方案详情
文
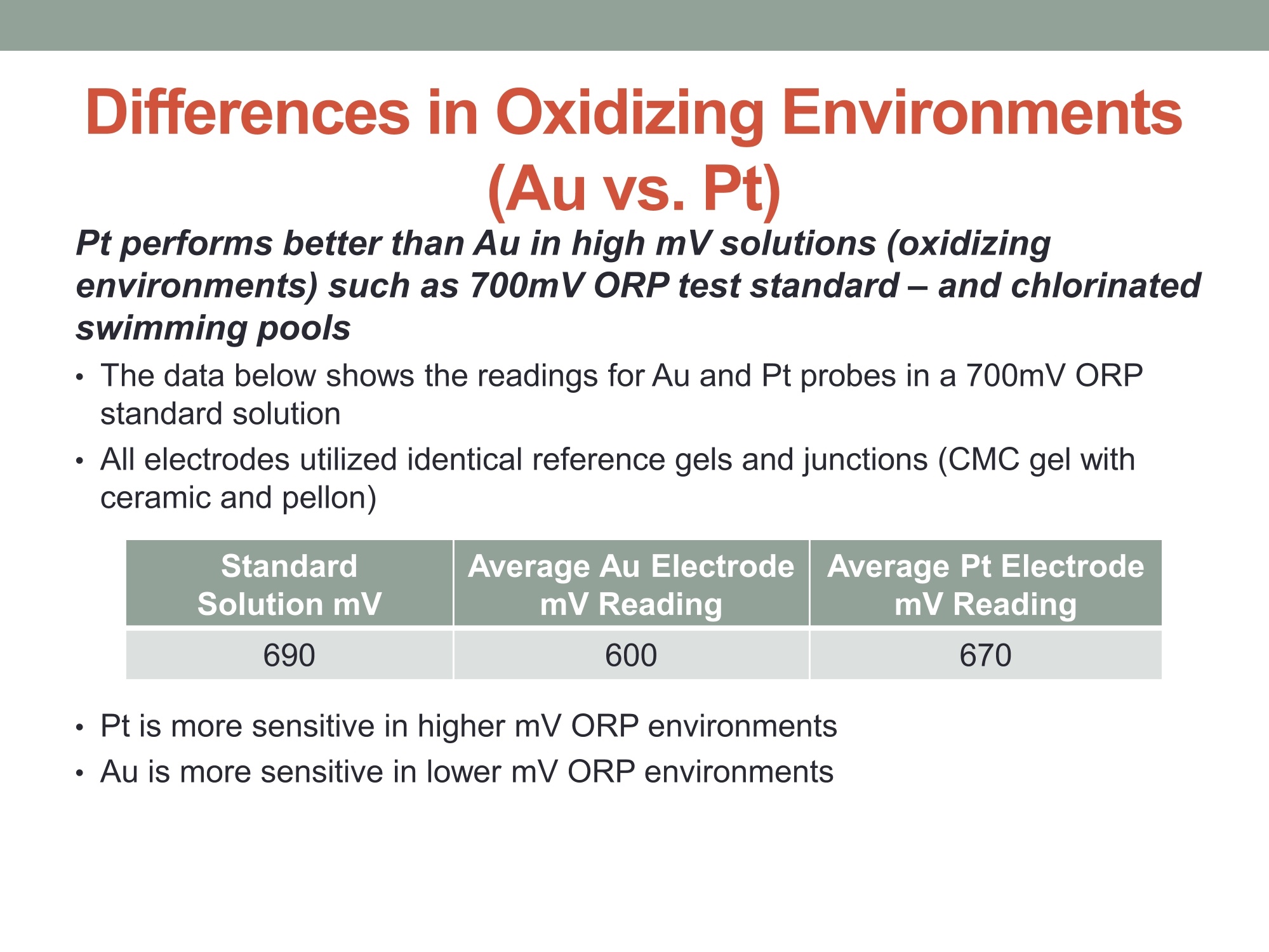
在高毫伏值的溶液(氧化环境)中铂金表现比黄金好,例如700mv的ORP标准测试溶液。含有氯气的游泳池水。
在高mv值的ORP环境中,铂金电极更加敏感。
在低mv值的ORP环境中,黄金电极更加敏感。
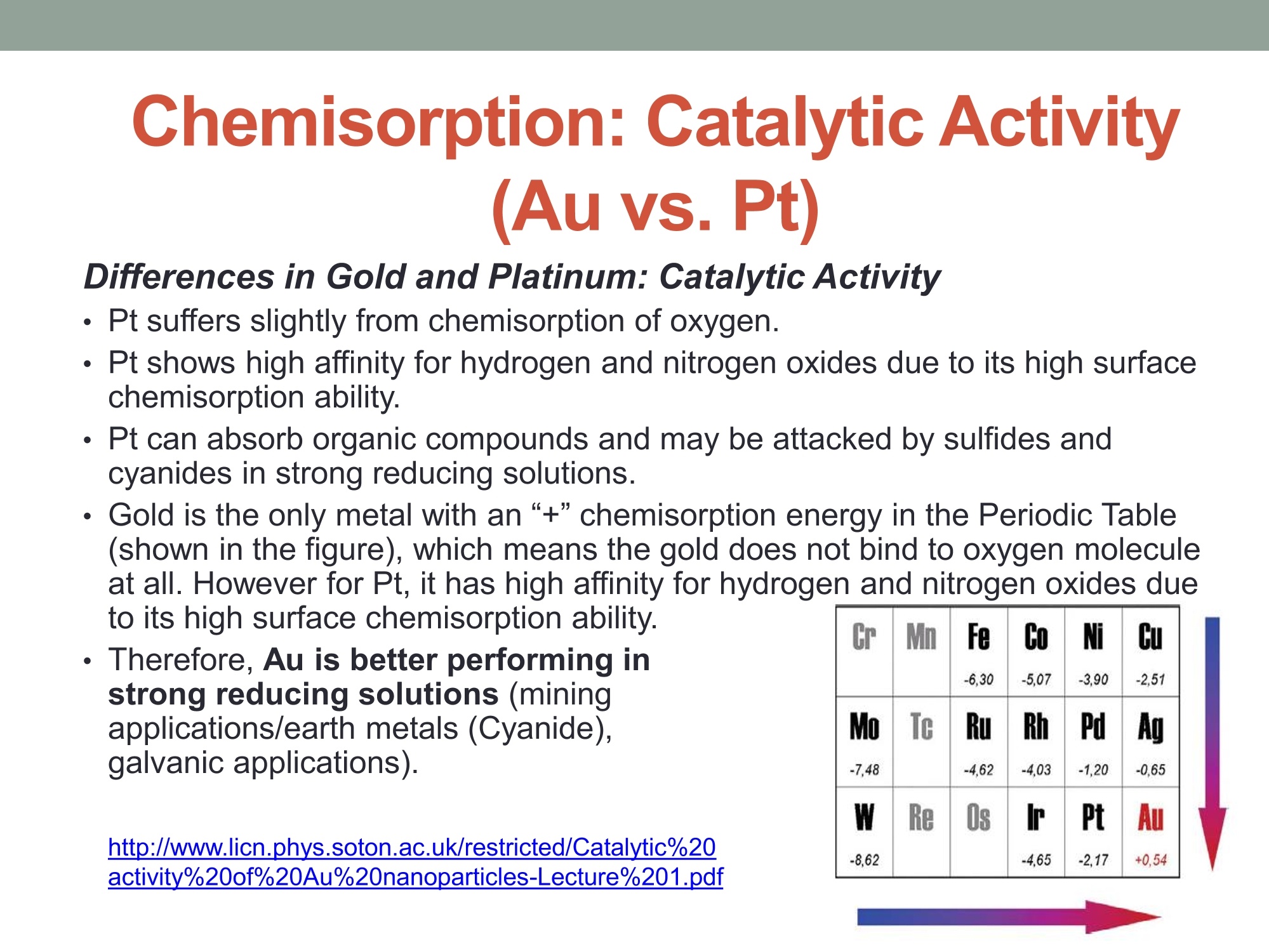
在强还原的溶液中,黄金表现的更好。
方案详情

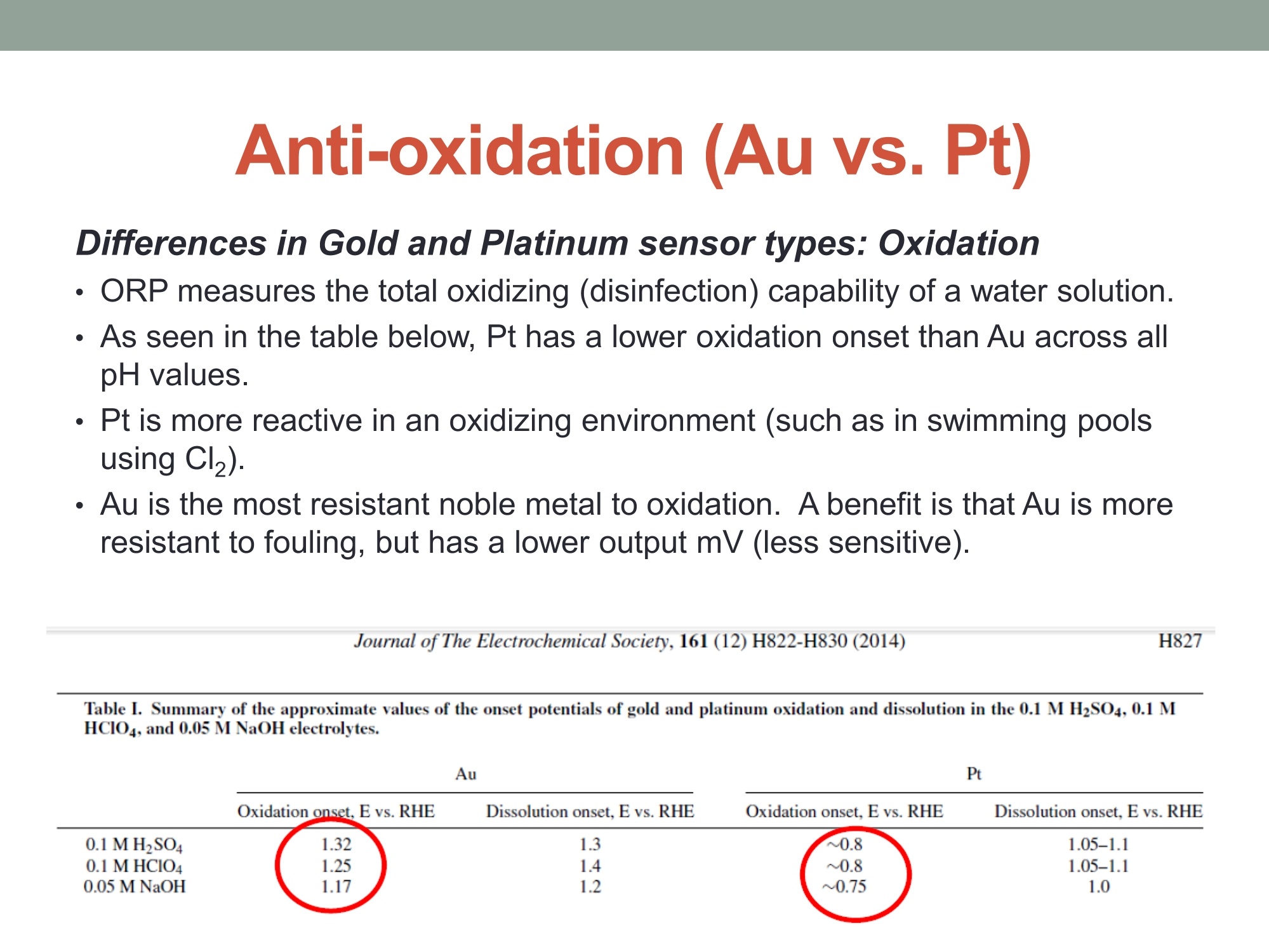
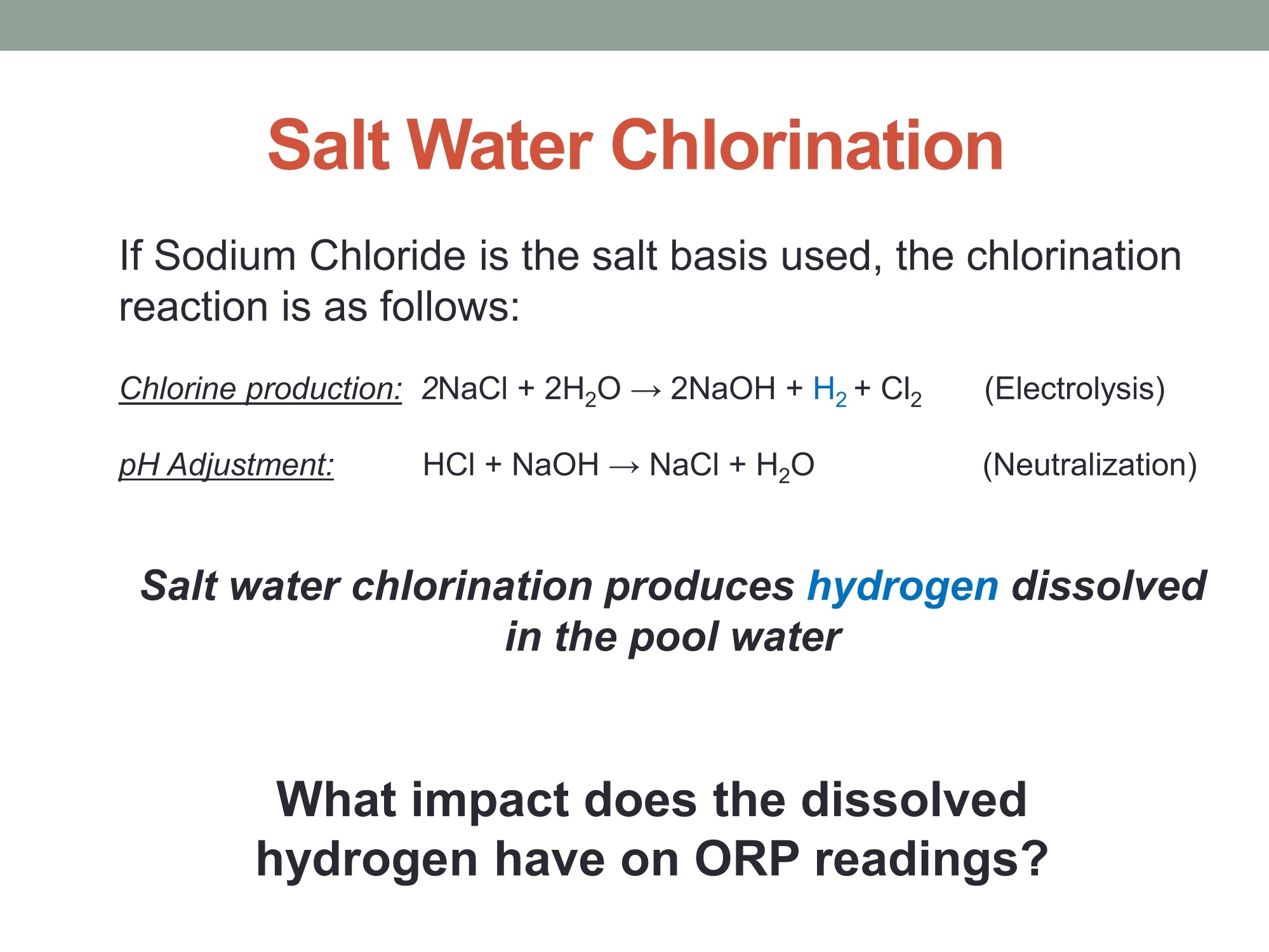
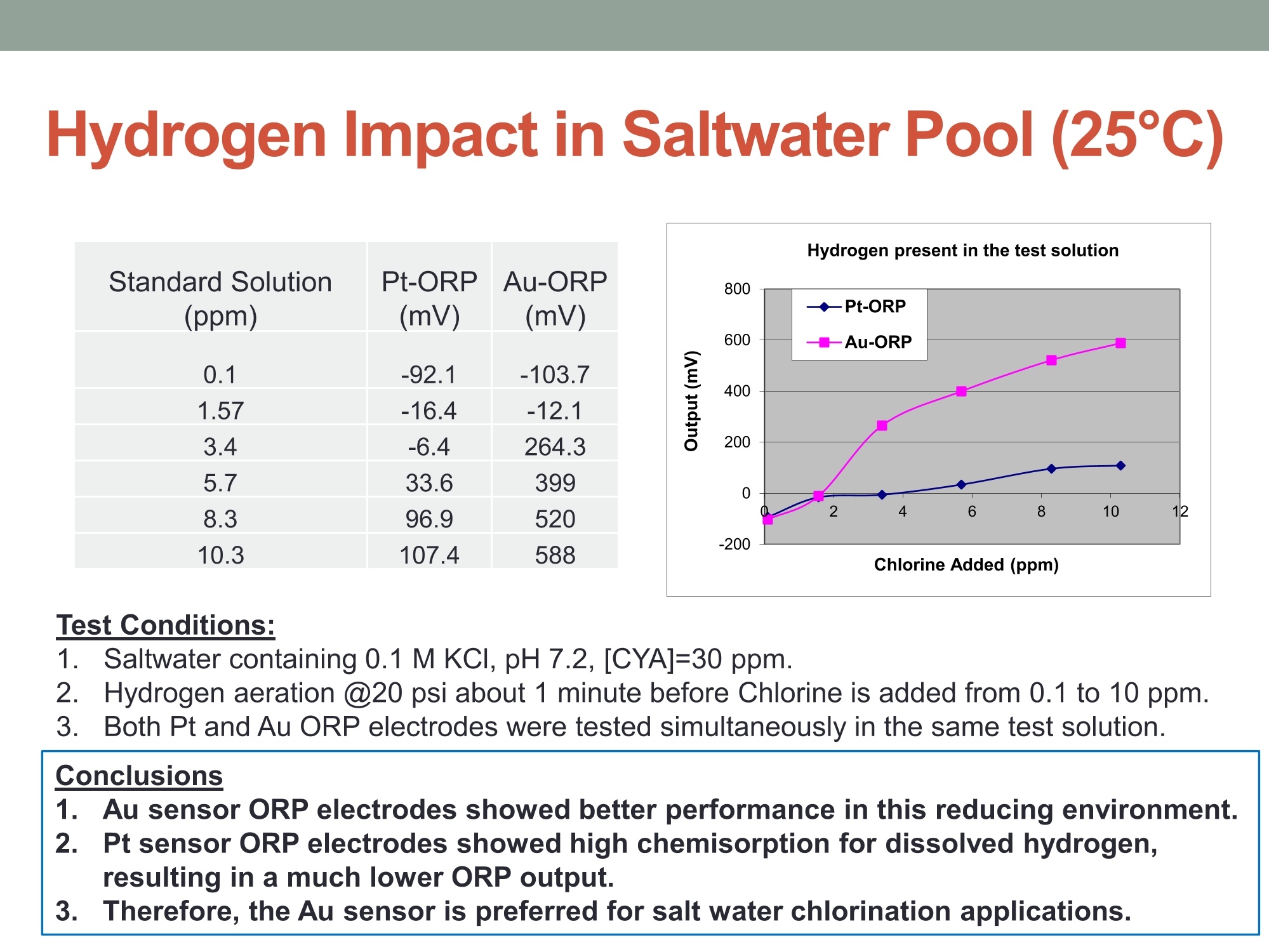
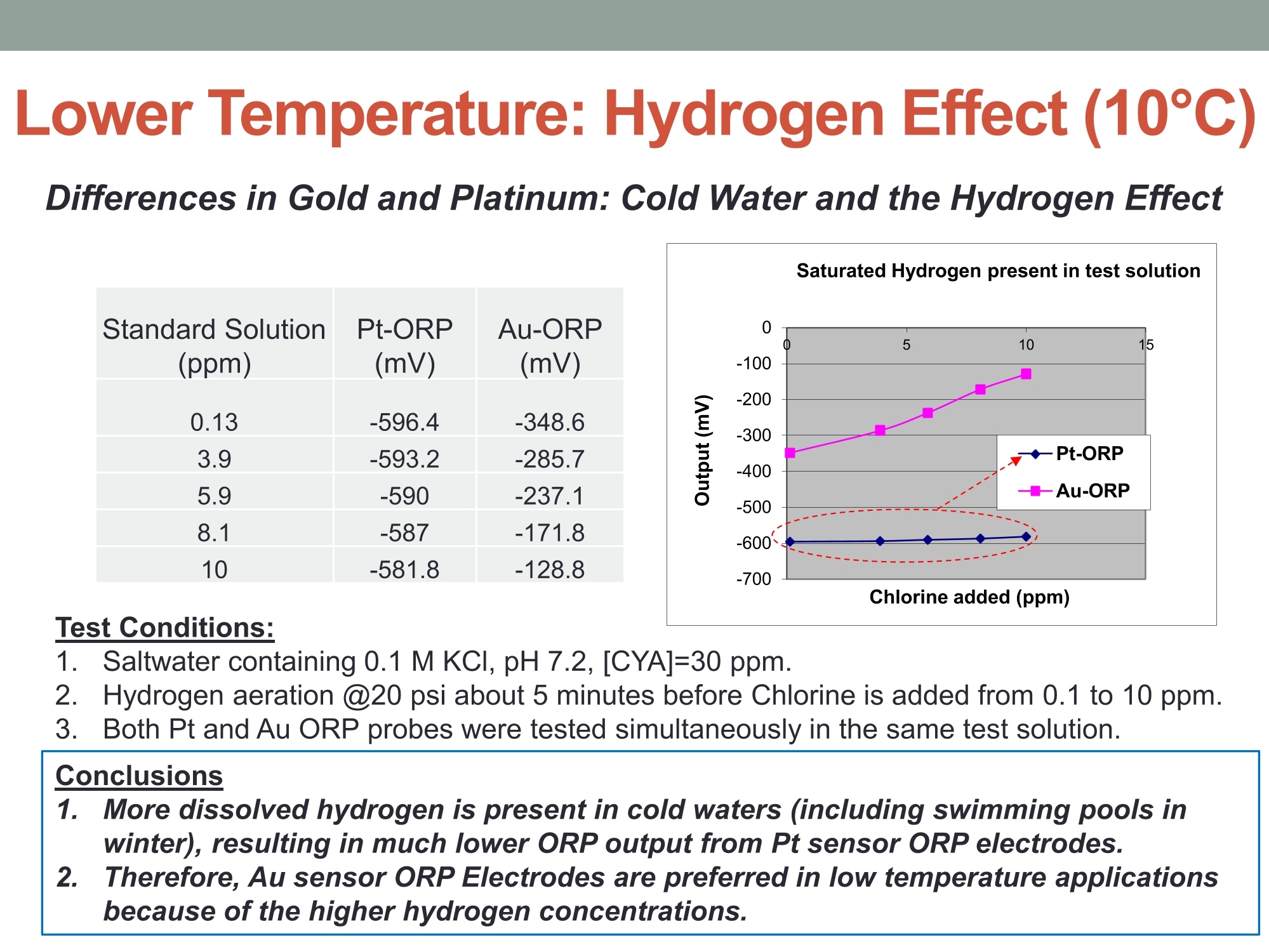
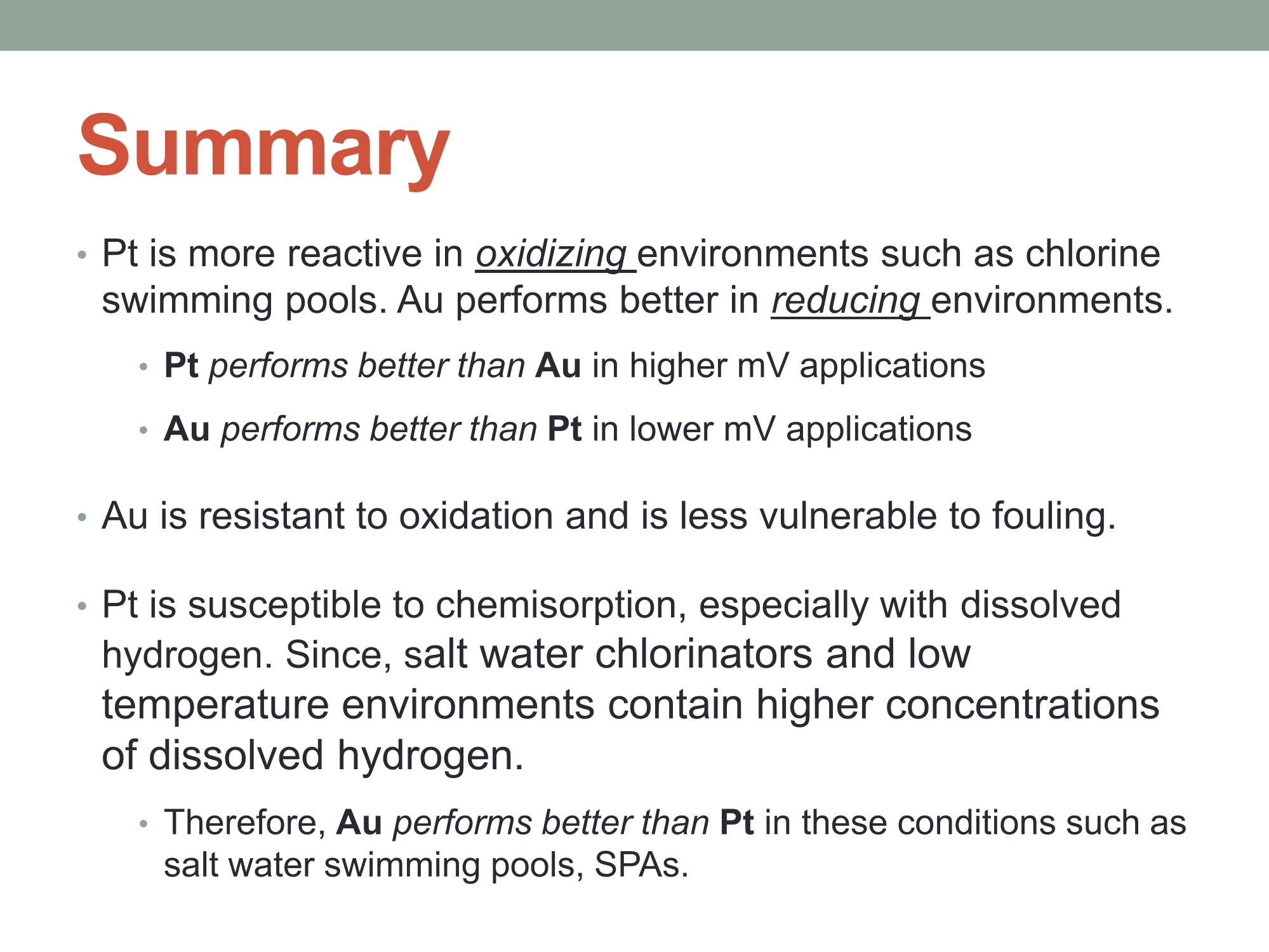
MEASURING YOUR SUCCESSSM Properties of Gold (Au) and Platinum (Pt) ORP Probes ·Oxidation Properties Catalytic activity (chemisorption capability) ·Implications in Various Swimming Pool Applications .Summary Anti-oxidation (Au vs. Pt) Differences in Gold and Platinum sensor types: Oxidation ORP measures the total oxidizing (disinfection) capability of a water solution. ·As seen in the table below, Pt has a lower oxidation onset than Au across allpH values. Pt is more reactive in an oxidizing environment (such as in swimming poolsusing CI,). Au is the most resistant noble metal to oxidation. A benefit is that Au is moreresistant to fouling, but has a lower output mV (less sensitive). Journal of The Electrochemical Society, 161 (12)H822-H830 (2014) H827 Table I. Summary of the approximate values of the onset potentials of gold and platinum oxidation and dissolution in the 0.1 M H2SO4,0.1 MHCIO4,and 0.05 M NaOH electrolytes. Au Pt Oxidation onset, E vs. RHE Dissolution onset, E vs. RHE Oxidation onset,E vs. RHE Dissolution onset. E vs. RHE 0.1 MHSO4 1.32 ~0.8 1.05-1.1 0.1 M HCIO4 1.25 ~0.8 1.05-1.1 0.05 M NaOH 1.17 ~0.75 1.0 Differences in Oxidizing Environments(Au vs. Pt) Pt performs better than Au in high mV solutions (oxidizing environments) such as 700mV ORP test standard - and chlorinatedswimmingpools The data below shows the readings for Au and Pt probes in a 700mV ORPstandard solution All electrodes utilized identical reference gels and junctions (CMC gel withceramic and pellon) StandardSolution mV Average Au Electrode mV Reading Average Pt ElectrodemV Reading 690 600 670 Pt is more sensitive in higher mV ORP environments ·Au is more sensitive in lower mV ORP environments Chemisorption: Catalytic ActivityAu vs. Pt) Differences in Gold and Platinum: Catalytic Activity ·Pt suffers slightly from chemisorption of oxygen. Pt shows high affinity for hydrogen and nitrogen oxides due to its high surfacechemisorption ability. Pt can absorb organic compounds and may be attacked by sulfides andcyanides in strong reducing solutions. Gold is the only metal with an“+”chemisorption energy in the Periodic Table(shown in the figure), which means the gold does not bind to oxygen moleculeat all. However for Pt, it has high affinity for hydrogen and nitrogen oxides dueto its high surface chemisorption ability. Therefore, Au is better performing instrong reducing solutions (miningapplications/earth metals (Cyanide),galvanic applications). http://www.licn.phys.soton.ac.uk/restricted/Catalytic%20activity%20of%20Au%20nanoparticles-Lecture%201.pdf Salt Water Chlorination If Sodium Chloride is the salt basis used, the chlorinationreaction is as follows: Chlorine production:;2NaCl+2H20→2NaOH+H2+Cl2 (Electrolysis)pHAdiustment: HCI+NaOH→NaCl+H,O (Neutralization) Salt water chlorination produces hydrogen dissolvedin the pool water What impact does the dissolvedhydrogen have on ORP readings? Hydrogen Impact in Saltwater Pool (25℃) Standard Solution Pt-ORP Au-ORP (ppm) (mV) (mV) 0.1 -103.7 -92.1 1.57 -16.4 -12.1 3.4 -6.4 264.3 5.7 33.6 399 8.3 96.9 520 10.3 107.4 588 Test Conditions: 1.Saltwater containing 0.1 M KCI, pH 7.2, [CYA]=30 ppm. 2.IHydrogen aeration @20 psi about 1 minute before Chlorine is added from 0.1 to 10 ppm.3.1Both Pt and Au ORP electrodes were tested simultaneously in the same test solution. Conclusions 1. Au sensor ORP electrodes showed better performance in this reducing environment.2. Pt sensor ORP electrodes showed high chemisorption for dissolved hydrogen,resulting in a much lower ORP output. 3.Therefore, the Au sensor is preferred for salt water chlorination applications. Lower Temperature: Hydrogen Effect (10℃ Differences in Gold and Platinum: Cold Water and the HydrogenEffect Saturated Hydrogen present in test solution Standard Solution Pt-ORP Au-ORP (ppm) (mV) (mV) O.13 -596.4 3.9 -593.2 -285.7 5.9 -590 -237.1 8.1 -587 -171.8 10 -581.8 -128.8 Test Conditions: 1. Saltwater containing 0.1 M KCI, pH 7.2, [CYA]=30 ppm. 2.Hydrogen aeration @20 psi about 5 minutes before Chlorine is added from 0.1 to 10 ppm.3.EBoth Pt and Au ORP probes were tested simultaneously in the same test solution. Conclusions 1. More dissolved hydrogen is present in cold waters (including swimming pools inwinter), resulting in much lower ORP output from Pt sensor ORP electrodes.2.Therefore, Au sensor ORP Electrodes are preferred in low temperature applicationsbecause of the higher hydrogen concentrations. Pt is more reactive in oxidizing environments such as chlorineswimming pools. Au performs better in reducing_environments. Pt performs better than Au in higher mV applications Au performs better than Pt in lower mV applications Au is resistant to oxidation and is less vulnerable to fouling. Pt is susceptible to chemisorption, especially with dissolvedhydrogen. Since, salt water chlorinators and lowtemperature environments contain higher concentrationsof dissolved hydrogen. Therefore, Au performs better than Pt in these conditions such assalt water swimming pools, SPAs. 铂金和黄金ORP电极的比较一、抗氧化黄金和铂金传感器的差异:氧化性。ORP测量水溶液总的氧化(消毒)能力。在所有的pH值中,铂金比黄金氧化性能要低。铂金在氧化的环境中反应更加剧烈。(例如在通入氯气的游泳池中。)二、在氧化环境中的差异在高毫伏值的溶液(氧化环境)中铂金表现比黄金好,例如700mv的ORP标准测试溶液。含有氯气的游泳池水。在高mv值的ORP环境中,铂金电极更加敏感。在低mv值的ORP环境中,黄金电极更加敏感。三、化学吸收作用:催化活性由于表面高的吸附能力,铂金显示对氢和氮氧化物的高的亲和能力且铂金能吸收有机化合物,可能在强还原溶液中被硫化物和氰化物的干扰。在元素周期表中金是唯一一个“+”化学吸收能量的金属。这意味着金不能和氧分子结合。然而对于铂金,由于它的表面高的化学吸收能力,它对于氢和氮氧化物有高的亲和力。因此,在强还原的溶液中,黄金表现的更好。四、盐水氯化如果氯化钠是主要的盐。则氯化反应如下:Chlorine production: 2NaCl + 2H2O → 2NaOH + H2 + Cl2pH调整: HCl + NaOH → NaCl + H2O (中和反应)盐水的氯化产生氢气溶解在游泳池水中。溶解氢气对ORP读数有什么影响?在盐水池中氢的影响(25摄氏度)测试情况1.盐水中包含0.1M KCl, pH 7.2, [CYA]=30 ppm。2.以20psi压力通入氢气大约1分钟,然后使氯含量从0.1ppm增加到10ppm。3.铂金电极和黄金电极同时在同样的溶液中测试。结论:1.黄金的ORP电极在还原环境中显示更好的性能。2.铂金的ORP电极对于溶解氢有高的化学吸收性,导致非常低的ORP输出。3.因此,黄金电极传感器首选用于盐水氯气消毒应用场合。五、低温:氢影响(10摄氏度)铂金和黄金差别:冷水和氢的影响。测试条件:1.盐水包含0.1 M KCl, pH 7.2, [CYA]=30 ppm。2.以20psi压力通入氢气大约5分钟,然后使氯含量从0.1ppm增加到10ppm。3.铂金和黄金ORP电极同时在同样的溶液中测试。结论:1.冷水中有更多的溶解氢,(包括冬天的游泳池)导致铂金ORP电极输出非常低。2.因此,由于氢的高浓度,首选黄金ORP电极应用于低温场合。总结•铂金在诸如含氯的游泳池的氧化环境中反应剧烈些,在还原环境中黄金的表现要好。•在高mv的应用场合铂金比黄金合适。•在低mv的应用场合黄金比铂金合适。•黄金具有抗氧化性,不容易污染。•铂金容易受到化学吸收作用,尤其受到溶解氢的作用,因为,盐水的氯化,低温环境包含高浓度的溶解氢。•因此,在这些使用场合(比如盐水游泳池、温泉),黄金比铂金表现的更好。
确定


还剩7页未读,是否继续阅读?
傲乐科学仪器(上海)有限公司为您提供《游泳池水中氧化还原电位检测方案 》,该方案主要用于环境水(除海水)中物理指标检测,参考标准--,《游泳池水中氧化还原电位检测方案 》用到的仪器有傲乐 R035型ORP复合电极(铂金针) 、傲乐 R035型ORP复合电极(铂金片)、傲乐 R035型ORP复合电极(铂金圈) 、傲乐 R035型ORP复合电极(黄金片)













