
方案详情
文
Rosmarinus officinalis from the Labiatae family comes from
...
方案详情

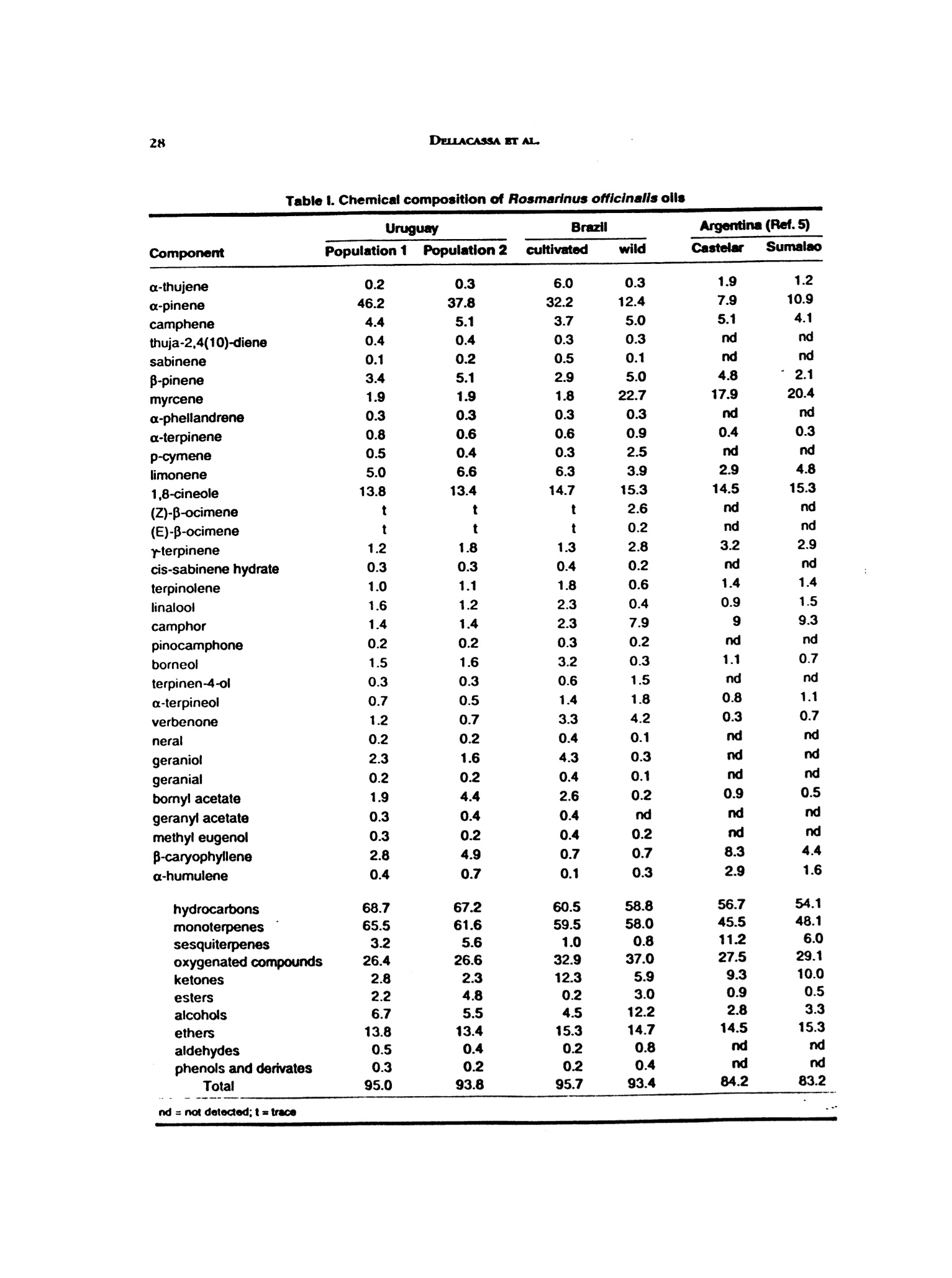
/. Essent. Otl Res., 11, 27-30 (Jan/Feb 1999) 28DELLACASSA BT AL. "Address for correspondenceRecelued: Oclober 1997Accepted: January 1998.1041-2905/99/0001-0027$04.00/001999 Allured Publishing Corp. Rosmarinus officinalis L. (Labiatae) Essential Oilsfrom the South of Brazil and Uruguay Eduardo Dellacassa,* Daniel Lorenzo and Patrick Moyna Caedra de Farmacognosia y Productos Naturales, Facultad de Quimica Universidad de la Repiiblica Oriental del Uruguay, Montevideo, Uruguay Caren D. Frizzo and Luciana Atti Serafini Instituto de Biotecnologia, Universidade de Caxias do Sul, Caxias do Sul, Rio Grande do Sul, Brazi Paola Dugo Dipartimento di Chimica Organica e Biologica, Facolta di Scienze FF. MM.NN. Universita di Messina, Italy Abstract Rosemary oils from cultivars of Rosmarinus officinalis L. growing in different areasof Uruguay and South of Brazil (Rio Grande do Sul State) were analyzed by GC andGC/MS. The oils from Uruguay were found to be rich in a-pinene (37.8-46.2%) and1,8-cineole (13.4-13.8%). The oil from R. officinalis which was cultivated in Brazilcontained a-pinene (32.2%) and 1,8-cineole (14.7%), while the oil from wild plantsfound in Brazil contained a-pinene (12.4%), myrcene (22.7%) and 1,8-cineole(15.3%). Key word Index Rosmarinus officinalis, Labiatae, essential oil composition, a-pinene, myrcene,1,8-cincole. Introduction Rosmarinus oficinalis from the Labiatae family comes from the South of Europe. It is cultivated inthe South of Spain, Morocco, Tunsia, Dalmacia (ex. Yugoeslavia), Portugal, Israel and Turkey (1-4).In South America its cultivation has been started in different countries, but without continuity in timefor the oil production (5). It grows as a small shrub with perennial foliage and small leaves. R. o/ficinalis oil is known for its antioxidants properties (6-9) and it is used in food industry, mainlymeat derivates, and in the perfumery and cosmetic industries. Most of its therapeutic applications arerelated to stimulant, antiseptic, diaphoretic, antispasmodic and anesthesic properties (2,8). Significative variations in the chemical composition of the R. officinalis oil has been reported withrelation to the geographic origin (10,11). This work studies the chemical composition of the Rosemary oils for samples collected in Uruguayand the South of Brazil, regions with similar phytogeographic characteristics. The results of the chemicalcomposition analysis were compared with those from the literature for Argentina oils (5). Table l. Chemical composition of Rosmarinus officinalis olls Experimental The fresh foliage of the analyzed plants were collected in April-May 1996. The samples from Brazilwere collected from wild and cultivated populations in Campestre da Serra (Rio Grande do Sul state).Those from Uruguay represent two populations of introduced plants in an experimental field by SESARS.A. as a part of an introduction garden to propagate this species. Voucher specimens (MVFQ 3542, 3543,3544, 3545) have been preserved in the Herbarium of Institute of Botanica, Faculty of Chemistry,University of Montevideo, Uruguay. The oils were isolated by hydrodistillation of the fresh plant materialusing a modified Clevenger-type apparatus. The oils were analyzed by GC and GC/MS. GC: Shimadzu cromatograph 14B equipped with a Shimadzu data processor EZ-Chrom; silica fusedcapillary column, 30 mx0.32 mm, coated with SE-52, 0.40-0.45 pm film thickness (Mega,Legnano,Italy);column temperature, 60℃ (8 min) to 100℃ at 3℃/min, to 130℃ at 2.5°C/min, to 180℃(10 min) at3°C/min, to 210℃ at 20°C/min; injector temperature 280℃; detector temperature280℃; injection mode,split; split ratio, 1:30; volume injected, 0.2 pil of the oil;carrier gas, H,, 55 KPa. GC/MS: Shimadzu QP 1100 equipped with Adams library (12), silica fused capillary column, 30 mx0.25 mm, coated with DB-5, 0.25 um film tickness (J&W, Folson,California, USA); column temperature,60°-240℃ at 3℃/min; injector temperature, 250℃; injection mode, split; split ratio, 1:20; volumeinjected, 0.2 uL of the oil; carrier gas, He, 50 KPa; interface temperature 250℃; ionization energy70 eV, acquisition mass range 40-300; solvent cut, 2 min. Results and Discussion Table I reports the chemical composition of all the oil analyzed, together with that repoted in theliterature for Rosemary oils from Argentina (5). As can be seen from Table I, 32 components were identified, representing about 93-96% of the wholeoils. For Argentinian oils, the identified components constitued 84.2% and 83.2% of the whole oils fromCastelar and Sumalao, respectively (5). All the samples analyzed and those from Argentina presenteda similar 1,8-cincole content, which ranges between 13.4% and 15.3%. The Uruguayan oils presented the highest values for monotepene hydrocarbons and esters, and thelowest values for ketones. a-Pinene was the main component for the samples from Uruguay and for the Brazilian oil fromcultivated plants (46.2%, 37.8% and 32.2% respectively). These three oils showed very similar chemicalcomposition, but the Brazilian sample showed higher values for alcohols (linalool, boreol, a-terpineoland geraniol) and for verbenone than the Uruguayan oils. Myrcene was the main component for the Brazilian oil from wild plants (22.7%), and this value wasvery similar to those found for myrcene in the Argentina oils (17.9% and 20.4%). These three oilsshowed similar percentages for some of their main components (a-pinene, camphor, camphene andy-terpinene). However the Brazilian oil can be distinguished from those from Argentina by its highercontent of verbenone and lower content of sesquiterpene hydrocarbon. Acknowledgments Research supported by Ministero per gli Affari Esteri, Direzione Generale per la Cooperazione e lo Sviluppo;Special Project: "Valorizzazione degli olii essenziali c dei derivati agrumari uruguaiani," under Istituto Italo LatinoAmericano; Scientific Director Prof. Giovanni Dugo, and by Comision Sectorial de Investigacion Cientifica de laUniversidad de la Repoeblica del Uruguay (CSIC 149). References 1..4A. F. Costa, Farmacognosia, Fundaccio Calouste Gulbenkian, Lisbua, Pontugal (1994). 2. L1. Manfred, Stete mil recelas botanicas a base de mil trescientas plantas medicinales Americanas. Editorial KierSA, Buenos Aires, Argentina (1992). 3W. C. Evans, "Farmacognosia."Editora Interamericana/McGraw-Hill, Mexico City, Mexico (1991). B. M. Lawrence, Essential Ofls 1992-1994. Allured Publ. Corp., Carol Stream, IL,LSA(1993). 5. I. Mizrahi, M. A. Juarez and A. L. Bandoni, Tbe essential oil ofRosmarinus oficinalis growing in Argentina.J. Essent. Oil Res., 3, 11-15 (1991). 6.S. M. Beckstrom-Stemberg and J. A. Duke, Potential for synergistic action of pbytocbemicals in spices. Dev.Food Sci., 34, 201-223(1994.). 7. S.G.Deans and K. P. Svoboda, Volatile oil crops: tbeir biology, biocbemistryandproduction Edits., R.K.H. Hay,P. G. Waterman, pp 113-136, Longman, Harlow, UK (1993). 8.E. De Vincenzi, E. Mancini and M. R. Dessi, Monograpbs on botanical flavouring substances used in foods.Part V. Fitoterapia, 67(3), 241-251 (1996). 9.F. Moreira, Asplantas que curam: a prevencao e cura das doencas pelas plantas. Libra-Empresa Editorial Ltda,Sio Paulo, Brasil (1994). 10. J.Granger,J. Passetand G. Arbouset, L'essence de Rosmarinusoficinalis L. I. Influence des facteursecologiqueset indivldueks. Parfum. Cosmet. Savons France, 3, 307-312(1973). 11. M. Boelens, Tbe essential oll from Rosmarinus officinalis L Perfum. Flavor. 10(5), 21-37 (1985). 12.2.R. P. Adams, Identification ofessenttal oil components bygas cbromatograpby/mass spectroscopy. Allured Publ.Corp., Carol Stream, IL, USA (1995).
确定



还剩2页未读,是否继续阅读?
华明科技(香港)有限公司为您提供《唇形科迷迭香精油中成分分析检测方案 》,该方案主要用于日用化学品/香精香料中成分分析检测,参考标准--,《唇形科迷迭香精油中成分分析检测方案 》用到的仪器有
相关方案
更多







