
方案详情
文
the composition of the volatile fraction and of non-volatile.....
方案详情

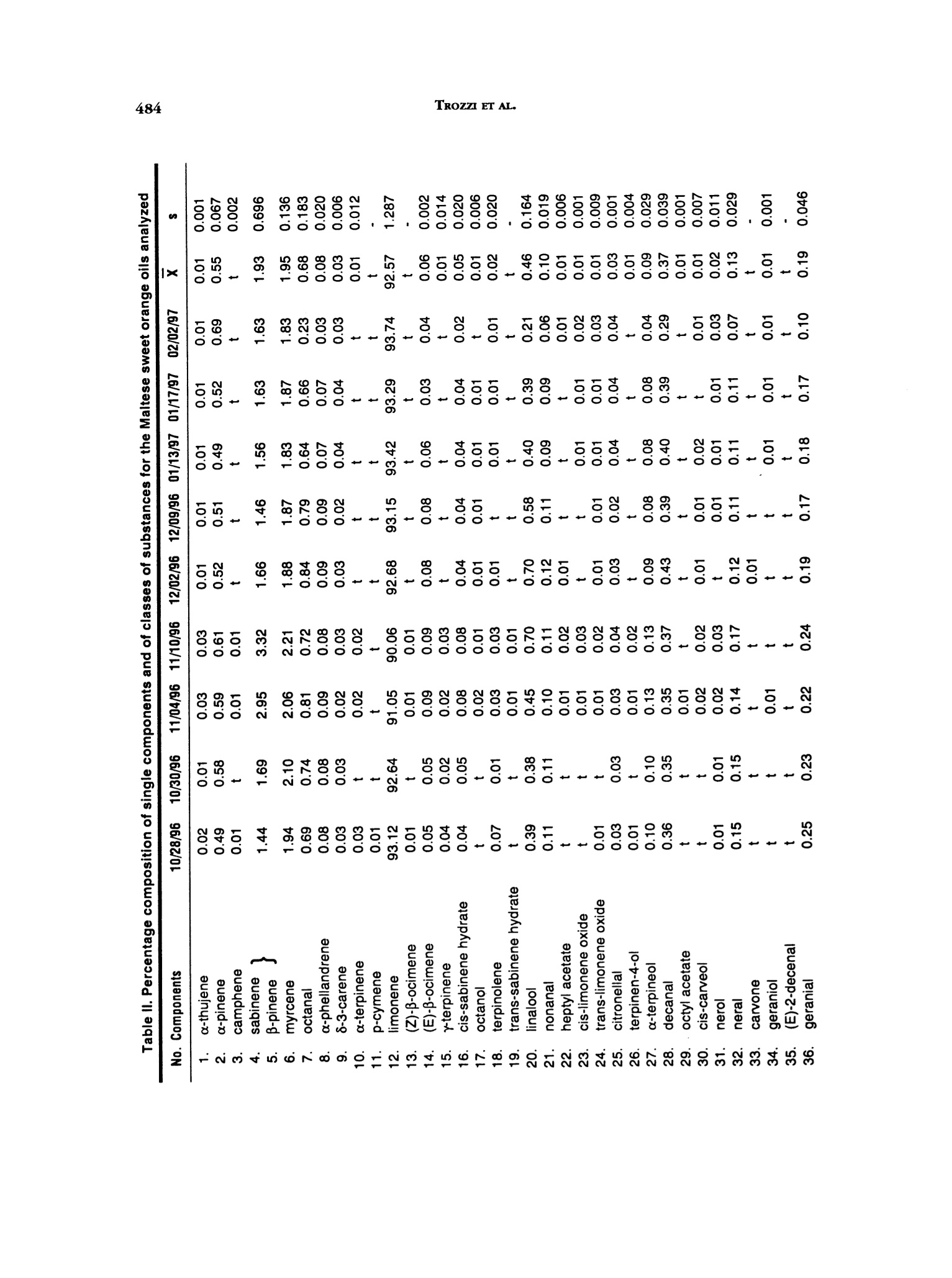
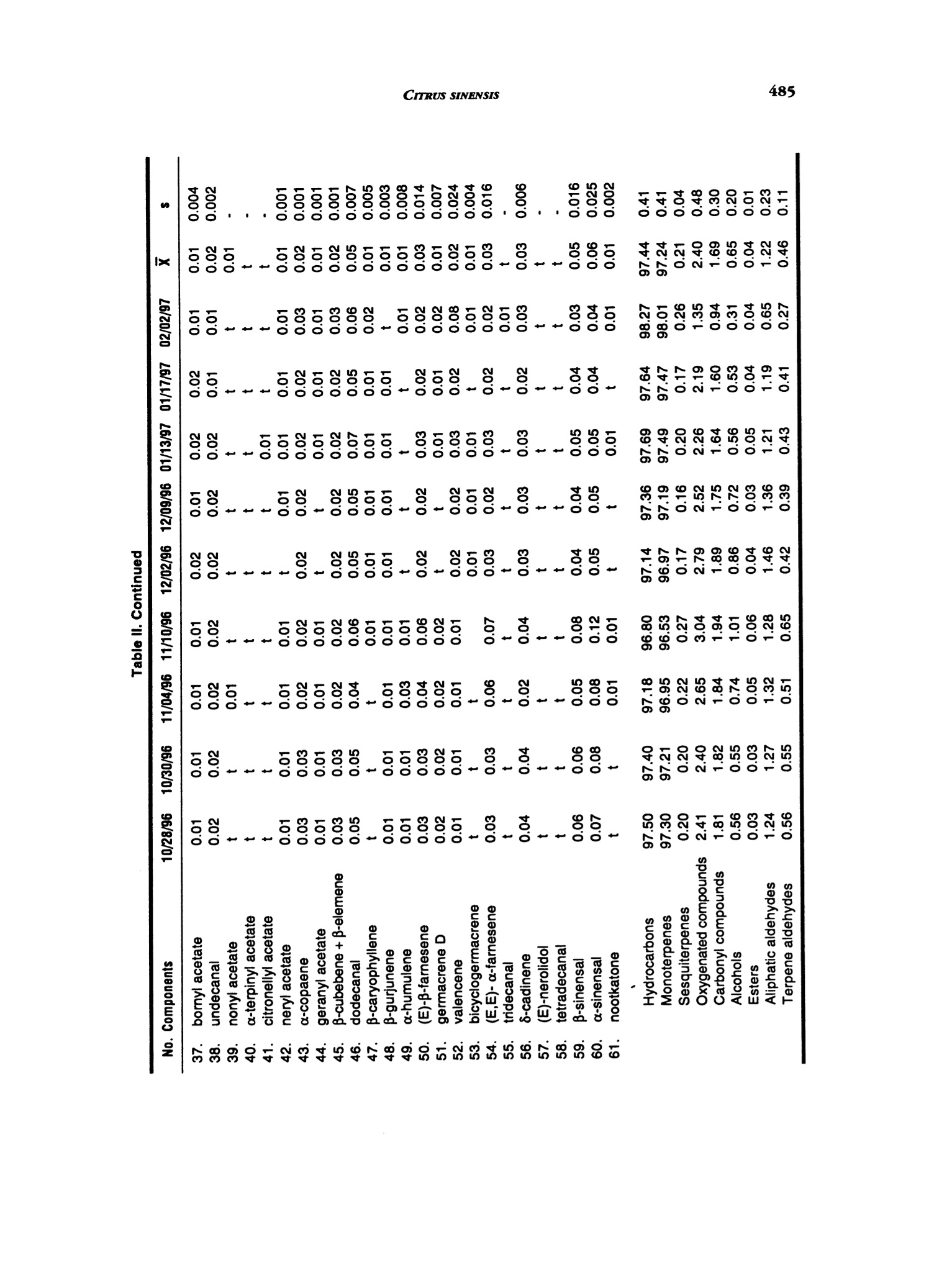
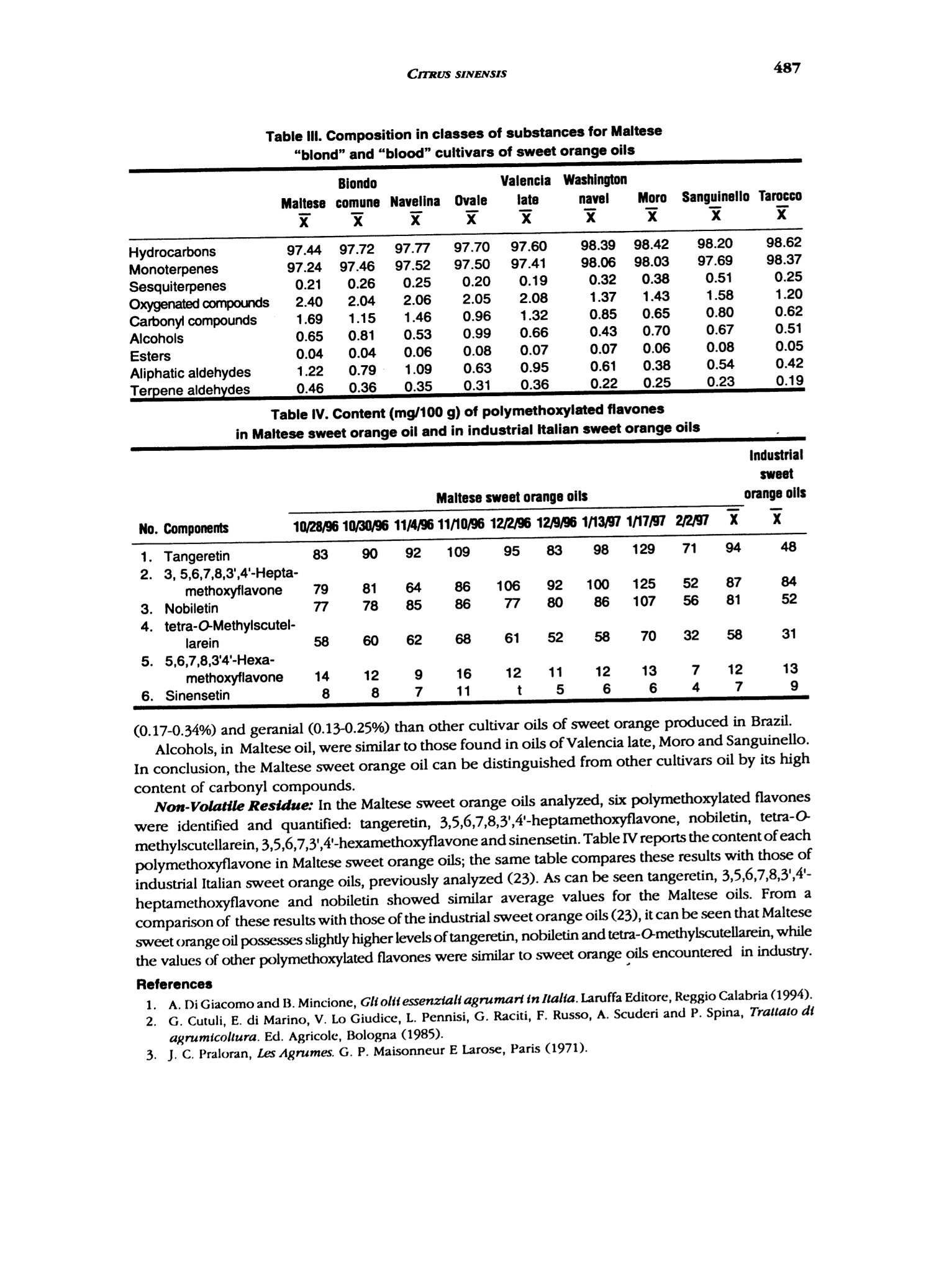
J. Essent. Oil Res.,11, 482-488 (Jul/Aug 1999) 483CITRUS SINENSIS 1041-2905/99/0004-0482$04.00/0—O1999 Allured Publishing Corp. Essential Oil Composition of Citrus sinensis (L.)Osbeck cv. Maltese Alessandra Trozzi Facolta di Farmacia, Universita di Catanzaro, Catanzaro, Italy Antonella Verzera* Instituto di Industrie Agrarie, Facolta di Agraria, Universita di Catania, Catania, Italy Giuseppe Lamonica Dipartimento Farmaco-chimico, Facolta di Farmacia, Universita di Messina, Messina, Italy Abstract The composition of the volatile fraction and of the non-volatile residue of theMaltese sweet orange oil (laboratory-prepared) was studied by GC, GC/MS andHPLC. Sixty-two components were identified in the volatile fraction. The maincomponent was limonene (92,6%); moreover, a high content of carbonylcompoundswas also found. Six polymethoxylated flavones were identified in the non-volatileresidue: 5,6,7,8,4'-pentamethoxyflavone (tangeretin), 3,5,6,7,8,3',4'-heptamethoxy-flavone, 5,6,7,8,3',4'-hexamethoxyflavone (nobiletin), 5,6,7,4'-tetramethoxyflavone(tetra-O-methylscutellarein), 3,5,6,7,3',4'-hexamethoxyflavone and 5,6,7,3'4'-pen-tamethoxyflavone (sinensetin). The Maltese oil composition was compared to thatof different cultivars of sweet orange previously analyzed. Key Word Index Citrus sinensis cv. Maltese, Rutaceae, sweet orange, essential oil composition,polymethoxylated flavones, GC, GC/MS, HPLC. Introduction In Italy, sweet orange (Citrus sinensis L. Osbeck) cultivars are distinguished as "blond" and "blood,"as previouslydescribed (1). There are some blond cultivars characterized by a sweet pulp (without acids) which can be relatedto orange “Douceatre" (France), to "Sucrena" or "Canamiel" or "Grana de Oro" or"Imperial" (Spain),to "Meski" (North Africa and Middle East), to “Moghrabi”(Middle East), to “Cok Kum"or“Tounisi"(Turkey), to“Sucari" (Egypt), to "Orange the Nice" (France, Cote d'Azur), to "Lima" or "Piralima"(Brazil), to "Mosambi"(India) (2,3). In Italy, these oranges are known as "Maltese" or "Vaniglia;"theyripen from October to February and they are reserved exclusively for the fresh fruit market. Malteseorange fruit has a sweetish taste, neither sour, nor pungent. The production is very limited and itdecreases continuously, because of the characteristic taste of the fruit, it is less requested on the market.The size of the fruits ranges from medium to large(180-250 g), the form from spherical to ovoid, therind is rough and very thick. At the time of ripeness the color is dark orange. Most of the papers pertinent to sweet orange oil composition were reviewed by Lawrence (4). Alarge "Address for correspondence Received: March 1998Reuised: june 1998Accepted: july 1998 Table l. Samples analyzed Sample no. Harvest period Ripeness Fruit color 1 10/28/96 unripe yellow-green 2 10/30/96 66 yellow-green 3 11/04/96 66 yellow-green 4 11/10/96 66 yellow-green 5 12/02/96 not very ripe yellow 6 12/09/96 not very ripe yellow 7 01/13/97 ripe yellow-orange 8 01/17/97 ripe yellow-orange 9 02/02/97 ripe yellow-orange number of papers deal with the composition of the volatile fraction of sweet orange oil and in many ofthem its differences in relation to the cultivars are reported with particular references to the differentcontent of aliphatic aldehydes and linalool (5-16). Few papers are, besides, pertinent to thenon-volatile residue (17-20). In the literature, no information could be found about"Maltese" sweet orange oil composition. Onlyone paper deals with the composition of“Piralima” oil, a cultivar from Brazil (21). In previous papers, we analyzed the volatile fraction and non-volatile residue composition ofindustrial Pelatrice and FMC sweet orange oils (22-23)and of laboratory-prepared Italian sweet orangeoils from different cultivars: Biondo comune, Navelina, Washington navel, Valencia late, Ovale,Tarocco, Moro, Sanguinello (24). In this paper, following our research on the composition of Italian sweet orange oils, we analyzedthe composition of Maltese sweet orange oils. This research focuses on the identification of newflavors that could have use in the perfumery and food industries. Experimental The research was carried out on nine samples of laboratory prepared Maltese orange oils. The freshfruits were collected near Taurianova (Reggio Calabria, Calabria), during the period October 1996-February 1997. Information about the samples analyzed are reported in Table I. The fruits were cold-pressed by applying a manual pressure on the rind to release the oil which was collected on a watchglass, transferred to a test tube, centrifuged and analyzed. GC: Volatile fraction was analyzed by GC on a Carlo Erba Gas Chromatograph 5160 Mega series,equipped with a Shimadzu data processor C-R3A using the following, previously described (25),experimental conditions: fused silica SE-52 column, 30 m x 0.32 mm; film thickness, 0.40-0.45 umMega, Legnano (MI), Italyl; column temperature, 45℃ (6 min) to 180℃ at 3℃/min; injection mode,split; detector, FID; injector and detector temperature, 280℃; carrier gas, He 95 kPa; injected volume,1 pL of neat oil. GC/MS: Samples were analyzed by GC/MS (EI) on a Fisons MD 800 (Milan, Italy) system coupledwith Adams'library (26) and FFC banks (27); GC conditions were: a DB-5 fused silica column, 30 mx 0.25 mm, film thickness, 0.25 pm; column temperature, 60℃ (6 min) to 240℃ at 3℃/min; injectortemperature, 250℃; injection mode, split; split ratio, 1:30; volume injected, 1 uL of a solution 1/100in pentane of the oil; carrier gas, He 61.6 kPa; linear velocity 33.5 mL/min; interface temperature,250°℃; detector 1.5 kV; acquisition mass range, 41-300 amu; solvent cut, 2 min. HPLC: All samples were analyzed by normal-phase HPLC, using a Waters Associates (W.A.)equipment composed of a model 519 pump; a 600 E gradient controller, a Rheodyne 9125 injector anda photo diode array detector model 996. Peak integration and quantitative calculations were performedby Millenium 2010 (W.A.) system using a calibration curve obtained for each standard componentagainst a coumarin standard (23). The column was a Zorbax silica column (25 cm x4.6 mm, particle size OOO NONN寸OO66寸666ONOONNNO9OOOOO0OOOOOOOOOOOOOOOOOO 寸 寸O O O NOONOOO OO O 6OOO OO O NO 寸' CO 一c' cca' cccCcEc>o' xoccoc >ac cc oc >>cccocc''co ccEcoccoococcooaN'c ccoaE co OO 6 O O 6OOO OO 66N6NNNOOOO寸NOONNOOOOOOOOOOO6 O O 66OO OO OO OO O寸NOO NOOOO O 69一 寸N O NOOO 寸6OO NO N 9一 四包NN N6O寸OO 寸69OOOO OO 寸寸一 一OO OO 6O寸OO 寸9OOO OO N寸6 NO O 6N 6O9O OO soscwscccoEococooaEo wcwcaooo3ox c oc'x oc o coEa coc c0co c dc oc m wwccwwcoococoooco工o c opoo coc Cao 7000 QN二 NN 3cco 7 um); mobile phase, hexane: ethyl alcohol, 95:5; flow rate, 1.6 mL/min; injection volume, 20 pL ofa solution obtained by diluting about 50 mg of each oil and 0.1 mL of a coumarin solution of knownconcentration in 1 mL of hexane: ethyl acetate (75:25). Detection was by UV absorbance at 315 nm.The UV spectra of eluting peaks were monitored with the PDA detector in the region 200-400 nm. Results and Discussion volatile Fraction Composition: Sixty-two components were identified in each oil. For eachsample we calculated the quantitative composition as a relative percentage of the peak area, as wellas, the total amount of hydrocarbons, monoterpenes, sesquiterpenes, oxygenated compounds,alcohols, esters, carbonyl compounds, aliphatic aldehydes and terpene aldehydes. The averagepercentage (X) and the standard deviation (s) of the single components and of classes of substanceswere also calculated. These data are reported in Table II. With respect to a previous research on sweetorange oil (24) the following additional components were identified: β-gurjunene and bicyclogermacrene.On the contrary, a-muurolene and y-muurolene were not identified. All samples analyzed showed similar composition characteristics. The most representative class ofsubstances was that of monoterpenes. The oils revealed a high content of limonene (X=92.57%) whichis typical for the orange oils; for other monoterpenes only sabinene, B-pinene and myrcene showeda percentage higher than 1%. Among the oxygenated compounds, the most representative classeswere the carbonyl compounds (X=1.69%) and alcohols (X=0.65%). For alcohols, the main componentwas linalool (X=0.46%), while octanal, nonanal and decanal represented more than 70% of carbonylcompound fraction with the most representative monoterpene aldehydes being neral (0.13%)andgeranial (0.19%). The content of a-sinensal (X=0.06%) was always higher than β-sinensal(X=0.05%)Moreover, this oil was characterized, as the other cultivars of sweet orange, by the presence of the.carene(x=0.03%), by a large number of sesquiterpenes and by a low content of esters(X=0.04%). Variation in tbe Composition of tbe Volatile Fraction during tbe Productive Season: During the productive season, quantitative differences in the composition of the oils were observed. Monoterpenes showed the highest values in the final period of the production season. Limoneneshowed a similar behavior. Carbonyl compounds showed a slight decrease during the productionseason, from October to January, and a clear reduction in February. Alcohols reached the highestvalues in November, then decreased, showing the lowest values at the end of the production season.Esters and sesquiterpenes also showed the highest values in November even if the variations duringthe production season were not very large. Comparison between tbe Volatile Fraction oftbe cu. Maltese Oil and"Blond"and"Blood”Cultivar Oils of Sweet Orange: Table III is compared to the average percentage composition inclasses of substances of Maltese sweet orange oils to that of oils obtained from the previously analyzedcultivars: Moro,Tarocco, Sanguinello (blood oranges), Navelina, Washington Navel, Biondo comune,Ovale, Valencia late (blond oranges) (24). The monoterpene content in the Maltese sweet orange oil was similar to that of oils obtained fromBiondo comune, Navelina, Ovale, Valencia late, while the sesquiterpene content was similar to thatfound in Ovale and Valencia sweet orange oils. The aliphatic and terpene aldehyde content was clearlyhigher than in other sweet orange cultivars, this was due to the content of each single aldehyde.For example, the Maltese sweet orange oil showed an average percentage of octanal equal to 0.68%;while the octanal highest content in oils of the other cultivars of sweet orange oils was 0.52%(Navelina oil) (24). Neral and geranial, together, never exceeded 0.2% in the sweet orange cultivar oils,previously analyzed (24). In Maltese sweet orange oil they reached 0.41% in November and had anaverage percentage composition of 0.32%. The high content of neral and geranial is probably a characteristic of oils obtained from cultivarswith sweet pulp without acids since a similar behavior is shown by the sweet orange "Piralima" oil fromBrazil (21). Piralima, in fact, possessed a higher content of carbonyl compounds, in particular of neral Table III. Composition in classes of substances for Maltese“blond”and“blood”cultivars of sweet orange oils Biondo Valencia Washington Maltese comune Navelina Ovale late navel Moro Sanguinello Tarocco x x x x Hydrocarbons 97.44 97.72 97.77 97.70 97.60 98.39 98.42 98.20 98.62 Monoterpenes 97.24 97.46 97.52 97.50 97.41 98.06 98.03 97.69 98.37 Sesquiterpenes 0.21 0.26 0.25 0.20 0.19 0.32 0.38 0.51 0.25 Oxygenated compounds 2.40 2.04 2.06 2.05 2.08 1.37 1.43 1.58 1.20 Carbonyl compounds 1.69 1.15 1.46 0.96 1.32 0.85 0.65 0.80 0.62 Alcohols 0.65 0.81 0.53 0.99 0.66 0.43 0.70 0.67 0.51 Esters 0.04 0.04 0.06 0.08 0.07 0.07 0.06 0.08 0.05 Aliphatic aldehydes 1.22 0.79 1.09 0.63 0.95 0.61 0.38 0.54 0.42 Terpene aldehydes 0.46 0.36 0.35 0.31 0.36 0.22 0.25 0.23 0.19 Table IV. Content (mg/100 g) of polymethoxylated flavonesin Maltese sweet orange oil and in industrial Italian sweet orange oils No. Components 10/28/96 10/30/96 11/4/96 11/10/96 12/2/96 12/9/96 1/13/97 1/17/97 2/2/97 x 1. Tangeretin 83 90 92 109 95 83 98 129 71 94 48 2. 3,5,6,7,8,3',4'-Hepta- methoxyflavone 79 81 64 86 106 92 100 125 52 87 84 3. Nobiletin 77 78 85 86 77 80 86 107 56 81 52 4. tetra-O-Methylscutel- larein 58 60 62 68 61 52 58 70 32 58 31 5. 5,6,7,8,3'4'-Hexa- methoxyflavone 14 12 9 16 12 11 12 13 7 12 13 6. Sinensetin 8 8 7 11 t 5 (0.17-0.34%) and geranial (0.13-0.25%) than other cultivar oils of sweet orange produced in Brazil.Alcohols, in Maltese oil, were similar to those found in oils of Valencia late, Moro and Sanguinello.In conclusion, the Maltese sweet orange oil can be distinguished from other cultivars oil by its highcontent of carbonyl compounds. Non-Volatile Residue: In the Maltese sweet orange oils analyzed, six polymethoxylated flavoneswere identified and quantified: tangeretin, 3,5,6,7,8,3',4'-heptamethoxyflavone, nobiletin, tetra-o-methylscutellarein, 3,5,6,7,3',4'-hexamethoxyflavone and sinensetin. Table IV reports the content of eachpolymethoxyflavone in Maltese sweet orange oils; the same table compares these results with those ofindustrial Italian sweet orange oils, previously analyzed (23). As can be seen tangeretin, 3,5,6,7,8,3',4'-heptamethoxyflavone and nobiletin showed similar average values for the Maltese oils. From acomparison of these results with those of the industrial sweet orange oils(23), it can be seen that Maltesesweet orange oil possesses slightly higher levels of tangeretin, nobiletin and tetra-O-methylscutellarein, whilethe values of other polymethoxylated flavones were similar to sweet orange oils encountered in industry. References ( 1. .1 A. D iGiacomo and B. Mincione,Gli o l it essenziali agrumarl in Italia. Laruffa Edit o re,Reggio Calabria (1994). ) ( 2.( G. Cutuli, E . d i Marino, V . L o Giudice, L. Pennisi, G. Raciti, F. Ru s so, A. Scu d eri and P. Spina, Trall a to dtagrumicollura. E d. Agricole, Bologna (1985). ) 3.JJ. C. Praloran, Les Agrumes. G. P. Maisonneur E Larose, Paris (1971). 4.B. M. Lawrence, Progress in essential oils. Perfum.Flavor., 1(1), 1-5(1976); 4(6), 31-36(1980);9(4), 37-48(1984);9(6), 61-71(1985);12(3),58-70(1987);15(6),45-64(1990);17(5),131-146(1992);19(4),35-37(1994). 5.A. Lifshitz, W. L. Stanley and Y. Stepak, Composition of Valencia essenzial oil from California, Florida andIsrael. J. Food Sci., 35,547-548(1970). 6 R. Huet and M. C. Murail, L’buile essentielle d'orange de type "Guinee."Fruits,27, 297-301 (1972). P. E. Shaw and R. L. Coleman, Quantitative composition ofcold-pressed orange oils. J. Agric. Food Chem.,22,785-787 (1974). 8.J. W. Kesterson and R. J. Braddock, Total peel oil content of the major Florida citrus cultivars. J. Food Sci.,40,931-933(1975). 9.C. W. Wilson and P. E. Shaw, Quantitation ofindividual and total aldebydes in citrus cold-pressed oils byfused silica capillary gas-cromatograpby.J. Agric. Food Chem., 32, 399-401 (1984). 10. H1. Boelens and R. Jimenez, The chemical composition of some Mediterraneam Citrus oils. J. Essent. Oil Res.1, 151-159(1989). 11. M. Ziegler, H. Brandauer, E. Ziegler and G. Ziegler, A different aging model for orange oil: deteriorationproducts. J. Essent. Oil Res., 3, 209-220(1991). 12.H. Sugisawa, M. Takeda, R. Hua Yang and N. Takagi, The comparison ofodor quality ofvolatiles in peel oilsof five kinds of Navel oranges. Nippon Shokuhin Kogyo Gakkaishi, 38, 668-674 (1991). 13).. M. Usai, G. Arras and F. Fronteddu, Effect ofcold storage on essential oil of peel of Thompson Navel Orange.J. Agric. Food Chem., 40, 271-275(1992). 14.A. Baaliouamer and B.-Y. Meklati, The cbemical composition of some cold-pressed Citrus oils produced inAlgeria.J. Essent. Oil Res., 4, 251-258(1992). 15..C. Blanco Tirado, E. E. Stashenko, M. Y. Combariza andJ. R. Martinez, Comparative study ofColombian citrusoils by bigb-resolution gas cbromatograpby and gas cbromatograpby-mass spectrometry.J. Chromatogr.,A.697,501-513(1995). 16.1L.R. Rouseff,J. W. Grosser, H. E. Nordby and H. D. Powell, Comparison offlavor volatiles in a somatic bybridfrom West Indian Lime (Citrus aurantifolia) and Valencia orange (C. sinensis) witb its parents. Spec. Publ.R. Soc. Chem., 197, 78-81 (1996). 17.J.] P. Bianchini and E. M. Gaydou, Role of water in qualitative and quantitative determination ofpolymetboxylated flavones bystraigbt-pbase bigb-performance liquid cbromatograpby: application to orangepeel oils. J. Chromatogr., 211,61-78(1981). 18..E. M.Gaydou,J. P. Bianchini and R. P. Randriamiharisoa, Orangeand mandarin peel oils differentiation usingpolymetboxylated flavone composition. J.Agric. Food Chem., 35, 525-529 (1987). 19.D1. McHale and J. B. Sheridan, The oxygen beterocyclic compounds of Citrus peel oils. J. Essent.Oil Res., 1,139-149(1989). 20. 1E. M. Gaydou, T. Berahia,J. C. Wallet andJ. P. Bianchini, Gas cbromatograpby of some polymetboxcylatedJlavones and their determination in orange peel oils. J. Chromatogr., 549,440-445 (1991). 21. M. Koketsu, M. T. Magalhaes, U. C. Wilberg and M. G. R. Donalisio, Oleos essenciais de frutos citricoscultivados no Brazil. Boletin de Pesquita, 7, 3-21 (1983). 22. (G. Dugo, A. Verzera, I. Stagno d'Alcontres, A. Cotroneo, A. Trozzi and L. Mondello, On tbe genuineness ofCitrus essential oils. Part XLIII. The composition ofthe volatile fraction ofitalian sweet orange oils. J. Essent.Oil Res., 6, 101-137(1994). ( 23 . P. Dugo, L. Mondello, E . Cogliandro, I. Stagno d'Alcontres and A. Co t roneo, On tbe genuineness of citrus essential oils. Part XLVI. Polymetboxylated Jlavones of tbe non volatile residue of ilalian swe e t orange and mandarin essential oils. F l av. F r agr. J., 9, 1 05-111 ( 1 994). ) ( 24. A. Verzera, A. Trozzi, I. Stagno d'Alcontres and A. Cotroneo, On tbe genuineness ofcitrus essential oils. Part XLvm. T he composition of lbe volatile fraction of some varleties ofsweet orange oils. J. Essent. Oil R e s., 8,159-170(1996). ) 25.A. Verzera, L.Mondello, A. Trozzi and P. Dugo, On the genuineness ofcitrus essential oils. Part LII. Chemicalcharacterization ofessential oil oftbree cultivars ofCitrus clementine Hort.Flav. Fragr. J., 12, 163-172(1997). 26. R. Adams, Identification ofessential oil componenis by gas cbromatograpby/mass spectroscopy. Allured Publ.Corp., Carol Stream, IL (1995). 27.LL. Mondello, P. Dugo, A. Basile, G. Dugo and K. D. Bartle, Interactive use of linear retention indices, on polar andapolar columns, witb a MS-library for reliable identification of complex mixlure. J. Microcol. Sep., 7, 581-591(1995).
确定



还剩5页未读,是否继续阅读?
华明科技(香港)有限公司为您提供《甜橙精油中化学成分检测方案 》,该方案主要用于日用化学品/香精香料中化学成分检测,参考标准--,《甜橙精油中化学成分检测方案 》用到的仪器有
相关方案
更多







