方案详情
文
Key Words
SMART Digest kit, SOLAμ, Vanquish, peptides, protein, bottom up
proteomics, proteomics, trypsin digestion, buffer, mAb, biomolecules,
biotherapeutics, biopharmaceutical, biocompatible UHPLC, Acclaim C18
RSLC, peptide mapping, in-solution digestion, UHPLC, accurate mass, QE,
Orbitrap, peptide quantitation, SPE, solid-phase extraction, Q Exactive Plus
方案详情

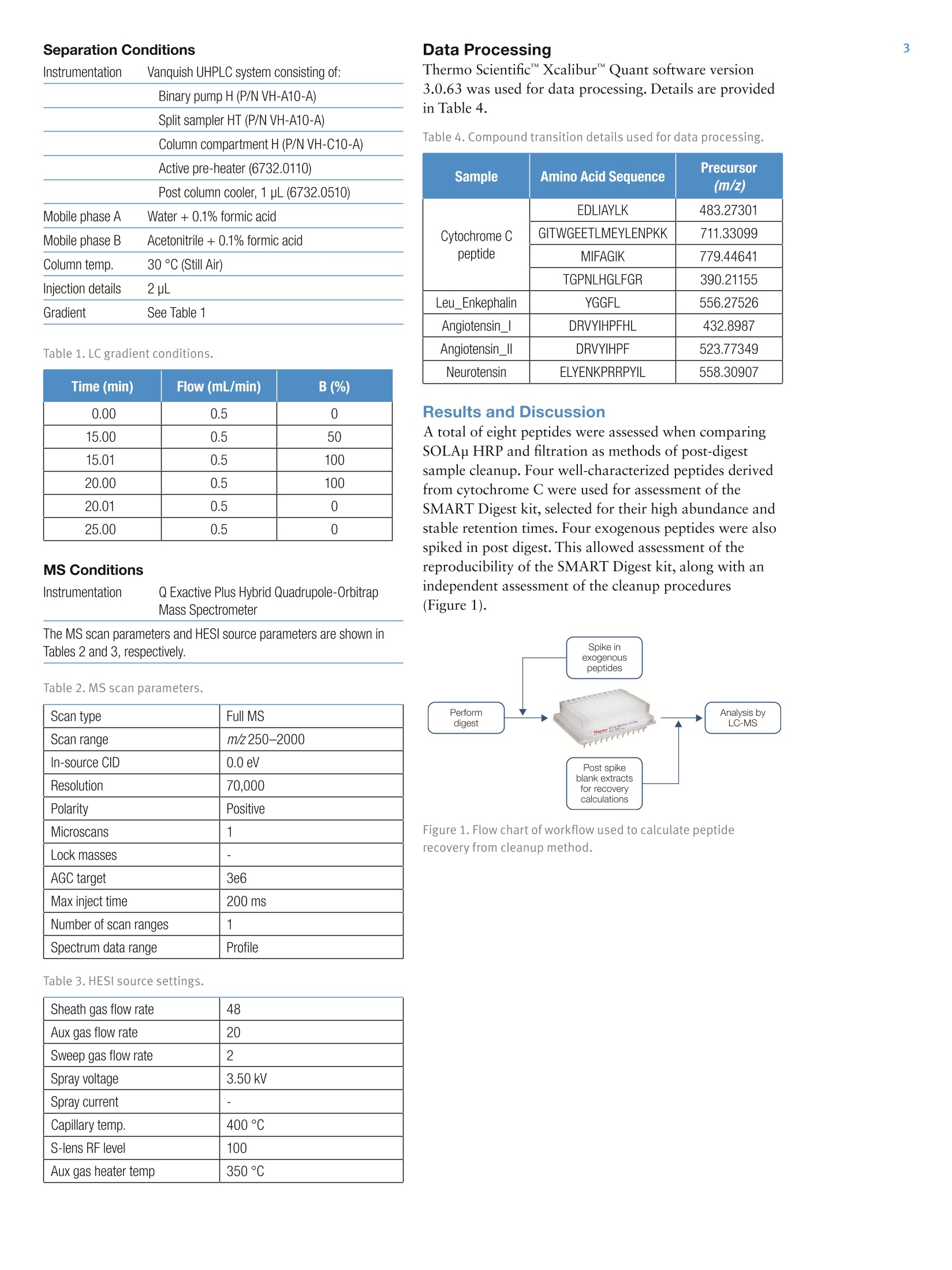
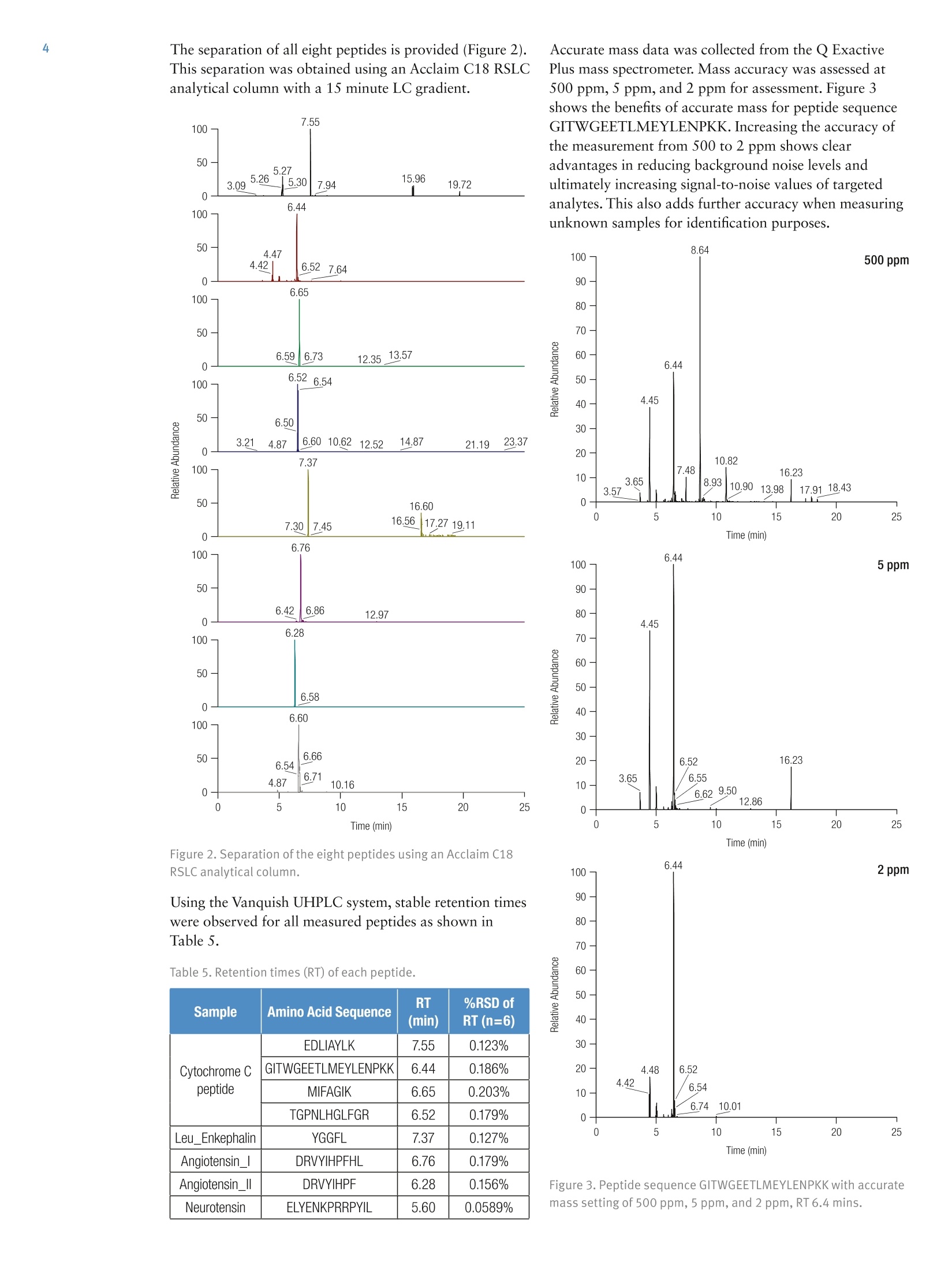
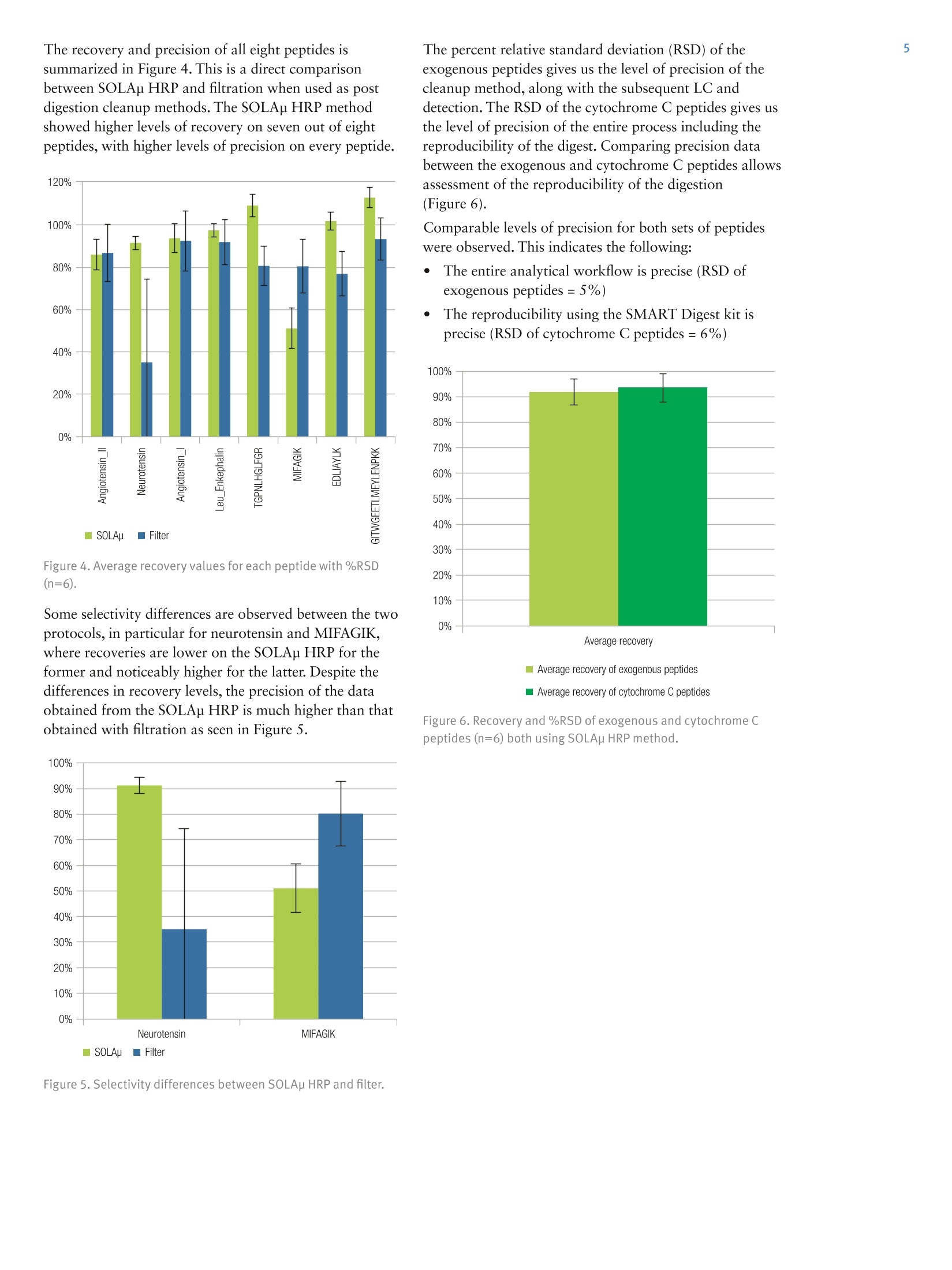
2 5A Thermo Fisher Scientific Brand Improvement in Speed and Reproducibilityof Protein Digestion and PeptideQuantitation, Utilizing Novel SamplePreparation Technology in a Full SolutionWorkflow Jon Bardsley, Joanne Jones, Phillip Humphryes, Thermo Fisher Scientific, Runcorn, UK Key Words SMART Digest kit, SOLAp, Vanquish, peptides, protein, bottom upproteomics, proteomics, trypsin digestion, buffer, mAb, biomolecules,biotherapeutics, biopharmaceutical, biocompatible UHPLC, Acclaim C18RSLC, peptide mapping, in-solution digestion, UHPLC, accurate mass, QE,Orbitrap, peptide quantitation, SPE, solid-phase extraction, QExactive Plus Goal To describe a rapid and precise protocol for peptide analysis utilizingimmobilized, heat stable trypsin and micro-elution solid-phaseextraction (SPE) coupled with next generation ultra-high pressure liquidchromatography and high resolution mass spectrometry (UHPLC-HRMS).Traditional trypsin digest protocols are time consuming, with some protocolstaking up to one and a half days to complete and involving multiple stepsincluding evaporation and reconstitution. This adds potential for a highdegree of variability. The Thermo Scientific"SMART Digest kit was used todigest cytochrome C with a 10 minute digestion protocol. This was followedby Thermo Scientific SOLAp SPE plates to clean and concentrate thedigest prior to injection onto the Thermo ScientificVanquishUHPLCsystem. Following separation on a Thermo ScientificAcclaimC18 RSLCanalytical column, high-resolution mass spectrometry was performed usinga Thermo Scientific Q ExactivePlus hybrid quadrupole-Orbitrap massspectrometer. A high-throughput, reproducible workflow was achievedthat can be applied to nontargeted, semitargeted, or targeted quantitativeenvironment for peptide analysis. Introduction A fundamental requirement of peptide mapping andquantitative analysis workflows is reproducibility. Thisenables users to confidently assign data differences to thesample and not the methodological conditions used. The current approach for peptide mapping andquantitative analysis involves in-solution trypsin digestionof proteins overnight. This protocol is time consumingand requires a number of different steps, including proteinassay, denaturation, alkylation, and reduction, which candiffer between laboratories and make method transfer anddata analysis between user groups problematic. Additionally, due to the number of steps required,in-solution digestion protocols are highly laborious,increasing the potential for user error. As a result,in-solution digestion often leads to irreproducible proteincleavage which manifests in variation in the chromatographic profile. This prevents the adoption ofrobust, generic methodologies resulting in reducedthroughput and return on investment. The SMART Digest kit eliminates these issues byproviding a protocol that is: ·Highly reproducible ·Quick and easy to use ·Detergent free ·Less prone to chemically-induced post translationalmodification (PTMs) Autolysis-free ·Highly amenable to automation Prior to analyzing digested samples it is common toperform sample cleanup such as centrifugation, filtration,or solid-phase extraction (SPE). This removes unwantedchemicals (such as detergents) that can interfere with thedownstream mass spectrometric detection. Centrifugation cannot always provide the level of cleanuprequired and exposes the detection system to unwantedlevels of contaminants. Consequences can be physical,such as a blocked injection needle, as well as analytical,such as reduced column life or detection variability. Filtration can be used to prevent the physical issues fromoccurring but offers little in the removal of excess buffersand reagents. Furthermore, the apparatus used in filtrationcan increase assay variability due to unpredictable bindingof molecules to sample handling devices. SPE provides a solution to both issues by filtering thedigest while selectively removing the reagents. A genericmethod can be employed for a nontargeted workflow,removing only the unwanted reagents from the digestwhile maintaining a high recovery and reproducibility. SOLAu SPE plates provide reproducibility, robustness,and ease of use at low elution volumes by utilizing therevolutionary Thermo Scientific"SOLASolid-PhaseExtraction technology. This removes the need for fritswhile delivering a robust, reproducible format whichensures highly consistent results at low elution volumesproviding: ·Lower sample failures due to high reproducibility atlow elution volumes · Increased sensitivity due to lower elution volumes ·The ability to process samples which are limited involume · Improved stability of bio-molecules by reduction ofadsorption and solvation issues SOLAp HRP is a micro-elution SPE device built on apolymeric backbone, containing both reversed-phase andpolar-retentive moieties. In addition to providing highlevels of recovery and reproducibility, it enables anadditional concentration factor to be achieved without theneed for evaporation and reconstitution. Removal of theevaporation step reduces non specific binding whencompared to traditional-scale SPE where evaporation andreconstitution are required. Here we compare two protocols of sample cleanupfollowing protein digestion with the SMART digest kit:filtration and SOLAp HRP SPE. A total of eight peptideswere assessed, four from a cytochrome C digest (to assessthe SMART digest) and four exogenous peptides spiked inpost digest (to assess the reproducibility of the cleanupmethods). Recovery and reproducibility of all eightpeptides were measured, allowing an assessment of eachstage of the workflow. All samples were analyzed using the Vanquish UHPLCsystem, which is optimized to reduce extra column banddispersion and allow users to significantly improve theseparation power of their analytical assays. The 1500 barpressure capability of the Vanquish pump enables anextended range of flow rates to be employed allowing forfaster separations and higher throughput. Separation onan Acclaim C18 RSLC analytical column was achievedwithin 15 minutes. Detection was performed on a Q Exactive Plus OrbitrapMS, a benchtop LC-MS system designed for high-performance, high-throughput screening, compoundidentification, and quantitative analysis. With itsOrbitrapmass analyzer, the Q Exactive Plus systemdelivers high-resolution, accurate-mass (HRAM) full-scanMS for fast, precise, and reproducible results withanalytical confidence. Experimental Digestion · SMART Digest kit (P/N 60109-101)SMART Digest with SOLAp HRP kit (P/N 60109-103) Chemicals ·Fisher ScientificOptimawater (P/N 10095164) ·Fisher Scientific Optima acetonitrile (ACN)(P/N 10001334) ●Thermo ScientificPierce"formic acid (FA)(P/N 10628654) ·Fisher Scientifictrifluoroacetic acid (TFA)(P/N 10294110) Cytochrome C, leu_enkephalin, angiotension 1,angiotensin 2, and neurotensin were purchased fromreputable sources. Sample Handling Equipment ·96 well square well microplate (P/N 60180-P202) ·96 well positive pressure manifold (P/N 60103-357) Separation · Acclaim RSLC 120, C18, 2.2 um analytical column(2.1×100 mm) (P/N 068982) It is also recommended that a heater/shaker equipped withPCR block be used to perform the digest. Sample Preparation Separation Conditions Binary pump H (P/NVH-A10-A) Split sampler HT (P/N VH-A10-A) Column compartment H(P/NVH-C10-A) Active pre-heater (6732.0110) Post column cooler, 1 pL (6732.0510) Mobile phase A Water + 0.1% formic acid Mobile phase B Acetonitrile+0.1% formic acid Column temp. 30°℃ (Still Air) Injection details 2uL Gradient See Table 1 Table 1. LC gradient conditions. Time (min) Flow (mL/min) B (%) 0.00 0.5 0 15.00 0.5 50 15.01 0.5 100 20.00 0.5 100 20.01 0.5 0 25.00 0.5 0 MS Conditions Instrumentation Q Exactive Plus Hybrid Quadrupole-OrbitrapMass Spectrometer The MS scan parameters and HESI source parameters are shown inTables 2 and 3, respectively. Table 2. MS scan parameters. Scan type Full MS Scan range m/z250-2000 In-source CID 0.0 eV Resolution 70,000 Polarity Positive Microscans 1 Lock masses -一 AGC target 3e6 Max inject time 200 ms Number of scan ranges 1 Spectrum data range Profile Table 3. HESI source settings. Sheath gas flow rate 48 Aux gas flow rate 20 Sweep gas flow rate 2 Spray voltage 3.50 kV Spray current 一一 Capillary temp. 400°℃ S-lens RF level 100 Aux gas heater temp 350°℃ Data Processing Thermo ScientificXcaliburQuant software version3.0.63 was used for data processing. Details are providedin Table 4. Table 4. Compound transition details used for data processing. Sample Amino Acid Sequence Precursor (m/z) Cytochrome Cpeptide EDLIAYLK 483.27301 GITWGEETLMEYLENPKK 711.33099 MIFAGIK 779.44641 TGPNLHGLFGR 390.21155 Leu_Enkephalin YGGFL 556.27526 Angiotensin_I DRVYIHPFHL 432.8987 Angiotensin_Il DRVYIHPF 523.77349 Neurotensin ELYENKPRRPYIL 558.30907 Results and Discussion A total of eight peptides were assessed when comparingSOLAu HRP and filtration as methods of post-digestsample cleanup. Four well-characterized peptides derivedfrom cytochrome C were used for assessment of theSMART Digest kit, selected for their high abundance andstable retention times. Four exogenous peptides were alsospiked in post digest. This allowed assessment of thereproducibility of the SMART Digest kit, along with anindependent assessment of the cleanup procedures(Figure 1). Figure 1. Flow chart of workflow used to calculate peptiderecovery from cleanup method. The separation of all eight peptides is provided (Figure 2).This separation was obtained using an Acclaim C18 RSLCanalytical column with a 15 minute LC gradient. Accurate mass data was collected from the Q ExactivePlus mass spectrometer. Mass accuracy was assessed at500 ppm, 5 ppm, and 2 ppm for assessment. Figure 3shows the benefits of accurate mass for peptide sequenceGITWGEETLMEYLENPKK. Increasing the accuracy ofthe measurement from 500 to 2 ppm shows clearadvantages in reducing background noise levels andultimately increasing signal-to-noise values of targetedanalytes. This also adds further accuracy when measuringunknown samples for identification purposes. 8.64 Time (min) Figure 2. Separation of the eight peptides using an Acclaim C18RSLC analytical column. 6.44 Using the Vanquish UHPLC system, stable retention timeswere observed for all measured peptides as shown inTable 5. Table 5. Retention times (RT) of each peptide. Sample Amino Acid Sequence RT (min) %RSD ofRT(n=6) Cytochrome Cpeptide EDLIAYLK 7.55 0.123% GITWGEETLMEYLENPKK 6.44 0.186% MIFAGIK 6.65 0.203% TGPNLHGLFGR 6.52 0.179% _eu_Enkephalin YGGFL 7.37 0.127% Angiotensin_I DRVYIHPFHL 6.76 0.179% Angiotensin_Ⅱl DRVYIHPF 6.28 0.156% Neurotensin ELYENKPRRPYIL 5.60 0.0589% Figure 3. Peptide sequence GITWGEETLMEYLENPKK with accuratemass setting of 500 ppm, 5 ppm, and 2 ppm, RT 6.4 mins. The recovery and precision of all eight peptides issummarized in Figure 4. This is a direct comparisonbetween SOLAp HRP and filtration when used as postdigestion cleanup methods. The SOLAp HRP methodshowed higher levels of recovery on seven out of eightpeptides, with higher levels of precision on every peptide. Figure 4. Average recovery values for each peptide with %RSD(n=6). Some selectivity differences are observed between the twoprotocols, in particular for neurotensin and MIFAGIK,where recoveries are lower on the SOLAp HRP for theformer and noticeably higher for the latter. Despite thedifferences in recovery levels, the precision of the dataobtained from the SOLAp HRP is much higher than thatobtained with filtration as seen in Figure 5. Figure 5. Selectivity differences between SOLAu HRP and filter. The percent relative standard deviation (RSD) of theexogenous peptides gives us the level of precision of thecleanup method, along with the subsequent LC anddetection. The RSD of the cytochrome C peptides gives usthe level of precision of the entire process including thereproducibility of the digest. Comparing precision databetween the exogenous and cytochrome C peptides allowsassessment of the reproducibility of the digestion(Figure 6). Comparable levels of precision for both sets of peptideswere observed. This indicates the following: ●TThe entire analytical workflow is precise (RSD ofexogenous peptides=5%) ●The reproducibility using the SMART Digest kit isprecise (RSD of cytochrome C peptides=6%) 图Average recovery of exogenous peptides ■Average recovery of cytochrome C peptides Figure 6. Recovery and %RSD of exogenous and cytochrome Cpeptides (n=6) both using SOLAp HRP method. This analysis demonstrates that use of the SMART Digestkit offers: ·A highly reproducible digest protocol Quick and easy use Detergent free digestion Post digest sample cleanup was achieved using filtrationand SOLAu HRP SPE: · Filtration provides a simple, fast workflow but offerslimited sample cleanup ·SPE provides a high precision workflow with optimizedsample cleanup, offering an additional sampleconcentration factor where required The benefits outlined above clearly demonstrate theadvantages of the SMART Digest kit with regards toworkflow efficiency and reproducibility. A choice ofsample clean-up is also demonstrated depending on theimportant factors for the analyst: speed and simplicity, oraccuracy and precision of data. The workflow describedallows for the introduction of fast, generic, and robustanalytical methods within a high-throughput,biopharmaceutical environment. thermoscientific.com/chromatography ◎2015 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries.This information is presented as an example of the capabilities of Thermo Fisher Scientific products. It is not intended to encourage use ofthese products in any manners that might infringe the intellectual property rights of others. Specifications, terms and pricing are subject to change.Not all products are available in all countries. Please consult your local sales representative for details. 十9 +91 27 1766 2352 AN21198-EN 0615S
确定



还剩4页未读,是否继续阅读?
赛默飞色谱与质谱为您提供《蛋白、多肽中酶解、定量检测方案(液相色谱仪)》,该方案主要用于其他中酶解、定量检测,参考标准--,《蛋白、多肽中酶解、定量检测方案(液相色谱仪)》用到的仪器有赛默飞优谱佳UHPLC+高效液相色谱系统、赛默飞 Vanquish™ UHPLC超高效液相色谱系统、赛默飞 Vanquish Flex UHPLC系统
推荐专场
相关方案
更多
该厂商其他方案
更多