方案详情
文
综合热分析仪用于测量有机高分子材料(塑料、橡胶、合成树脂、纤维、涂料、油脂)无机材料(陶瓷、玻璃、建材、耐火材料、金属及合金)的热分析
方案详情

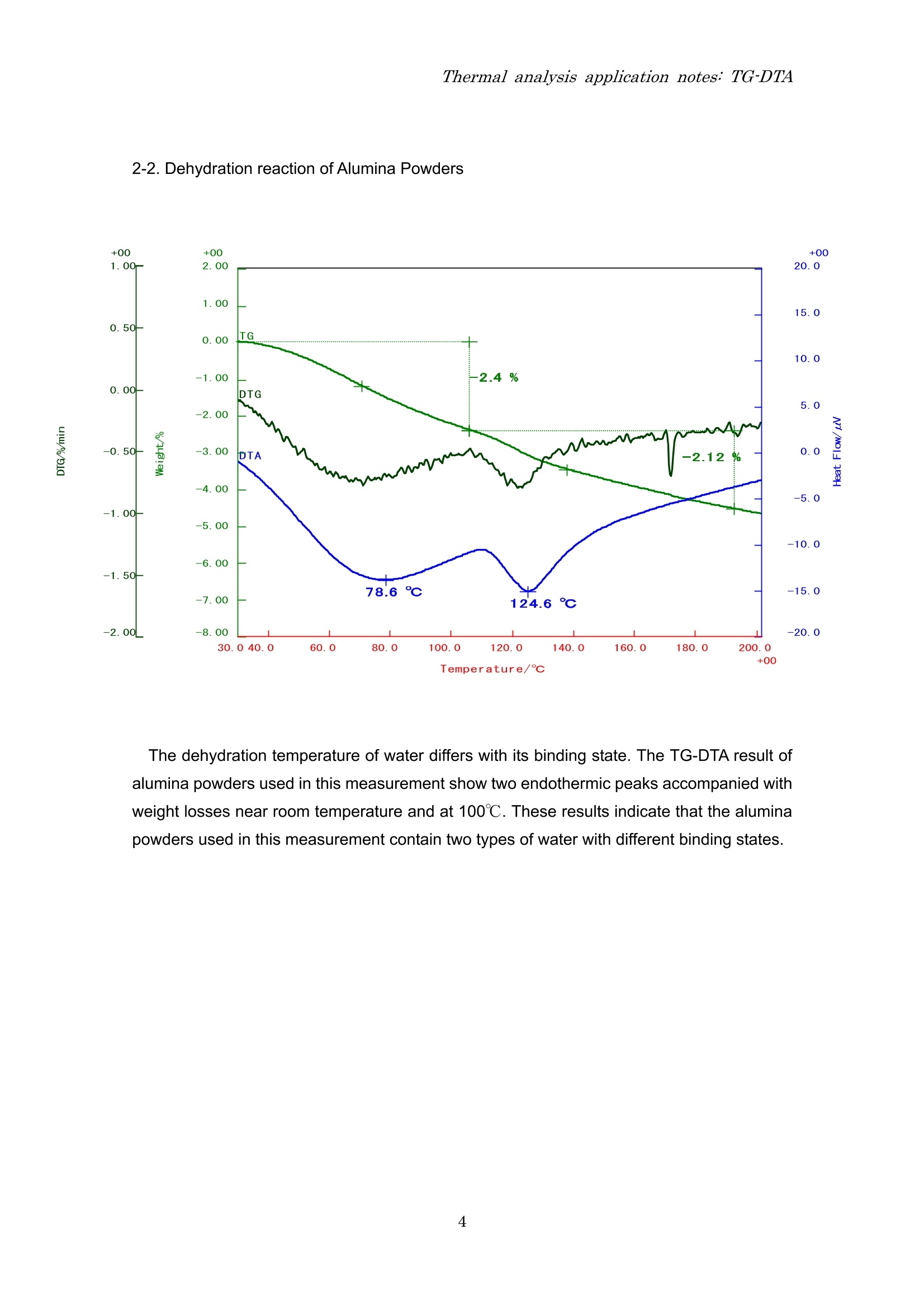
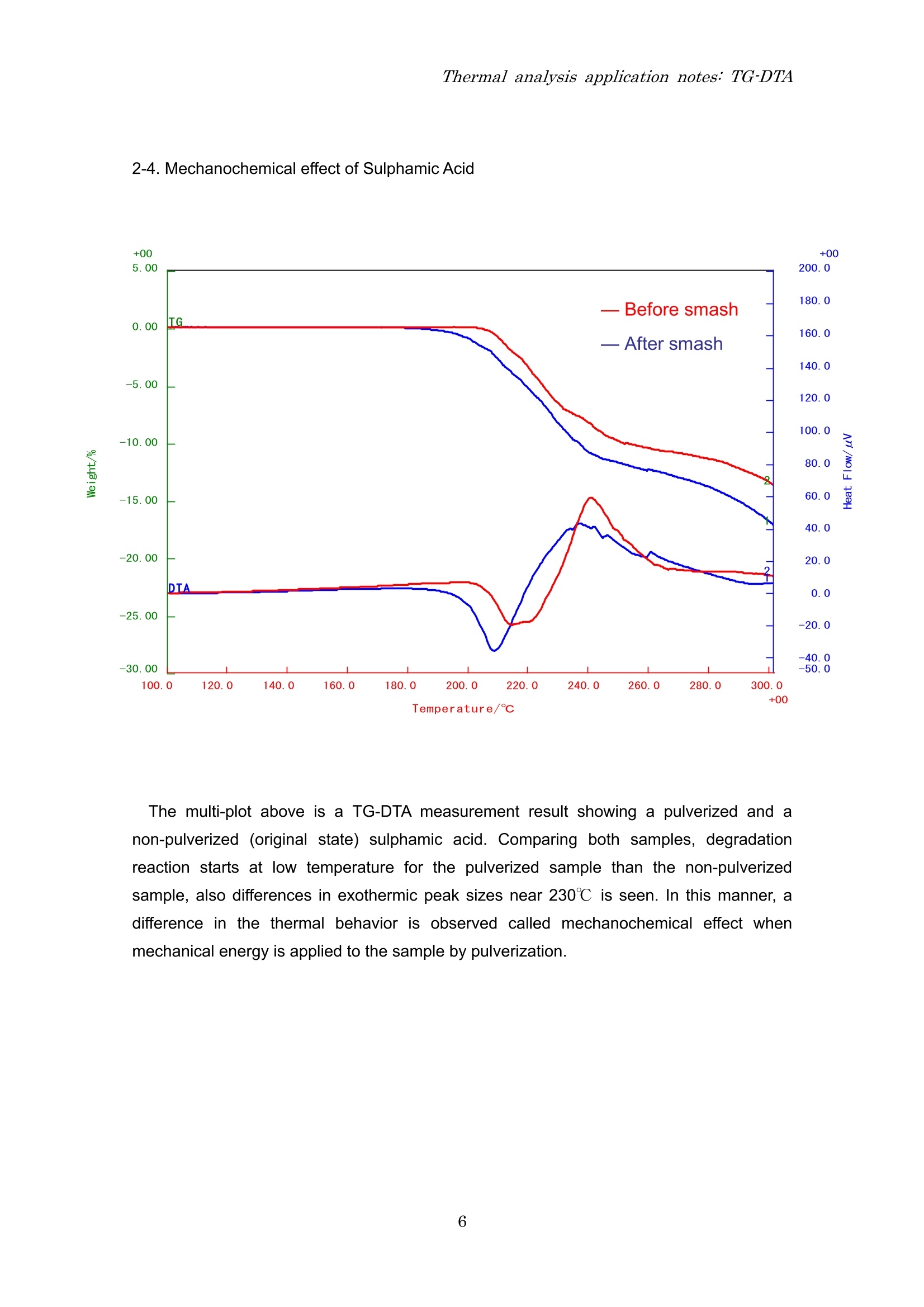
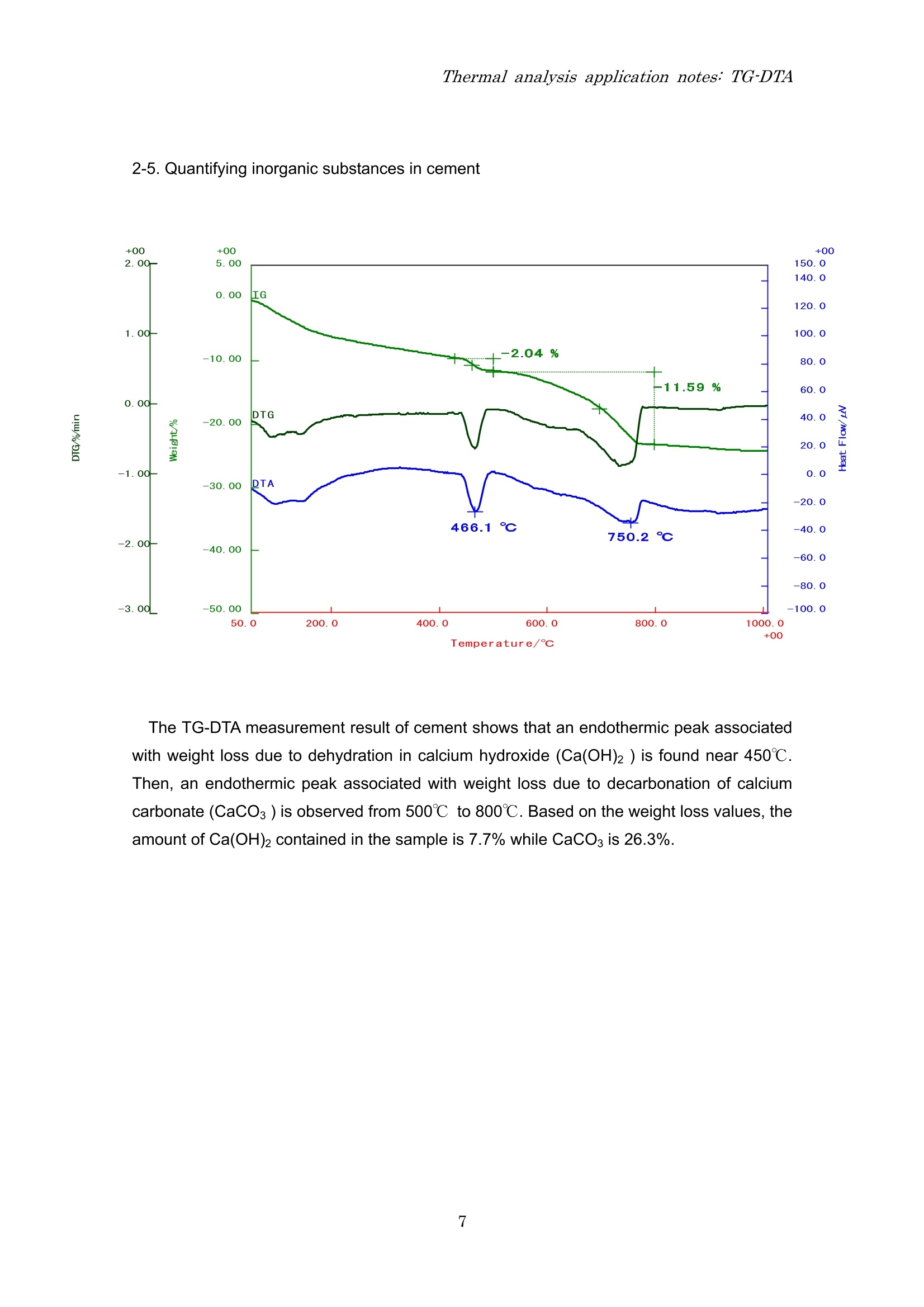
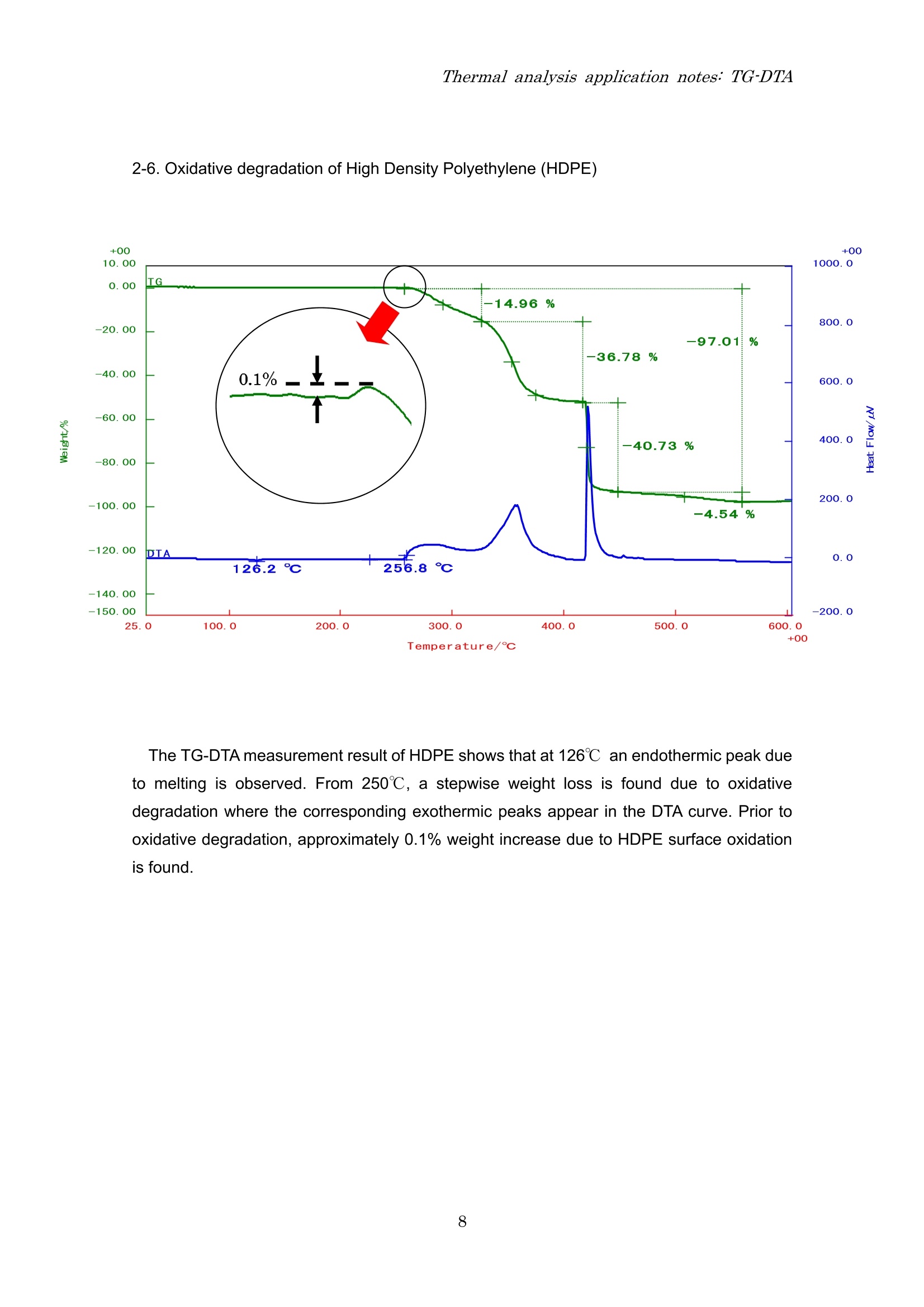
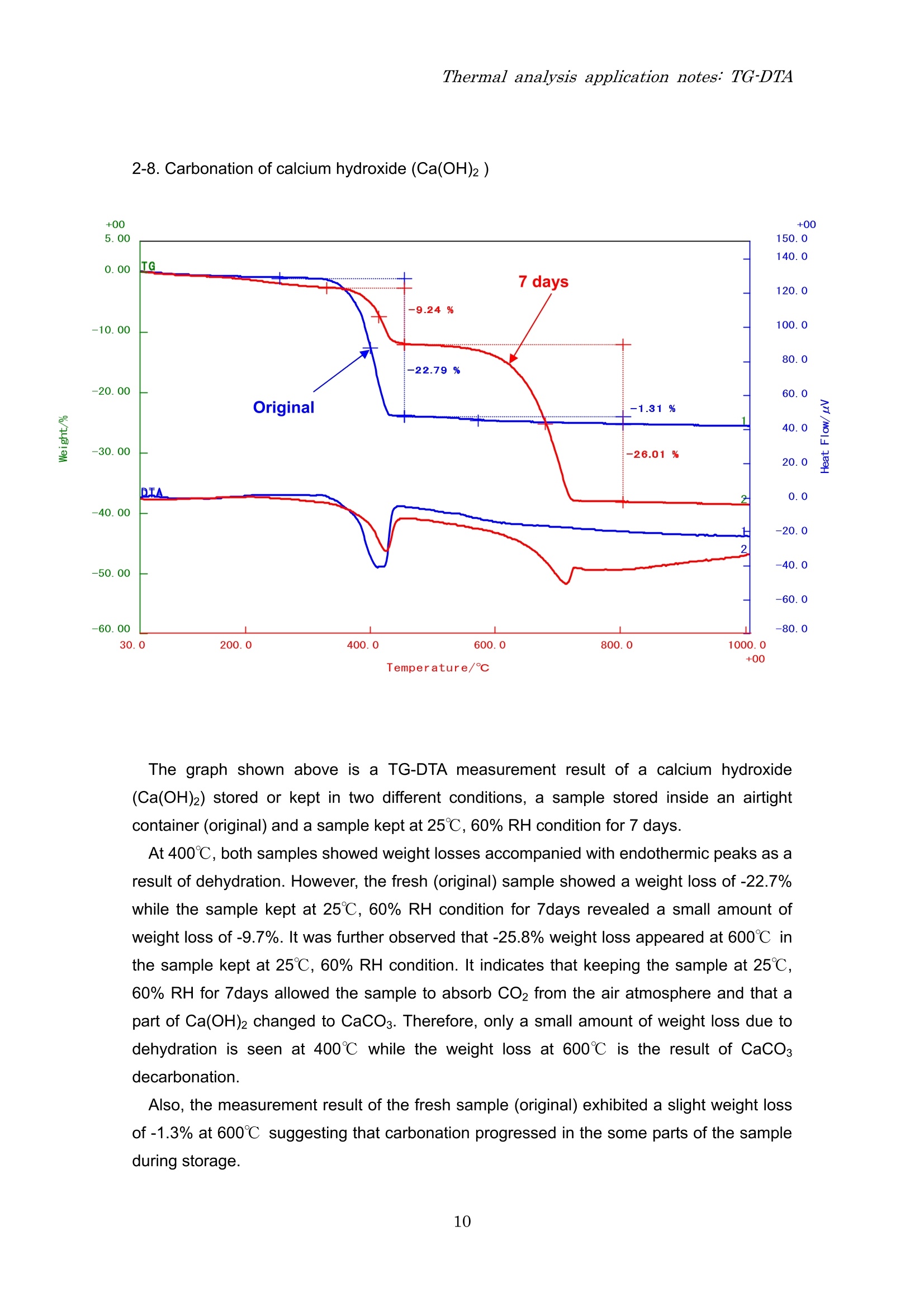
Thermal analysis application notes:TG-DTA Thermal analysis application notes;TG-DTA Tthermogravimetry-differentialthermal analyzer RigokuRigaku Corporation Contents 1. Outline Thermo plus EVO I seriesare tthermal analysis equipment developed ffor theimprovement of the stability of data, safety, operativeness, andthe fundamentalperformance. The thermal analysis equipment is a necessary and an indispensable tool toobtain basic data on the thermophysical property of amaterials and is used in various fieldsuch as new materials development and product evaluation; QC (quality control),etc. High precision differential type thermogravimetry-differential thermal analyzer is a modulethat detects the mass and energy changes which occurs in a material when heated at aconstant temperature program. Measurementshave (good reproducibility/evenincomparatively small amount of sample. EVOllTG8120 series 2. Application notes 2-1. The sublimation, melting and evaporation reactions of benzoic acid 3 Temperature/c The TG-DTA measurement result of benzoic acid shows that a weight loss accompaniedwith an endothermic peak started near 90℃ and then another endothermic peak due tomelting is found near 121℃. These thermal behaviors were due to the sublimation ofbenzoic acid followed by melting. After melting was observed, a weight loss accompaniedwith an endothermic peak due to evaporation was found. From this measurement result, it isunderstood that sublimation, melting and evaporation reactions continuously occur inbenzoic acid. 2-2. Dehydration reaction of Alumina Powders The dehydration temperature of water differs with its binding state. The TG-DTA result ofalumina powders used in this measurement show two endothermic peaks accompanied withweight losses near room temperature and at 100℃. These results indicate that the aluminapowders used in this measurement contain two types of water with different binding states. 2-3. Oxidation-reduction reaction of Palladium powders The TG-DTA result of the palladium used in this measurement shows that at 200℃ anexothermic peak is seen accompanied with increase in %weight due to oxidation reaction.As the temperature increases, an endothermic peak associated with weight loss due toreduction reaction is seen from 800℃ onwards. In this manner, the palladium oxidizes astemperature increases and at high temperature it reduces. 2-4. Mechanochemical effect of Sulphamic Acid Temperature/℃ The multi-plot above is a TG-DTA measurement result showing a pulverized and anon-pulverized (original state) sulphamic acid. Comparing both samples, degradationreaction starts at low temperature for the pulverized sample than the non-pulverizedsample, also differences in exothermic peak sizes near 230℃ is seen. In this manner, adifference in the thermal behavior is observed called mechanochemical effect whenmechanical energy is applied to the sample by pulverization. 2-5. Quantifying inorganic substances in cement The TG-DTA measurement result of cement shows that an endothermic peak associatedwith weight loss due to dehydration in calcium hydroxide (Ca(OH)2) is found near 450℃.Then, an endothermic peak associated with weight loss due to decarbonation of calciumcarbonate (CaCO3) is observed from 500℃ to 800℃. Based on the weight loss values, theamount of Ca(OH)2 contained in the sample is 7.7% while CaCO3 is 26.3%. 2-6. Oxidative degradation of High Density Polyethylene (HDPE) Temperature/C The TG-DTAmeasurement result of HDPE shows that at 126℃ an endothermic peak dueto melting is observed. From 250℃, a stepwise weight loss is found due to oxidativedegradation where the corresponding exothermic peaks appear in the DTA curve. Prior tooxidative degradation, approximately 0.1% weight increase due to HDPE surface oxidationis found. 2-7. Quantifying carbon content in rubber Shown above is a quantified measurement result of carbon content in rubber. First, the s:ample was heated up to 800℃ under N2 atmosphere to allow degradation ofthe resin component in rubber. In this measurement, the TG curve indicated a three-stepweight loss at 214 due to degradation of the resin content. When the temperaturereached 800℃, it was cooled down to 200℃ and then the atmosphere was changed to airatmosphere.Finally, it was re-heated again to allow combustion of the carbon. The carboncontent can be quantified using the weight loss ratio in the re-heating process. The abovemeasurement result shows a two-step weight loss as a result of combustion. The weightloss found in the low temperature was due to the carbonized component of the resin(carbonaceous residue) while the weight loss in the high temperature was due to the carboncontent. Therefore, the total organic, carbon black and ash contents in this sample are 82.8%,3.8% and 13.4%, respectively. 2-8. Carbonation of calcium hydroxide (Ca(OH)2) Temperature/℃ The graph shown above is a TG-DTA measurement result of a calcium hydroxide(Ca(OH)2) stored or kept in two different conditions, a sample stored inside an airtightcontainer (original) and a sample kept at 25℃, 60% RH condition for 7 days. At 400℃, both samples showed weight losses accompanied with endothermic peaks as aresult of dehydration. However, the fresh (original) sample showed a weight loss of -22.7%while the sample kept at 25℃, 60% RH condition for 7days revealed a small amount ofweight loss of -9.7%. It was further observed that -25.8% weight loss appeared at 600℃ inthe sample kept at 25℃, 60% RH condition. It indicates that keeping the sample at 25℃,60% RH for 7days allowed the sample to absorb CO2 from the air atmosphere and that apart of Ca(OH)2 changed to CaCO3. Therefore, only a small amount of weight loss due todehydration is seen at 400℃ while the weight loss at 600℃ is the result of CaCOsdecarbonation. Also, the measurement result of the fresh sample (original) exhibited a slight weight lossof-1.3% at 600℃ suggesting that carbonation progressed in the some parts of the sampleduring storage. Headquarters and factory 3-9-12 Matsubaracho Akishima-shi Tokyo Japan 196-8666 Telephone (042)545-8111 FAX (042)544-9795 Thermal Analysis Division Telephone (042)545-8127(direct) FAX (042)545-7984 Tokyo branch 4-14-4 Sendagaya Shibuyaku Tokyo T151-0051 Tel (03)3479-6011 FAX(03)3479-6171Osaka branch 14-8 Akaojimachi Takatsuki City Osaka T569-1146 Tel (0726)96-3387 FAX(0726)94-5852Tohoku office 1-2-16 Omachi Aobaku Sendai City MiyagiiT980-0804 Tel (022)264-0446 FAX (022)223-1977 Nagoya office 35-16 Daikancho Higashiku Nagoya City Aichi T461-0002 Tel (052)931-8441 FAX (052)931-2689 Kyushu office 2-1-1 Sakaicho Kokurakitaku Kitakyusyu City FukuokaT802-0005 Tel (093)541-5111 FAX(093)541-5288
确定





还剩9页未读,是否继续阅读?
北京赛思蒙仪器有限公司为您提供《有机物、无机物中热稳定性、热历史性能检测方案(热重分析仪)》,该方案主要用于化工原料中热稳定性、热历史性能检测,参考标准--,《有机物、无机物中热稳定性、热历史性能检测方案(热重分析仪)》用到的仪器有
相关方案
更多








