方案详情
文
bergamot cultivation statred in Italy at the beginning of....
方案详情

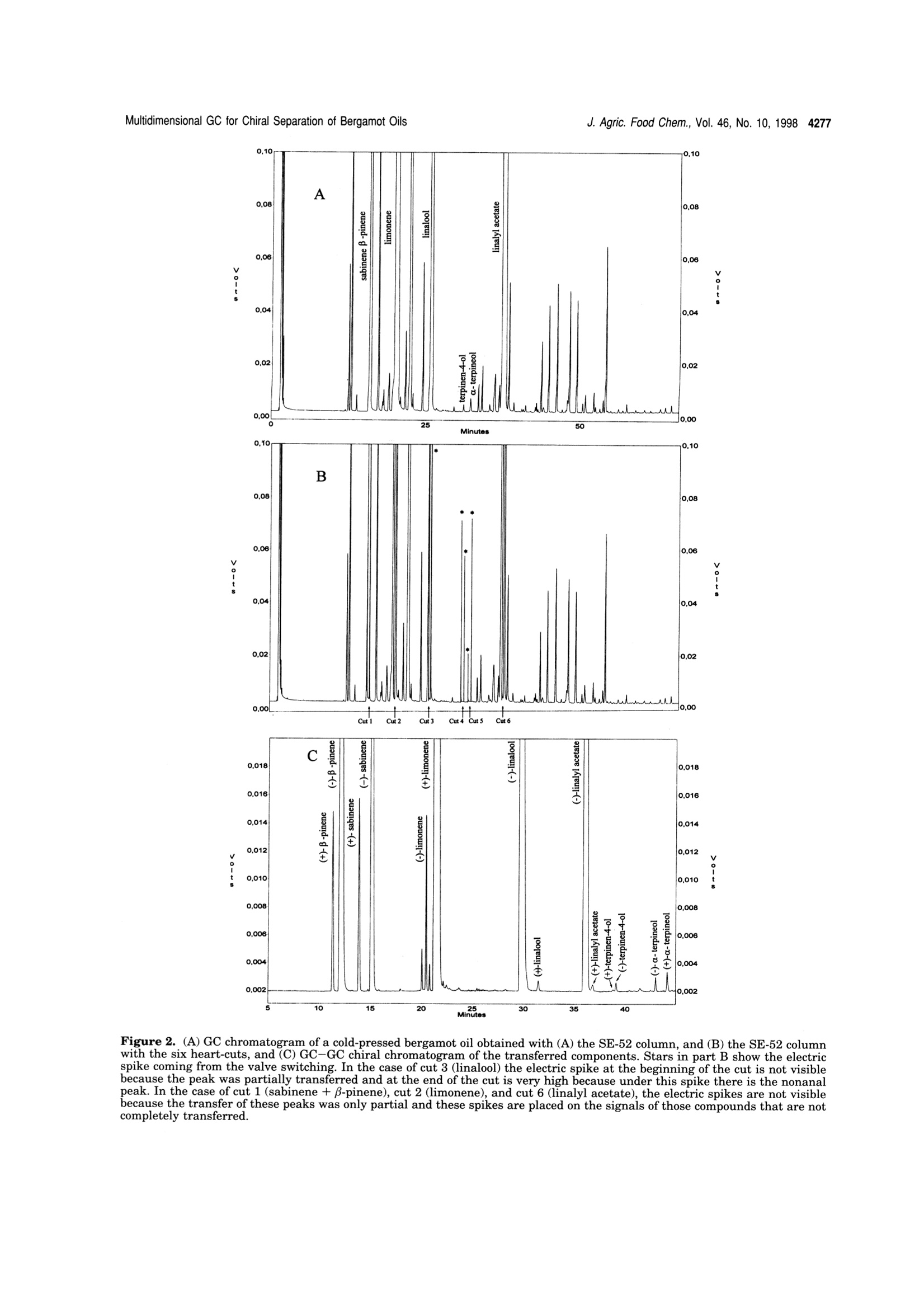
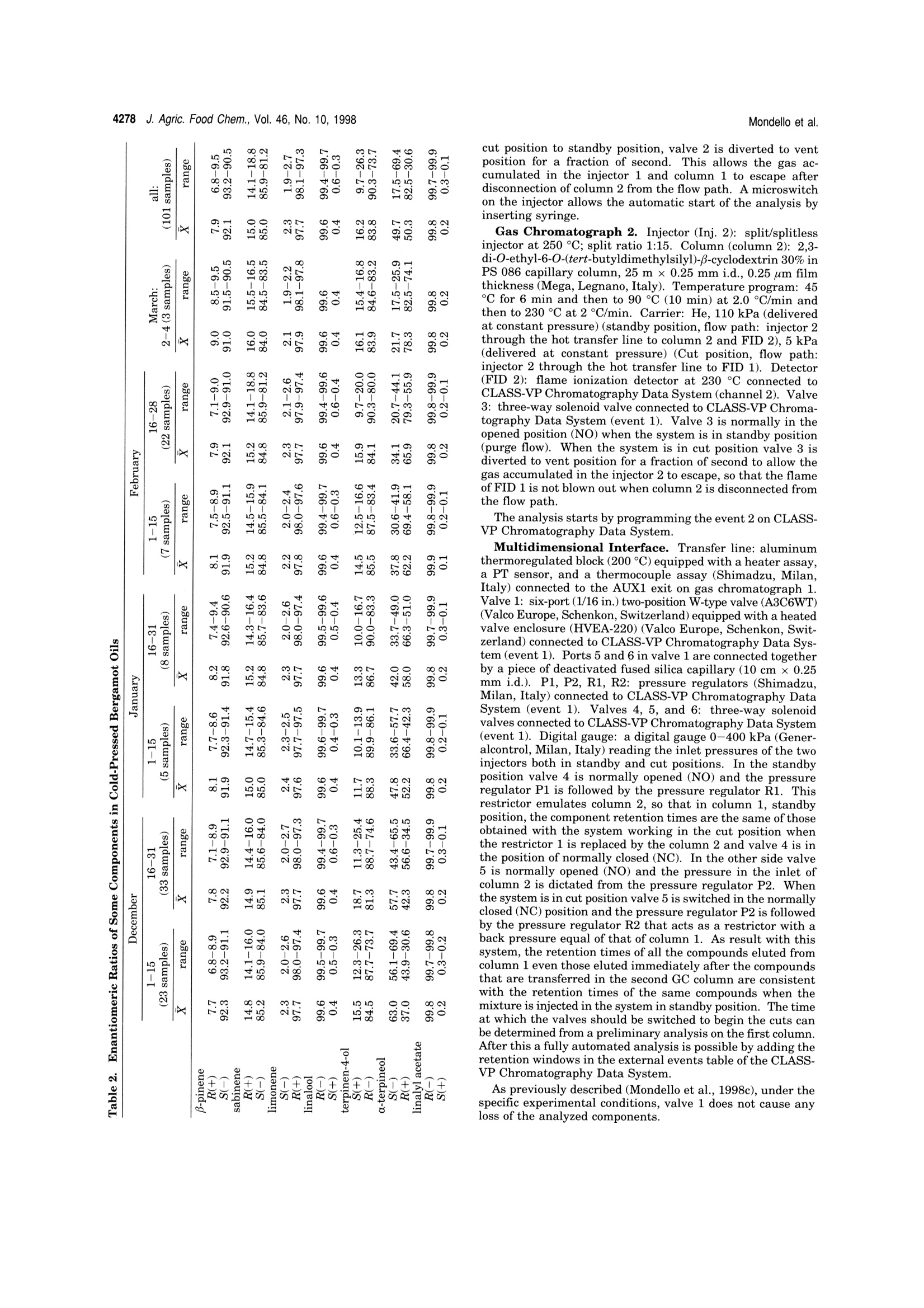
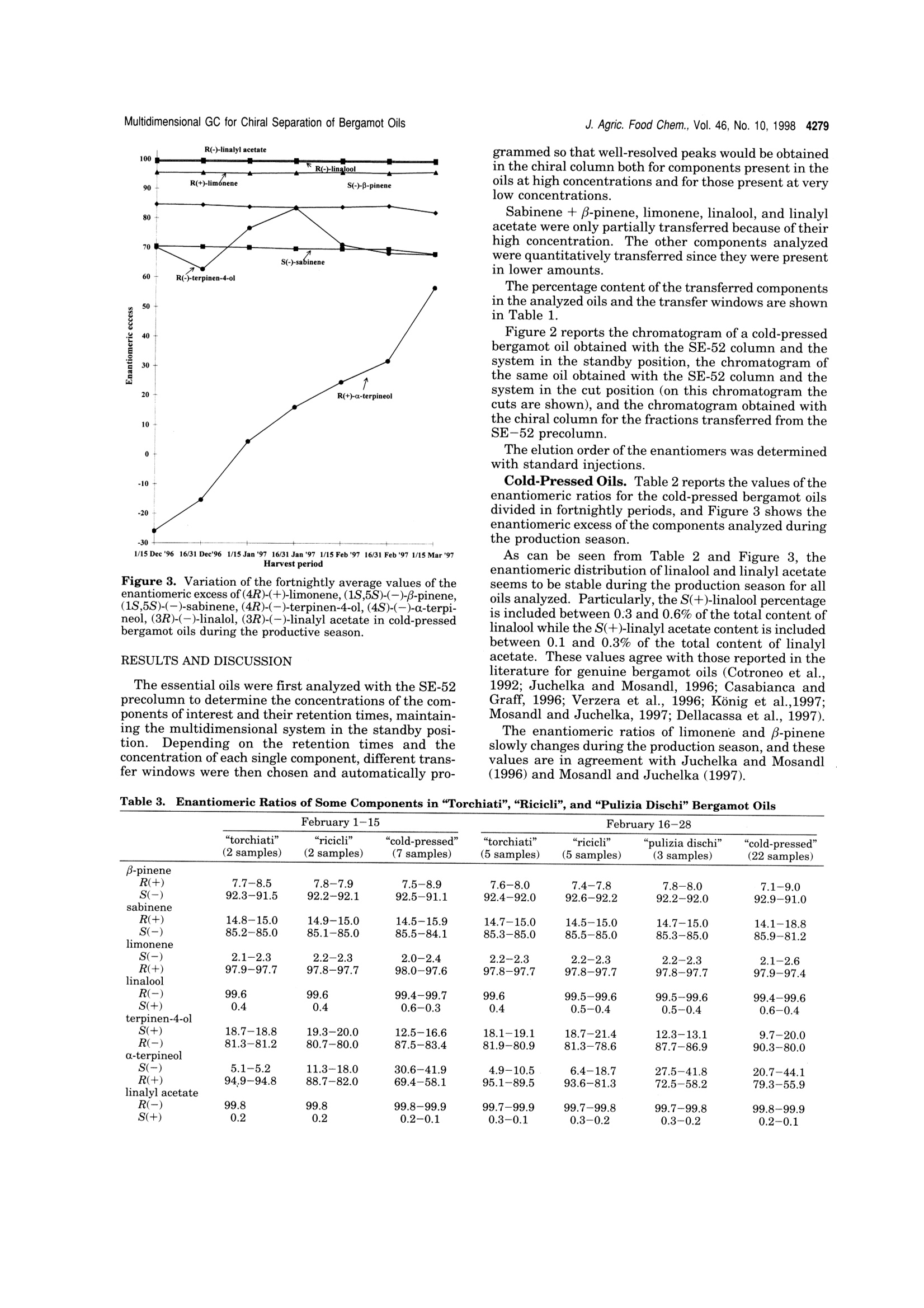
4275J. Agric. Food Chem. 1998, 46, 4275-4282 4276 J. Agric. Food Chem., Vol. 46, No. 10, 1998 10.1021/jf980228u CCC: $15.00C 1998 American Chemical SocietyPublished on Web 09/12/1998 Multidimensional Capillary GC-GC for the Analysis of ComplexSamples. 5. Enantiomeric Distribution of MonoterpeneHydrocarbons, Monoterpene Alcohols, and Linalyl Acetate ofBergamot (Citrus bergamia Risso et Poiteau) Oils Luigi Mondello,t Antonella Verzera,t Piero Previti,* Francesco Crispo, and Giovanni Dugo*f Dipartimento Farmaco-chimico and Dipartimento Farmaco-biologico, Facolta di Farmacia,Universita di Messina, viale Annunziata 98168 Messina, Italy, and Consorzio del Bergamotto,89100 Reggio Calabria, Italy The enantiomeric distribution of β-pinene, sabinene, limonene, linalool, terpinen-4-ol,a-terpineol,and linalyl acetate in cold-pressed, “torchiati","ricicli",“pulizia dischi",“distilled", and "bergapten-free" bergamot oils has been determined using a tully automated.a dnoaamanai-G Th lows fractions to be multitransferred during the same GC analysisand the use of the two GCs independently when the multitransfer option is not used. The resultsobtained allowed the characterization of the cold-pressed bergamot oils, to determine the variationsof the enantiomeric distribution of some components during the whole production season, and tocorrelate the enantiomeric ratios with the technology of oil isolation. Keywords: Multidimensional GC-GC; enantioselective gas chromatography; bergamot oils; Citrusbergamia Risso et Poiteau; monoterpene hydrocarbons; monoterpene alcohols; linalyl acetate INTRODUCTION Bergamot (Citrus bergamia Risso et Poiteau) cultiva-tion started in Italy at the beginning of the 18th century.Italian bergamot production is limited to approximately2000 hectares in a narrow strip of the Calabrian coast,near Reggio Calabria, along the Ionian and the Thyr-renian seas. The plant is also cultivated in the IvoryCoast, Brazil, Argentina, and,more recently, Uruguay(Dellacassa et al.,1997). Bergamot is mainly cultivatedfor the essential oil representing an important rawmaterial for cosmetic and food industries. In Italy, the“Consorzio del Bergamotto di ReggioCalabria"and the private industries use “Pelatricemachines to obtain the essential oil. The oil is obtainedfirst by scraping the peel fruit under streams of spoutingwater,and then, by centrifugation it is separated fromthe water and the solid residues. The centrifugation ofthe oil is accomplished in two different steps: a firstseparator, where the aqueous emulsion increases in oilcontent up to about 70-90%; and then, in a secondaryseparator, the pure essential oil is recovered. Watermust be recycled to avoid the loss of polar compounds,which are partly soluble in water. Generally, the samerecycled water is used during the entire processing day.Small amounts of less valuable bergamot oil can alsobe recovered by processing the residues of the cold-pressed oil extraction. These oils are named dependingon the process used: "Pulizia dischi”oils are oils recovered by decantactionof the liquid residues and by centrifugation of the solidresidues of the drum of the secondary separator at the ( * To whom c o rrespondence s h ould be addressed (phone, +3 9-90-356022; fax, +39-90-6766532; e-mail , gdugo@pharma. unime.it). ) ( D ipartimento Farmaco-chimico. ) ( # Dipartimento Farmaco-biologico. ) ( C o nsorzio del B ergamotto. ) end of an entire processing day. The yield of the oil, soobtained, is about 0.5% of the total oil. “Torchiati"oils are oils recovered by pressing the solidresidues of the secondary separator after centrifugationwith little hydraulic presses. The yield is 3-4% of thetotal oil. This name for citrus essential oil, other thanbergamot oils, indicates a different extraction process.In fact,"Torchiati oils” are usually those obtained bycold-pressing the whole fruit; therefore, an acid productis obtained at a pH depending on the citrus fruit (pH ofcitrus juices is always less than 3.7). In this processthe oil is separated centrifuging the acid product. “Ricicli" oils are oils recovered by centrifugation of therecycle water at the end of an entire processing day.The yield of the oil, so obtained, is about 1.0% of thetotal oil. The oils recovered using these techniques can beseparately marked or, more frequently, they are addedto cold-pressed oils. “Distilled”oils are oils recovered by distillation of thesemifluid wastes (“feccie”) ejected by the first separatorwhich is supplied with an automated discharge. Previ-ously, the distillation was carried out at atmosphericpressure while, presently, it is carried out at reducedpressure using the "Peratoner”method (Di Giacomo,1974). The yield of the oil, so obtained, is about 3% ofthe total oil. In our laboratory, bergamot volatile fraction composi-tion has been widely studied (Dugo et al., 1987, 1991;Lamonica et al., 1990; Verzera et al., 1996; Mondello etal., 1994, 1998d). During the production season largequantitative differences ofeach component are observed.Therefore quantitative data are not always useful todetermine the genuineness and the quality of a berga-mot oil,as is possible for other citrus oils, such as lemonor mandarin (Dugo, 1986; Dugo et al., 1990). Thequantitative determination of flavor components, to- Figure 1. Pneumatic and electronic scheme of the GC-GCsystem, in the standby position. gether with their enantiomeric distribution, can provideuseful information about the genuineness of the oils andtheir quality. For example, in genuine cold-pressedbergamot oils,the percentage of S(+) enantiomer, bothfor linalool and linalyl acetate, is lower than 1% of thetotal content of the two isomers (Cotroneo et al., 1992;Verzera et al., 1996; Juchelka and Mosandl, 1996;Casabianca and Graff, 1996; Konig et al., 1997; Mosandland Juchelka, 1997; Dellacassa et al., 1997); in com-mercial oils (Mosandl and Schubert, 1990; Schubert andMosandl, 1991; Bernreuther and Schreier, 1991; Wein-rich and Nitz, 1992; Casabianca and Graff, 1994, 1996;Casabianca et al., 1995; Juchelka and Mosandl, 1996;Konig et al.,1997) andh a a tilat owere chela andMosandl, 1996; Konig et al., 1997), this percentage ismore than 1%. Therefore, an S enantiomer content of linalool andlinalyl acetate of more than 1% allows the presence ofextraneous products in bergamot oil to be detected, buta value less than to 1% is not always due to a genuinebergamot oil; in fact it is possible to prepare reconsti-tuted bergamot oils in which the enantiomeric ratio oflinalool shows the same values of the genuine oils(Juchelka and Mosandl, 1996;Konig et al., 1997). In literature, the enantiomeric ratios of some mono-terpenes, such as a-pinene, B-pinene, and limonene are reported for bergamot oils (Mosandl et al., 1990;Hener et al., 1990;Mosandl,1995; Juchelka and Mosandl,1996). These results, because of the low number ofsamples which are related and their similarity to othercitrus essential oils are not able to be used to character-ize a genuine bergamot oil.In this paper, the results relative to the enantiomeric ratios of B-pinene, sabinene, limonene, linalool, ter-pinen-4-ol, a-terpineol, and linalyl acetate for cold-pressed, recovered, and bergapten-free bergamot oils are9111reported. The data can be used to determine thegenuineness and the quality of the bergamot oils.Analyses were carried out using a fully automated, multidimensional double oven GC system, made in ourlaboratory (Mondello et al., 1998e) which is an improve-ment of a system previously described (Mondello et al.,1997;1998a-c). The system, using an SE-52 precolumnand a chiral main column, allows the determination ofthe enantiomeric ratios of each component during asingle analysis.Previously, the advantages of the multidimensional system were widely discussed (Mondello et al., 1997;1998b,c,d,e) MATERIALS AND METHODS The research was carried out on 101 genuine cold-pressed bergamot oil samples obtained by“Pelatrice” machines duringthe entire 1996/97 production season; 7 “torchiati"bergamotoil samples; 7 "ricicli"bergamot oil samples; 3 “pulizia dischi"bergamot oil samples; 2 “distilled"bergamot oil samples; 7“bergapten-free"bergamot oils obtained by distillation; and 7011"bergapten-free" bergamot oil samples obtained by NaOHtreatment.The multidimensional system used in this study was an improved model (Mondello et al., 1998e), made in our labora-tory, of a developmental instrument formerly described indetail (Mondello et al., 1997, 1998a-c). The instrument setupand the experimental conditions used are described below andin Figure 1.All the samples of the essential oils were analyzed by injecting 1 uL of a 10% (v/v) solution of essential oil inn-pentane with a split ratio of 1:10. Gas Chromatograph 1. Injector (Inj. 1): split/splitless injector at 250 ℃; split ratio 1:10. Column (column 1): SE52 capillary column, 30 m x 0.32 mm i.d., 0.40-0.45 um filmIIthickness (Mega, Legnano, Italy). Temperature program: 45C for 6 min and then to 240℃ at 2.0C/min. Carrier: He,90 kPa (delivered at constant pressure) (standby position, flowpath: injector 1 to column 1 through the hot transfer line toFID 1), 170 kPa (delivered at constant pressure) (Cut position,flow path: injector 1 to column 1 through the hot transfer lineto column 2 and FID 2). Detector (FID 1): flame ionizationdetector at 280°℃ connected to CLASS-VP ChromatographyData System (channel 1) (Shimadzu, Milan, Italy). Valve 2:three-way solenoid valve (SIRAI, Milan, Italy) connected toCLASS-VP Chromatography Data System (event 1) (Shi-madzu, Milan, Italy). Valve 2 is normally in the openedposition (NO) when the system is both in standby position andin cut position (purge flow). When the system is switched from Table 1. Minimum to Maximum Percentage Values of the Analyzed Component in Bergamot Oils and Their TransferWindows “bergaptene-free” transfer B-pinene+ sabinene 4.8-12.7 7.5-9.8 6.2-9.3 6.6-8.9 4.2-7.9 7.1-7.9 6.9-8.3 15.30-16.00 limonene 25.4-45.4 29.7-33.2 30.3-34.7 27.2-34.4 23.1-35.1 32.8-35.9 33.5-35.7 20.00-20.20 linalool 3.6-22.7 5.7-10.3 7.3-11.2 7.9-12.2 25.4-36.9 6.8-7.7 6.9-7.6 25.00-25.55 terpinen-4-ol tr tr tr tr 0.4-0.5 30.60-31.20 a-terpineol tr-0.1 tr-0.1 0.1-0.5 0.1-0.3 1.3-4.0 31.60-32.35 linalyl acetate 21.8-41.4 35.5-40.1 34.3-37.9 33.2-41.8 8.9-22.8 31.3-32.6 30.2-32.8 36.80-37.10 Minutes Figure 2. (A) GC chromatogram of a cold-pressed bergamot oil obtained with (A) the SE-52 column, and (B) the SE-52 columnwith the six heart-cuts, and (C)GC-GC chiral chromatogram of the transferred components. Stars in part B show the electricspike coming from the valve switching. In the case of cut 3 (linalool) the electric spike at the beginning of the cut is not visiblebecause the peak was partially transferred and at the end of the cut is very high because under this spike there is the nonanaltse of cut 1 (sabinene+B-pi cut 2 (limonebecause the transrer or these peaks was oniy partial and these spikes are placed on tne steteals of those compoenas that are n cut position to standby position, valve 2 is diverted to ventposition for a fraction of second. This allows the gas ac-cumulated in the injector 1 and column 1 to escape afterdisconnection of column 2 from the flow path. A microswitchon the injector allows the automatic start of the analysis byinserting syringe. Gas Chromatograph 2. Injector (Inj. 2): split/splitlessinjector at 250 C; split ratio 1:15. Column (column 2): 2,3-di-O-ethyl-6-O-(tert-butyldimethylsilyl)-B-cyclodextrin 30% inPS 086 capillary column, 25 m x0.25 mm i.d., 0.25 um filmthickness (Mega, Legnano, Italy). Temperature program: 45℃ for 6 min and then to 90℃ (10 min) at 2.0C/min andthen to 230°℃ at2C/min. Carrier: He, 110 kPa (deliveredat constant pressure) (standby position, flow path: injector 2through the hot transfer line to column 2 and FID 2), 5 kPa(delivered at constant pressure) (Cut position, flow path:injector 2 through the hot transfer line to FID 1). Detector(FID2): flame ionization detector at 230 C connected toCLASS-VP Chromatography Data System (channel 2). Valve3: three-way solenoid valve connected to CLASS-VP Chroma-tography Data System (event 1). Valve 3 is normally in theopened position (NO) when the system is in standby position(purge flow). When the system is in cut position valve 3 isdiverted to vent position for a fraction of second to allow thegas accumulated in the injector 2 to escape, so that the flameofFID 1 is not blown out when column 2 is disconnected fromthe flow path. The analysis starts by programming the event 2 on CLASS-VP Chromatography Data System. Multidimensional Interface. Transfer line: aluminumthermoregulated block (200C) equipped with a heater assay,a PT sensor, and a thermocouple assay (Shimadzu, Milan,Italy) connected to the AUX1 exit on gas chromatograph 1.Valve 1: six-port (1/16 in.) two-position W-type valve (A3C6WT)(Valco Europe, Schenkon,Switzerland) equipped with a heatedvalve enclosure (HVEA-220) (Valco Europe, Schenkon, Swit-zerland) connected to CLASS-VP Chromatography Data Sys-tem (event 1). Ports 5 and 6 in valve 1 are connected togetherby a piece of deactivated fused silica capillary (10 cm x0.25mm i.d.). P1, P2, R1, R2: pressure regulators (Shimadzu,Milan, Italy) connected to CLASS-VP Chromatography DataSystem (event 1). Valves 4, 5, and 6: three-way solenoidvalves connected to CLASS-VP Chromatography Data System(event 1). Digital gauge: a digital gauge 0-400 kPa (Gener-alcontrol, Milan, Italy)reading the inlet pressures of the twoinjectors both in standby and cut positions. In the standbyposition valve 4 is normally opened (NO) and the pressureregulator P1 is followed by the pressure regulator R1. Thisrestrictor emulates column 2, so that in column 1, standbyposition, the component retention times are the same of thoseobtained with the system working in the cut position whenthe restrictor 1 is replaced by the column 2 and valve 4 is inthe position of normally closed (NC). In the other side valve5 is normally opened (NO) and the pressure in the inlet ofcolumn 2 is dictated from the pressure regulator P2. Whenthe system is in cut position valve 5 is switched in the normallyclosed (NC) position and the pressure regulator P2 is followedby the pressure regulator R2 that acts as a restrictor with aback pressure equal of that of column 1. As result with thissystem, the retention times of all the compounds eluted fromcolumn 1 even those eluted immediately after the compoundsthat are transferred in the second GC column are consistentwith the retention times of the same compounds when themixture is injected in the system in standby position. The timeat which the valves should be switched to begin the cuts canbe determined from a preliminary analysis on the first column.SAfter this a fully automated analysis is possible by adding theretention windows in the external events table ofthe CLASS-VP Chromatography Data System. As previously described (Mondello et al., 1998c), under thespecific experimental conditions, valve 1 does not cause anyloss of the analyzed components. Multidimensional GC for Chiral Separation of Bergamot Oils Figure 3. Variation of the fortnightly average values of theenantiomeric excess of (4R)-(+)-limonene,(1S,5S)-(-)-B-pinene,(1S,5S)-(-)-sabinene, (4R)-(-)-terpinen-4-ol,(4S)-(-)-a-terpi-neol, (3R)-(-)-linalol, (3R)-(-)-linalyl acetate in cold-pressedbergamot oils during the productive season. RESULTS AND DISCUSSION The essential oils were first analyzed with the SE-52precolumn to determine the concentrations of the com-ponents of interest and their retention times, maintain-ing the multidimensional system in the standby posi-tion.Depending on the retention times and theconcentration of each single component,different trans-fer windows were then chosen and automatically pro- grammed so that well-resolved peaks would be obtainedin the chiral column both for components present in theoils at high concentrations and for those present at verylow concentrations. Sabinene +β-pinene, limonene, linalool, and linalylacetate were only partially transferred because of theirhigh concentration. The other components analyzedwere quantitatively transferred since they were presentin lower amounts. The percentage content of the transferred componentsin the analyzed oils and the transfer windows are shownin Table 1. Figure 2 reports the chromatogram of a cold-pressedbergamot oil obtained with the SE-52 column and thesystem in the standby position, the chromatogram ofthe same oil obtained with the SE-52 column and thesystem in the cut position (on this chromatogram thecuts are shown), and the chromatogram obtained withthe chiral column for the fractions transferred from theSE-52 precolumn. The elution order of the enantiomers was determinedwith standard injections. Cold-Pressed Oils. Table 2reports the values of theenantiomeric ratios for the cold-pressed bergamot oilsdivided in fortnightly periods, and Figure 3 shows theenantiomeric excess of the components analyzed duringthe production season. As can be seen from Table 2 and Figure 3, theenantiomeric distribution of linalool and linalyl acetateseems to be stable during the production season for alloils analyzed. Particularly, the S(+)-linalool percentageis included between 0.3 and 0.6% of the total content oflinalool while the S(+)-linalyl acetate content is includedbetween 0.1 and 0.3% of the total content of linalylacetate. These values agree with those reported in theliterature for genuine bergamot oils (Cotroneo et al.,1992; Juchelka and Mosandl, 1996;Casabianca andGraff, 1996; Verzera et al., 1996; Konig et al.,1997;Mosandl and Juchelka, 1997; Dellacassa et al., 1997). The enantiomeric ratios of limonene and B-pineneslowly changes during the production season, and thesevalues are in agreement with Juchelka and Mosandl(1996) and Mosandl and Juchelka (1997). “torchiati” “ricicli” “cold-pressed” “torchiati” “ricicli” “pulizia dischi” "cold-pressed” (2 samples) (2 samples) (7 samples) (5 samples) (5 samples) (3 samples) (22 samples) β-pinene R(+) 7.7-8.5 7.8-7.9 7.5-8.9 7.6-8.0 7.4-7.8 7.8-8.0 7.1-9.0 S(-) 92.3-91.5 92.2-92.1 92.5-91.1 92.4-92.0 92.6-92.2 92.2-92.0 92.9-91.0 sabinene R(+) 14.8-15.0 14.9-15.0 14.5-15.9 14.7-15.0 14.5-15.0 14.7-15.0 14.1-18.8 S(-) 85.2-85.0 85.1-85.0 85.5-84.1 85.3-85.0 85.5-85.0 85.3-85.0 85.9-81.2 limonene S(-) 2.1-2.3 2.2-2.3 2.0-2.4 2.2-2.3 2.2-2.3 2.2-2.3 2.1-2.6 R(+) 97.9-97.7 97.8-97.7 98.0-97.6 97.8-97.7 97.8-97.7 97.8-97.7 97.9-97.4 linalool R(-) 99.6 99.6 99.4-99.7 99.6 99.5-99.6 99.5-99.6 99.4-99.6 S(+) 0.4 0.4 0.6-0.3 0.4 0.5-0.4 0.5-0.4 0.6-0.4 terpinen-4-ol S(+) 18.7-18.8 19.3-20.0 12.5-16.6 18.1-19.1 18.7-21.4 12.3-13.1 9.7-20.0 R(-) 81.3-81.2 80.7-80.0 87.5-83.4 81.9-80.9 81.3-78.6 87.7-86.9 90.3-80.0 a-terpineol S(-) 5.1-5.2 11.3-18.0 30.6-41.9 4.9-10.5 6.4-18.7 27.5-41.8 20.7-44.1 R(+) 94.9-94.8 88.7-82.0 69.4-58.1 95.1-89.5 93.6-81.3 72.5-58.2 79.3-55.9 linalyl acetate R(-) 99.8 99.8 99.8-99.9 99.7-99.9 99.7-99.8 99.7-99.8 99.8-99.9 S(+) 0.2 0.2 0.2-0.1 0.3-0.1 0.3-0.2 0.3-0.2 0.2-0.1 Table 4. Enantiomeric Ratios of Some Components in“Distilled” Bergamot Oils distillation at atmospheric “feccie”oil distillation at reduced cold-pressed oils (all samples) pressure, 04/29/97 pressure, 04/29/97 range β-pinene R(+) 8.9 8.2 7.9 6.8-9.5 S(-) 91.1 91.8 92.1 93.2-90.5 sabinene R(+) 15.9 15.2 15.0 14.1-18.8 S(-) 84.1 84.8 85.0 85.9-81.2 limonene S(-) 2.0 2.3 2.3 1.9-2.7 R(+) 98.0 97.7 97.7 98.1-97.3 linalool R(-) 81.6 98.7 99.6 99.4-99.7 S(+) 18.4 1.3 0.4 0.6-0.3 terpinen-4-ol S(+) 31.8 27.1 16.2 9.7-26.3 R(-) 68.2 72.9 83.8 90.3-73.7 a-terpineol S(-) 26.6 11.2 49.7 17.5-69.4 R(+) 73.4 88.8 50.3 82.5-30.6 linalyl acetate R(-) 98.9 99.1 99.8 99.7-99.9 S(+) 1.1 0.9 0.2 0.3-0.1 Table 5. Enantiomeric Ratios of Some Components in“Bergapten-Free”Bergamot Oils, Both Distilled and Treatedwith NaOH treated with NaOH cold-pressed oils (all samples) distilled range β-pinene R(+) 8.2-8.7 8.1-9.2 7.9 6.8-9.5 S(-) 91.8-91.3 91.9-90.8 92.1 93.2-90.5 sabinene R(+) 15.3-15.7 15.1-16.0 15.0 14.1-18.8 S(-) 84.7-84.3 84.9-84.0 85.0 85.9-81.2 limonene S(-) 2.1 2.1-2.2 2.3 1.9-2.7 R(+) 97.9 97.9-97.8 97.7 98.1-97.3 linalool R(-) 99.6 99.5-99.6 99.6 99.4-99.7 S(+) 0.4 0.5-0.4 0.4 0.6-0.3 terpinen-4-ol S(+) 18.4-20.1 19.8-21.5 16.2 9.7-26.3 R(-) 81.6-79.9 80.2-78.5 83.8 90.3-73.7 a-terpineol S(-) 31.4-52.1 41.4-50.5 49.7 17.5-69.4 R(+) 68.6-47.9 58.6-49.5 50.3 82.5-30.6 linalyl acetate R(-) 99.7-99.8 99.7-99.9 99.8 99.7-99.9 S(+) 0.3-0.2 0.3-0.1 0.2 0.3-0.1 The values of the enantiomeric ratio oflimonene (1.9/98.1 to 2.7/97.3) are similar to those of lemon (1.5/98.5to 2.0/98.0) (Mondello et al.,1998e) and mandarin oils(2.0/98.0 to 2.3/97.7) (Mondello et al., 1998a), while thevalues of B-pinene (6.8/93.2 to 9.5/90.5) are similar tothose of lemon oils (4.2/95.8 to 7.0/93.0) (Mondello etal., 1998e) but very different from those of mandarinoils (97,0/3.0 to 98.8/1.2)(Mondello et al., 1998a). The enantiomeric ratio of sabinene seems to be stableenough during the production season; the ratio R(+)/S(-)-sabinene is included from 14.1/85.9 to 18.8/81.2.These values are similar to those of lemon oils (13.3/86.7 to 15.3/84.7) (Mondello et al., 1998e) and differentfrom those of mandarin oils (76.2/23.8 to 80.5/19.5)(Mondello et al., 1998a), from those of sweet orange oils(94.6/5.4 to 97.9/2.1) and from those of bitter orange oils(44.4/55.6 to 54.5/45.5) (Mondello et al., 1997). The enantiomeric ratio of S(+)/R(-)-terpinen-4-olchanges irregularly during the production season. Thevalues of S(+)/R(-)-terpinen-4-ol enantiomeric ratio inbergamot oils (9.7/90.3 to 26.3/73.7) are similar to those of mandarin oils (10.0/90.0 to 19.2/81.8)(Mondello et al.,1998a) and to those of lemon oils (15.9/84.1 to 26.7/73.3)(Mondello et al., 1998e). The enantiomeric ratio of S(-)/R(+)-a-terpineolchanges greatly during the production season and itsfortnightly average values are included from 63.0/37.0at the beginning of the season to 21.7/78.3 at the end ofthe season. Lemon and mandarin oils always have ahigh content of S(-)-a-terpineol isomer (Mondello et al.,1998a,e). With regard to the values of sabinene, terpinen-4-ol,and a-terpineol enantiomeric ratios no data are reportedin the literature, except for our preliminary results Table 3 reports the values of the enantiomeric ratiosfor “pulizia dischi”, “torchiati”, and “ricicli”oils. Thesame table compares the results obtained for cold-pressed oils produced in the same period. “Pulizia Dischi”Oil. As can be seen from Table 3,values of the enantiomeric distribution for all the“pulizia dischi"oils are in the ranges observed for cold- pressed oils produced in the same period, so the systemused for the recovery of these oils does not seem toinfluence the enantiomeric distribution of the compo-nents analyzed. “Torchiati”Oils. As can be seen from Table 3, theenantiomeric distribution of β-pinene, sabinene, li-monene, linalool, and linalyl acetate for sample pro-duced in the first half and in the second half ofFebruary, are in the ranges observed for the cold-pressed oils produced in the same period. The isomerS(+)-of terpinen-4-ol shows values for"torchiati oils”produced in the first half of February, higher than thehighest value shown by cold-pressed oils, while for"torchiati oils”produced in the second half of February,it shows values near to the highest value shown by cold-pressed oils produced in the same period. This resultseems to show that the contact with the solid residues.that are acidic, before the recovery of the oil by pressing,causes a partial racemization of terpinen-4-ol. The S(-) isomer of a-terpineol for “torchiati oils”produced both in the first and in the second half ofFebruary, shows values much lower than the minimumvalues presented by a cold-pressed oils. “Ricicli”Oils”.As can be observed in Table 3.“ricicli”oils behave as "torchiati"oils. In fact, terpinen-4-ol shows a slight trend to the racemization, while theS(-)isomer of a-terpineol shows values lower than thelowest values of cold-pressed oils produced in the sameperiod. The behavior of a-terpineol in "torchiati”oils and“ricicli"oils cannot be explained with the contact of theoil before its recovery with the acid, solid, and liquidresidues. This could have caused a trend to the race-mization but not the increase of the enantiomeric excessof R(+) isomer. In fact, as observed in our laboratory,the warm treatment in an aqueous medium acidic forcitric acid (pH 3) of a mixture of S(-)- and R(+)-a-terpineol (30:70) led to the racemization after 4 h. This phenomenon can be explained with the hypoth-esis that R(+)-a-terpineol can be produced by microor-ganisms. And in fact, recycling water and solid residuesof the cold extraction are good culture mediums (Mur-dock et al., 1969). Another hypothesis could be that ofthe acid or enzymatic hydrolysis of glycosidically bondedR(+)-a-terpineol. In fact, the analyzed percentage ofa-terpineol in “torchiati” and in “ricicli”oils variesbetween 0.1 and 0.47 and between 0.1 and 0.30 respec-tively, while in cold-pressed oils produced in the sameperiod ranged between 0.04 and 0.12. “Distilled” Oils.. Table 4 reports values of theenantiomeric distribution of two oils recovered by distil-lation of the semifluid wastes ejected by the firstseparator. The same table compares the results ob-tained for cold-pressed bergamot oils. As can be seen from Table 4, the enantiomericdistribution of β-pinene, sabinene, and limonene is notinfluenced by the distillation process. Linalool, linalylacetate, and terpinen-4-ol show a trend to the racem-ization. For the linalool this trend was previouslyobserved by other authors (Konig et al.1997; Hener etal., 1990). The phenomenon is more evident for thesample obtained by distillation at atmospheric pressure.In this sample, S(-)-a-terpineol shows a value lowerthan the lowest values observed for the cold-pressedbergamot oils, as previously observed for"torchiati”and“ricicli" oils. In the sample obtained by distillation atreduced pressure this latter phenomenon is not evident. “Bergapten-Free” Oils. Table 5 reports values ofthe enantiomeric distribution of“bergapten-free"ber-gamot oils, obtained by distillation or by treatment withNaOH. The same table reports the results obtained forcold-pressed bergamot oils. As can be seen from Table5, the enantiomeric ratios of all the samples of“ber-gapten-free" oil are in the ranges observed for cold-pressed oils, so the systems used for the elimination ofbergapten do not seem to influence the enantiomericdistribution of the components under examination. ACKNOWLEDGMENT We thank Shimadzu Italia for their cooperationduring the development of this work. LITERATURE CITED Bernreuther, A.; Schreier, P. Multidimensional Gas Chroma-tography/Mass Spectrometry: a powerful tool for the directchiral evaluation of aroma compounds in plant tissues. II.Linalool in essential oil and fruits. Phytochem. Anal. 1991,2,167-170. Casabianca, H.; Graff,J. B. Separation of linalyl acetateenantiomers: application to the authentication of bergamotfood products. J. High Resolut. Chromatogr. 1994,17,184-186. Casabianca, H.; Graff,J. B. Chiral analysis of linalool andlinalyl acetate in various plants. EPPOS 1996, 7, 227-243. Casabianca, H.; Graff, J. B.; Jame, P.; Perrucchietti, C.;Chastrette, M. Application of hyphenated techniques to thechromatographic authentication of flavours in food productsand perfumes. J. High Resolut. Chromatogr. 1995, 18, 279-285. Cotroneo, A.; Stagno d'Alcontres, I.; Trozzi, A. On the genuine-ness of citrus essential oils. Part XXXIV. Detection of addedreconstituted bergamot oil in genuine bergamot oil by high-resolution gas chromatography with chiral capillary col-umns. Flauour Fragrance J. 1992, 7, 15-17. Dellacassa, E.; Lorenzo,D.;Moyna, P.; Verzera, A.; Cavazza,A. Uruguayan essential oils. Part. V. Composition of ber-gamot oil. J. Essent. Oil Res. 1997,9,419-426. Di Giacomo, A. Gli olii essenziali degli agrumi (Citrus essentialoils);EPPOS: Milan, Italy, 1974. Dugo, G.L’huile essentielle de citron sicilien (The essentialoil of Sicilian lemons). Parfums, Cosmet., Aromes 1986, 68,95-105. ( Dugo, G.; Lamonica, G. ; Cotroneo, A.; Tr o zzi, A. ; Crispo, F. ; Licandro, G .; Gioffre, D. Nota XVII. La composizione dellafrazione v olatile dellessenza di bergamotto ( O n the ge n u- i neness of c itrus essentials oils. Part XVII. Composition o f t he v olatile f raction of the e s sential o i l o f t h e C a labrian bergamot). E ssenze, Deriv. Agrum. 1 987, 57, 456- 5 34. ) ( Dugo, G .; Rouzet, M., Verzera, A.; Cotroneo,A.; Merenda, I.La p urete des essences d'agr m u e m s e . s. w o N o o n t e e o X y X . I V c . o C e o r m en po d s a i ron tionde I huile essentielle italienne de mandarine (On t he genu- ineness of citrus essential oils. Part XXIV. C omposition of y umycosmeractomes iso. saan mandarin orange). P a r ) Dugo, G.; Cotroneo, A.; Verzera, A.; Donato, M. G.; Del Duce,R.; Licandro, G.; Crispo, F. Genuineness characters ofCalabrian bergamot essential oil. Flauour Fragrance J.1991,6,39-56. ( Hener, U.; H ollnagel, A.; Kreiss, P. ; Maas, B. ; Schmarr, H.G.; Schubert, V.;Rettinger,K.; Weber, B.; Mosandl, A. Directenantiomer s e paration o f chiral v o latiles from c o mplexmatrixes b y m ultidimensional gas c h romatography. InFlauor S cience a nd Technology; W i ley: C h ichester, WestSussex, England,1990. ) ( Juchelka, D .; Mosandl, A . Authenticity p rofiles of bergamotoil. Pharmazie 1996,51, 417 - 422. ) ( K onig, W. A.; F ricke, C.; Saritas, Y.; Momeni,B.; H ohenfeld,G. Adulteration or natural variability? Enantioselective gas ) chromatography in purity control of essential oils. J.HighResolut. Chromatogr. 1997,20, 55-61. Lamonica, G.; Stagno d'Alcontres, I.; Donato, M. G.; Merenda,I. On the Calabrian bergamot essential oil. Chim. Oggi 1990,5,59-63. Mondello, L.; Bartle, K. D.; Dugo, P.; Gans, P.; Dugo, G.Automated LC-GC; a powerful method for essential oilsanalysis. Part ⅣV. Coupled LC-GC-MS (ITD) for bergamotoil analysis. J. Microcolumn Sep. 1994, 6,237-244. Mondello, L.; Catalfamo, M.; Dugo, P.; Proteggente, A. R.;Dugo, G. La gascromatografia multidimensionale per lanalisidi miscele complesse. Nota preliminare. Determinazionedella distribuzione enantiomerica di componenti degli oliessenziali agrumari (Multidimensional capillary GC/GC forthe analysis of real complex samples. Enantiomeric distri-bution of some components of citrus essential oil). Essenze,Deriu. Agrum.1997, 67, 62-85. Mondello, L.; Catalfamo, M.; Proteggente, A. R.; Bonaccorsi,I; Dugo, G. Multidimensional capillary GC-GC system forthe analysis of real complex samples. 3. Enantiomericdistribution of monoterpene hydrocarbons and monoterpenealcohols of mandarin oils. J. Agric. Food Chem. 1998a,46,54-61. Mondello, L.; Catalfamo, M.;Dugo, G.; Dugo, P. Multidimen-sional capillary GC-GC for the analysis of real complexsamples. Part II. Enantiomeric distribution of monoterpenehydrocarbons and monoterpene alcohols of cold-pressed anddistilled lime oils. J. Microcolumn Sep. 1998b,10,203-212. Mondello, L.; Catalfamo,M.; Dugo, P.; Dugo, G. Multidimen-sional capillary GC-GC system for the analysis of realcomplex samples. Part I. Development of a fully automatedGC-GC system. J. Chromatogr. Sci. 1998c, 36,201-209. Mondello,L.; Dugo, P.; Cotroneo, A.; Proteggente, A. R.; Dugo,G. Multidimensional advanced techniques for the analysisof bergamot oil. EPPOS 1998d, in press. Mondello L.; Catalfamo M.; Cotroneo A.; Dugo G.; DugoGiacomo; McNair H. Multidimensional capillary GC-GC forthe analysis of real complex samples. Part IV. Enantiomericdistribution of monoterpene hydrocarbons and monoterpene alcohols of lemon oils. J. High Resolut. Chromatogr.1998e,submitted for publication. Mosandl, A. Enantioselective capillary gas chromatographyand stable isotope ratio mass spectrometry in the authentic-ity control of flavours and essential oils. Food Reu. Int. 1995,11(4),597-664. (4Mosandl, A.; Shubert, V. Stereoisomeric flavour compoundsXXXVII: Enantiomer separation of 1-alken -3-yl esters andtheir chirality evaluation from essential oils using multi-dimensional gas chromatography (MDGC). J. Essent. OilRes. 1990, 2, 121-132. Mosandl, A.; Juchelka, D. Advances in the AuthenticityAssessment of Citrus Oils.B .J. Essent. Oil Res.1997,9,5-12.Mosandl, A.; Hener,U.; Kreis, P.; Schmarr, H. G. Enantiomeric distribution of a-pinene, p-pinene and limonene in essentialoils and extracts. Part I. Rutaceae and Gramineae. FlauourFragrance J. 1990, 5,193-199. Murdock, D. I.;Hunter, G. L. K.; Buceck, W. A.; Brent,J. A.Relation of bacterial contamination in orange oil recoverysystem to quality of finished product. Food Technol. 1969,23(5),98-100. Shubert, V.; Mosandl, A. Chiral compounds of essential oils.VIII: stereodifferentiation of linalool using multidimen-11Isional gas chromatography. Phytochem. Anal. 1991,2,171-174. Verzera, A.;Lamonica, G.; Mondello, L.; Trozzi, A.; Dugo, G.The composition of bergamot oil. Perfum. Flauor. 1996,21,19-34. Weinrich, B.; Nitz, S. Influences of processing on the enan-tiomeric distribution of chiral flavour compounds. Part A:Linalyl acetate and terpene alcohols. Chem. Mikrobiol.Technol. Lebensm. 1992, 14,117-124. Received for review March 9, 1998. Revised manuscriptreceived July 6, 1998. Accepted July 9, 1998. This researchwas supported by“Ministero dellUniversita e della RicercaScientifica"of Italy (60% and 40% research funds). ( JF980228U )
确定





还剩6页未读,是否继续阅读?
华明科技(香港)有限公司为您提供《佛手柑油中使用多维毛细管分析检测方案 》,该方案主要用于日用化学品/香精香料中使用多维毛细管分析检测,参考标准--,《佛手柑油中使用多维毛细管分析检测方案 》用到的仪器有
相关方案
更多








