (11),1347-1355. ACS Applied Materials & Interfaces 16. Schermelleh, L.; Heintzmann, R.; Leonhardt, H., A Guide to Super-Resolution Fluorescence Microscopy. J. Cell Biol. 2010, 190 (2),165-175. 17. Bates, M.: Huang,B.; Dempsey,G.T;2Zhuang, X. W., Multicolor Super-Resolution Imaging with Photo-Switchable Fluorescent Probes. Science 2007,317 (5845), 1749-1753. 18. Betzig, E.; Patterson, G. H.; Sougrat , R.; Lindwasser, O. W.; Olenych, S.;Bonifacino, J. S.; Davidson, M. W.; Lippincott-Schwartz, J.; Hess, H. F., Imaging Intracel l ular Fluorescent Proteins a t Nanometer Resolution. Science 2006,313 (5793),1642-1645. 19. Dempsey, G. T; Vaughan, J. C.; Chen, K. H.; Bates, M.; Zhuang, X. W.,Evaluation of Fluorophore es s f for Optimal Performance in Localizat i on-based Super-Resolution Imaging. Nat. Methods 2011,8(12),1027-1036. 20. Huang, B.; Wang,V W. Q.; Bates, M.; Zhuang, X.. W., Three-Dimensional Super-Resolution Imaging by Stochastic Optical Reconstruction Microscopy. Science 2008,319 (5864), 810-813. 21. Heilemann, M.; van de Linde, S.; Schuttpelz, M.; Kasper, R.; Seefeldt, B.;Mukherjee, A.; T i nnefeld, P.; Sauer, M., Subdiffraction-Resolution Fluorescence Imaging with Conventional Fluorescent Probes. Angew. Chem. Int. Ed. 2008, 47 (33),6172-6176. 22. Folling, J.; Belov, V; Kunetsky, R.; Medda, R.; Schonle, A.; Egner, A.; Eggeling,C.; Bossi, M.; Hell, S. W. Photochromic Rhodamines Provide Nanoscopy with Optical Sectioning. Angew. Chem. Int. Ed. 2007,46(33), 6266-6270. 23. Patterson, G.; Davidson, M.; Manley, S.; Lippincott-Schwartz, J., Superresolution Imaging Using Single-Molecule Localization. In Annu. Rev. Phys. Chem., Vol 61,Leone, S. R.; Cremer, P. S.; Groves, J. T.; Johnson, M. A.; Richmond, G., Eds. 2010,pp 345-367. 24. Chien, F. C.; Kuo, C. w.; Chen, P. L., Localization Imaging Using Blinking Quantum Dots. Analyst. 2011, 136(8),1608-1613. 25. Hoyer, P.; Staudt, T .; Engelhardt, J; Hell, s. W., Quantum Dot Blueing and Blinking Enables Fluorescence Nanoscopy. Nano Lett. 2011, 11 (1),245-250. 26. Wang, Y.; Fruhwirth, G.; Cai, E.; Ng, T.; Selvin, P. R., 3D Super-Resolution Imaging with Bl i nking Quantum Dots. Nano Lett. 2013, 13 (11),5233-5241. 27. Lippincott-Schwartz, J.; Patterson, G. H., Photoactivatable Fluorescent Proteins for Diffraction-Limited and Super-Resolution Imaging. Trends Cell Biol. 2009, 19(11),555-565. 28. Sydor, A. M.; Czymmek, K. J.; Puchner, E. M.; Mennel l a, V., Super-Resolution Microscopy: From Single Molecules to Supramolecular Assemblies. Trends Cell Biol.2015,25(12),730-748. 29. Huang, B.; Bates, M.; Zhuang, X. W., Super-Resolution Fluorescence Microscopy.In Annu. Rev. Biochem., 2009, pp 993-1016. 30. Aldras, A. M.; Orenstein, J. M.; Kotler, D. P.; Shadduck, J. A.; Didier, E. S.,Detection of Microsporidia by Indirect Immunofluorescence Antibody-Test Using Polyclonal and Monoclonal-Ant i bodies. J. Clin. Microbiol. 1994, 32(3),608-612. 31. Lawrence, D. M. P.; Elhamouly, W.; Archer, S.; Leary, J. F.; Bidlack, J. M., ACS Applied Materials & Interfaces Identif i cation of Kappa-Opioid Receptors s in tthe Immune-System by Indirect Immunofluorescence. Proc. Natl. Acad. Sci. U.S.A. 1995, 92 (4),1062-1066. 32. Giepmans, B. N. G.; Adams, S. R.; Ellisman, M. H.; Tsien, R. Y., Review - The Fluorescent Toolbox for Assessing Protein Location and Function. Science 2006,312(5771),217-224. 33. Zhuang, X. W., Nano-Imaging with STORM. Nature Photon. 2009, 3(7),365-367. 34. Lasser, C.;Eldh,M.;Lotval l ,J Isolation and 1Characterization of RNA-Containing Exosomes. J. Vis. Exp. 2012, (59). 35. Cheruvanky, A.; Zhou, H.; Pisitkun, T .; Kopp, J. B.; Knepper, M. A.; Yuen, P. S.T.;Star, R. A., Rapid 1Isolation of Urinary Exosomal Biomarkers Using 2a Nanomembrane Ultrafi l tration Concentrator. Am. J. Physiol. Renal. Physiol. 2007,292 (5), F1657-F1661. 36. Dragovic, R. A.; Collett, G. P.; Hole, P.; Ferguson, D. J. P.; Redman, C. w.;Sargent, I . L.; Tannetta, D. S., Isolation of Syncytiotrophoblast Microvesicles and Exosomes and The e i i r r Characterisation by y Multicolouirr Flow Cytometry and Fluorescence Nanoparticle Tracking Analysis. Methods 2015, 87,64-74. 37. Rolfo, C. D.; Giallombardo, M.; Castiglia, M.; Chacartegui, J. J .; Provencio, M.;Alessandro, R.; Carreca, A. P.; Roca, P.; Oliver, J.; Bover, I.; Van Meerbeeck, J. P.;Russo, A.; Peeters, M.; Pauwels, P., Exosomes Isolation and Characterization i n Nonsmall Cell Lung Carcinoma Patients: Proof of Concept Study. J. Clin. Oncol.2015,33(15). ACS Applied Materials & Interfaces 38. Bennit, H. R. F.; Valenzuela, M. M. A.; Jutzy, J. S.; Wall, N. R., B-Cell Lymphoma-Derived Exosomes are Reservoirs of Inhibitors of Apoptosis. Cancer Res.2014,74(19). 39. Cheng, Y; Schorey, J. S., Exosomes Carrying Mycobacterial Antigens Can Protect Mice against Mycobacterium Tuberculosis Infection. Eur J. Immunol. 2013,43 (12), 3279-3290. 40. Kadiu, I; Narayanasamy, P; Dash, P. K.; Zhang,. W.; Gendelman, H. E.,Biochemical and Biologic Characterization of Exosomes a a nd Microvesicles S:as Faci l itators of HIV-1 Infection i n Macrophages. J. Immunol. 2012,189(2),744-754. 41. Farr, A. D., Saunders Dictionary and Encyclopedia of Laboratory Medicine and Technology-Bennington,JL. Med. Lab. Sci. 1984,41(3), 318-318. 42. Vonbartheld, c. S.; Cunningham, D. E.; Rubel , E. W., Neuronal Tracing with Dii-Decalcification, Cryosectioning, and Photoconversion for Light and Electron-Microscopic Analysis. J. Histochem. Cytochem. 1990, 38(5),725-733. 43. Shroff, H.; Galbraith, C. G.; Galbraith, J . A.; White, H.; Gillette, J .; Olenych, S.;Davidson, M. W; Betzig, E., Dual-Color Superresolution I maging of Genetically Expressed Probes within Individual Adhesion Complexes. Proc. Natl. Acad. Sci.U.S.A. 2007,104 (51),20308-20313. 44. Perou, C. M.; Sorlie, T.; Eisen, M. B.; van de Rijn, M.; Jeffrey, S. S.; Rees, C. A.;Pollack, J. R.; Ross, D. T.; Johnsen, H.; Akslen, L. A.; Fluge, O.; Pergamenschikov, A.;Will i ams, C.; Zhu, S. X.; Lonning, P. E.; Borresen-Dale, A. L.; Brown, P. O.; Botstein,D., Molecular Portraits of Human Breast Tumours. Nature 2000,406 (6797),747-752. ACS Applied Materials & Interfaces 45. Sorlie, T.; Perou, C. M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie,T .; Eisen, M. B.; van de Rijn, M.; Jeffrey, S. S.; Thorsen, T.; Quist, H.; Matese, J. C .;Brown, P. O.; Botstein, D.; Lonning, P. E.; Borresen-Dale, A. L., Gene Expression Patterns of f 1Breastt Carcinomas DistinguishTumor Subclasses with Clinical Implications. Proc. Natl. Acad. Sci. U. S. 4. 2001,98(19), 10869-10874. 46. Aung, P. P.; Walter, B. A.; Garcia, C.; Bratslavsky, G.; Merino, M. J. In Potential Role of HER2/Neu as a Molecular Target in the Treatment of High Grade Urothelial Cancer. Determination of Gene Ampli f ication by FISH, CISH and IHC, Lab. Invest.,Feb; 2011; pp 179A-179A. 47. Ciravolo, V.; Huber, V.; Ghedini, G. C.; Venturelli, E.; Bianchi, F.; Campiglio, M.;Morelli, D.; Villa, A.; Della Mina, P.; Menard, S.; Filipazzi, P.; Rivolt i ni, L.; Tagliabue, E.; Pupa, S. M., Potential Role of HER2-Overexpressing Exosomes in Countering Trastuzumab-Based Therapy. J. Cell. Physiol. 2012, 227(2),658-667. 48. Robbins, P. D.; Morel l i , A. E., Regulation of Immune Responses by Extracellular Vesicles. Nat. Rev. Immunol. 2014,14(3), 195-208. 49. Schorey, J. S.; Bhatnagar, S., Exosome Function: From Tumor Immunology to Pathogen Biology. Traffic 2008, 9 (6),871-881. 50. van Niel, G.; Porto-Carreiro, I.; Simoes, S.; Raposo, G., Exosomes: A Common Pathway for a Specialized Function.J. Biochem. 2006,140(1),13-21. 51. Hoshino, A.; Costa-Silva, B.; Shen, T . L.; Rodrigues, G.; Hashimoto, A.; Mark,M. T .; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; Singh, S.; Williams, C.; Soplop, N.; Uryu, K.; Pharmer, L.; King, T.; Bojmar, L.; Davies, A. E.; Ararso, Y; Zhang, T.; Zhang, H.; Hernandez, J.; Weiss, J . M.; Dumont-Cole, V. D.; Kramer, K.; Wexler, L. H.; Narendran, A.; Schwartz, G. K.; Healey, J. H.; Sandstrom, P .; Labori, K. J.; Kure, E. H.; Grandgenett, P. M.; Hollingsworth, M. A.; de Sousa, M.; Kaur, S.; Jain, M.; Mallya, K.; Batra, S. K.; Jarnagin, W. R.; Brady, M. S.; Fodstad, O.; Muller, V; Pantel, K.; Minn, A. J.; Bissel l , M. J; Garcia, B. A.; Kang, Y.; Rajasekhar, V. K.;Ghajar, C. M.; Matei, I .; Peinado, H.; Bromberg, J.; Lyden, D., Tumour Exosome Integrins Determine Organotropic Metastasis. Nature 2015, 527 (7578), 329-335. 52. Zhang, L.; Zhan, S. Y.; Yao, J.; Lowery, F. J.; Zhang, Q. L.; Huang, W. C.; Li , P;Li, M.; Wang, X.; Zhang, C. Y.; Wang, H.; Ellis, K.; Cheerathodi, M.; McCarty, J. H.; Palmieri, D.; Saunus, J .; Lakhani, S.; Huang, S. Y.; Sahin, A. A.; Aldape, K. D.; Steeg,P. S.; Yu, D. H., Microenvironment-Induced PTEN Loss by Exosomal MicroRNA Primes Brain Metastasis Outgrowth. Nature 2015,527(7576),100-104. 53. Axelrod, D., Total Internal Reflection Fluorescence Microscopy in Cell Biology.Traffic 2001, 2 (11),764-774. 54. Raposo,G.; Stoorvogel, W., Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 2013,200 (4),373-383. 55. Tian, T .; Zhu, Y. L.; Hu, F. H.; Wang, Y. Y.; Huang, N. P .; Xiao, Z. D., Dynamics of Exosome Internalization n and d Trafficking. J. Cell 1.. F Physiol. 22013,2288(7),1487-1495. 56. Tian, T.; Zhu, Y. L.; Zhou, Y. Y; Liang, G. F.; Wang, Y. Y.; Hu, F. H.; Xiao, Z. D.,Exosome Uptake through Clathrin-Mediated Endocytosis and Macropinocytosis and Mediating miR-21 Delivery. J. Biol. Chem. 2014, 289(32),22258-22267. ACS Applied Materials & Interfaces 57. Tian, T. A.; Wang, Y. Y.; Wang, H. T.; Zhu, Z. Q.; Xiao, Z. D., Visual i zing of t he Cellular Uptake and Intracellular Trafficking of Exosomes by L i ve-Cell Microscopy.J.Cell. Biochem. 2010,111(2),488-496. 58. Shim, S. H.; Xia, C. L.; Zhong, G. S.; Babcock, H. P; Vaughan, J. C.; Huang, B.;Wang, X.; Xu, C.; Bi, G. Q.; Zhuang, X. W., Super-Resolution Fluorescence Imaging of Organelles in Live Cells with Photoswitchable Membrane Probes. Proc. Natl. Acad.Sci. U. S. 4. 2012,109(35),13978-13983. For TOC only 1pm 5pm 190x191mm (300×300 DPI) 168x82mm (300×300 DPI) TIRF and PALM/STORM i maging of HeLa exosomes. (a) Schematic illustration of indirect IF l abeling of CD63and exosome membrane stained with CM-Dil . (b) TIRF image of HeLa-derived exosomes stained with CM-Dil. (c) IF labeling of CD63 with Alexa Fluor 647, conventional diffraction-limited image (upper right) and PALM/STORM image (low left). (d) Immunofluorescence control sample without primary antibody for specificity detection, CM-Dil channel. (e) Immunofluorescence control sample without primary antibody for specificity detection, Alexa Fluor 647 channel. (f) Wide field TIRF image of CD63 on a single exosome, (g)PALM image of the same region i n (f). (h) Cross-sectional profiles of the exosome shown in f and g. Figure 2 162x108mm (300×300 DPI) Tmaaina of two adiacent exosomes (a) TIRF image; (b) PALM/STORM image . (c ) Cross-sectional profiles of the two adjacent exosomes shown i n a and b. Figure 3 191x221mm (300×300 DPI) Dual-color super-resolution imaging of CD63 and HER2 in SKBR3-derived exosomes. Fluorescence labeling of CD63 with Alexa Fluor 647, conventional diffraction-limited i mage (a) and PALM/STORM image (d).Fluorescence labeling of HER2 with Alexa Fluor 488, conventional diffraction-limited i mage (b) and PALM/STORM image (e). (c) SKBR3-derived exosome membrane stained with CM-Dil. (f) Merged image of the two super-resolution channels. (g-i) Blank control sample, fluorescence image of Alexa Fluor 647 channel (g), Alexa Fluor 488 channel (h) and CM-Dil channel (i). Figure 4 121x120mm (300×300 DPI) 386x193mm (150×150 DPI) 309x150mm (300×300 DPI)
确定































还剩38页未读,是否继续阅读?
宁波力显智能科技有限公司为您提供《【应用实例】基于单分子定位的超分辨率显微镜对癌源性外泌体的成像和细胞内追踪(申请试用)》,该方案主要用于其他中超分辨率显微镜检测,参考标准--,《【应用实例】基于单分子定位的超分辨率显微镜对癌源性外泌体的成像和细胞内追踪(申请试用)》用到的仪器有力显智能科技INVIEW激光共聚焦超分辨STORM原理显微镜iSTORM、赛乐微培养箱中实时监测细胞生长的智能监控助手、超高分辨显微镜iSTORM
推荐专场
相关方案
更多