
Distilled lime oil is obtained from the distillation of....
方案详情

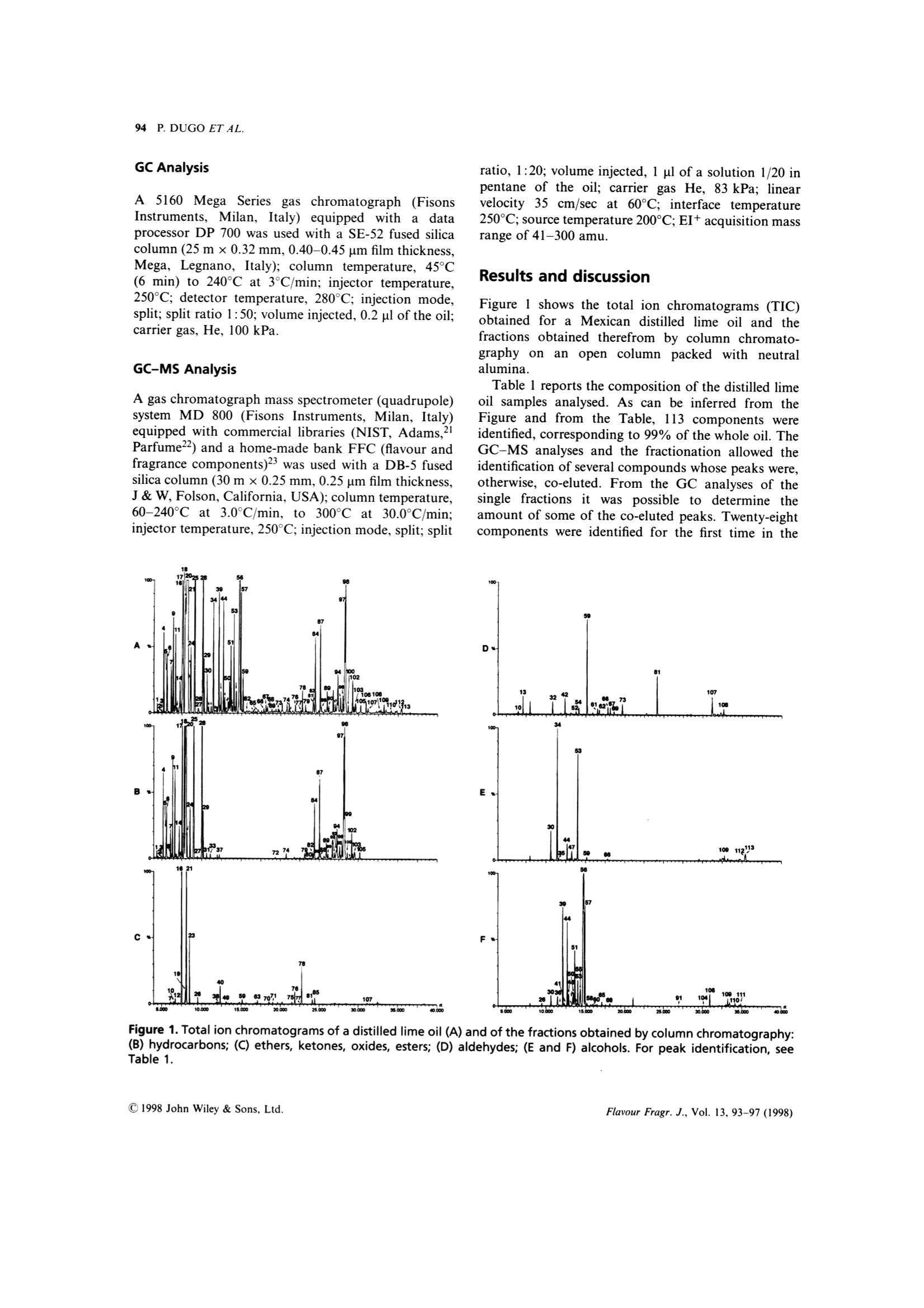
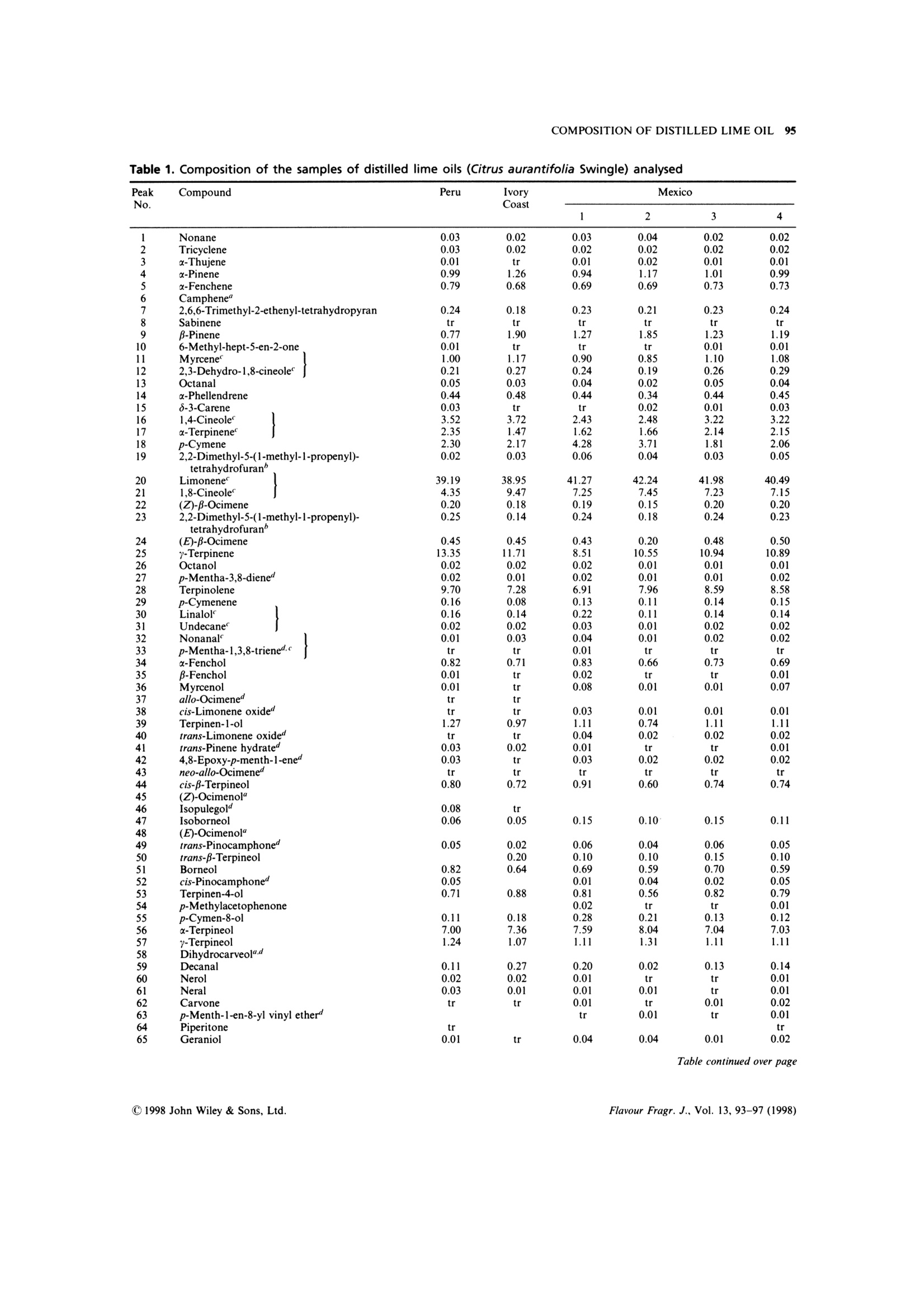
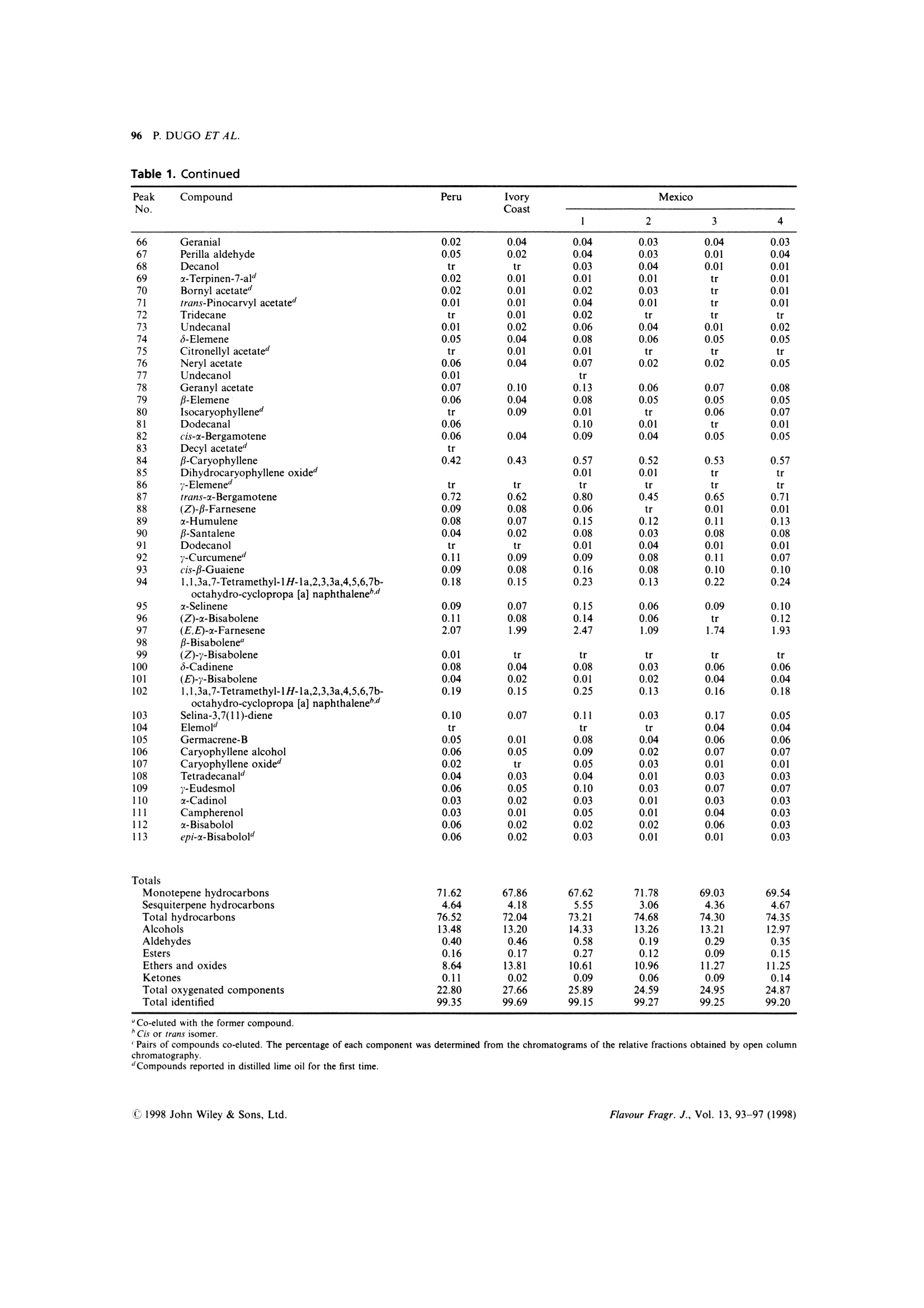
FLAVOUR AND FRAGRANCE JOURNAL, VOL. 13,93-97 (1998) 94 P. DUGO ET AL. On the Genuineness of Citrus Essential Oils. Part LVII.+The Composition of Distilled Lime Oil Paola Dugo,1 Antonella Cotroneo,2* lvana Bonaccorsi² and Luigi Mondello 1Dipartimento di Chimica Organica e Biologica, Facolta di Scienze MM.FF.NN.,Universita di Messina, Salita Sperone 98165,Messina, Italy Dipartimento Farmaco-chimico, Facolta di Farmacia, Universita di Messina, Viale Annunziata 98168, Messina, Italy .Received 22 March 1997 Accepted 22 May 1997 ABSTRACT: The composition of distilled lime oil has been investigated. The analytical techniques used tofractionate the oils and identify each component were: open column chromatography, gas chromatography, gaschromatography-mass spectrometry. Among the 113 compounds identified, 28 are reported to be presentin distilled lime oil for the first time. The main differences between the cold-pressed and the distilled lime oil arealso discussed.C 1998 John Wiley & Sons, Ltd. Flavour Fragr. J., 13, 93-97 (1998) KEY WORDS: Citrus aurantifolia Swingle; lime;distilled lime oil composition; column chromatography;HRGC-MS Introduction Distilled lime oil is obtained from the distillation of theoil/juice emulsion produced by pressing whole lime,Citrus aurantifolia Swingle, fruits2.3 through a screw-press which crushes the fruits. The complexity of itscomposition4-20 is due to the reactions, catalysed by theacidic medium, that occur during the distillation. The chemistry involved during these transformationshas been the subject of different studies,11,19,20 mainlyregarding the monoterpene bicyclic hydrocarbonsa-thujene, u-pinene, -pinene and sabinene and theisomerization and hydration reactions of limoneneforming alcohols, aldehydes and oxides.l Moreover,under the conditions used for the distillation, neral andgeranial undergo cyclization/oxidation and polymer-ization reactions. The complexity of the distilled limeoil makes it difficult to resolve and identify all of thecomponents in a single gas chromatographic analysis.Therefore, we first separated the oil into simplermixtures by column chromatography before gaschromatographic analysis, avoiding the inconveniencerepresented by too many overlapping peaks.17,20 This research note gives the composition of severaldistilled lime oils of different geographical origins,obtained by opencolumnchromatography,gas ( "Correspondence to: A. Cotroneo ) ( tFor Part L VI,see Ref. 1 ) ( Contract grant sponsor: Ministero de l l'Universita e della Ricerca Scientifica eTecnologica, Italy. ) chromatography and gas chromatography-mass spec-trometry. Experimental The research was carried out on six distilled lime oilsamples, four of which were obtained from Mexico, onefrom Peru and one from the Ivory Coast. All of thesamples were analysed by GC and GC-MS. One of theMexican samples and the samples from Peru and IvoryCoast were also fractionated on a column packed withneutral Al,O,. The fractions so obtained were analysedby GC-MS in the same conditions used for the wholeoils. Column Chromatography The essential oil (100 pl) was introduced on to a column(1.6 cm i.d.) containing neutral alumina, grade IIactivity (20 g) in light petroleum (b.p. 30-50℃).Elution with light petroleum (150 ml) gave fraction 1(hydrocarbons); elution with light petroleum:diethylether (90:10 v:v, 100 ml) gave fraction 2 (ethers,ketones, oxides,esters); elution with light petroleum:-diethyl ether (80:20, 100 ml) gave fraction 3 (alde-hydes); elution with light petroleum:diethyl ether(50:50,100 ml) gave fraction 4 (alcohols); elution withdiethyl ether (100 ml) gave fraction 5 (alcohols). GC Analysis A5160 Mega Series gas chromatographh (FisonsInstruments, Milan, Italy) equipped with a dataprocessor DP 700 was used with a SE-52 fused silicacolumn (25 m x0.32 mm, 0.40-0.45 um film thickness,Mega, Legnano, Italy); column temperature, 45C(6 min) to 240℃ at 3°℃/min; injector temperature,250C; detector temperature, 280℃; injection mode,split; split ratio 1:50; volume injected, 0.2ul of the oil;carrier gas, He, 100 kPa. GC-MS AnalySis A gas chromatograph mass spectrometer (quadrupole)system MD 800 (Fisons Instruments, Milan, Italy)equipped with commercial libraries (NIST, Adams,Parfume²2) and a home-made bank FFC (flavour andfragrance components)23 was used with a DB-5 fusedsilica column (30 m ×0.25 mm, 0.25 pm film thickness,J & W, Folson, California, USA); column temperature,60-240℃ at 3.0℃/min, to 300℃ at 30.0°℃/min:injector temperature, 250°C; injection mode, split; split ratio, 1:20; volume injected, 1 pl of a solution 1/20 inpentane of the oil; carrier gas He, 83 kPa; linearvelocity 35 cm/sec at 60℃; interface temperature250℃; source temperature 200℃; EI+acquisition massrange of 41-300 amu. Results and discussion Figure 1 shows the total ion chromatograms (TIC)obtained for a Mexican distilled lime oil and thefractions obtained therefrom by column chromato-graphy on anopen column packed with neutralalumina. Table 1 reports the composition of the distilled limeoil samples analysed. As can be inferred from theFigure and from the Table, 113 components wereidentified, corresponding to 99% of the whole oil. TheGC-MS analyses and the fractionation allowed theidentification of several compounds whose peaks were,otherwise, co-eluted. From the GC analyses of thesingle fractions it was possible to determine theamount of some of the co-eluted peaks. Twenty-eightcomponents were identified for the first time in the Fiqure 1. Total ion chromatograms of a distilled lime oil (A) and of the fractions obtained by column chromatography:(B) hydrocarbons; (C) ethers, ketones, oxides, esters; (D) aldehydes; (E and F) alcohols. For peak identification, seeTable 1. Table 1. Composition of the samples of distilled lime oils (Citrus aurantifolia Swingle) analysed Table 1. Continued Pairs of compounds co-eluted. The percentage of each component was determined from the chromatograms of the relative fractions obtained by open columnchromatography.“Compounds reported in distilled lime oil for the first time. Table 2. Average composition in classes of substances ofKey lime cold-pressed oil, type A, and distilled lime oil Cold pressed lime Distilled oil type A" lime oil (%) (%) Monoterpene hydrocarbons 86.33 69.58 Bicyclic monoterpene hydrocarbons 26.22 3.17 Sesquiterpene hydrocarbons 7.61 4.41 Monoterpene aldehydes 2.77 0.09 Aliphatic aldehydes 0.55 0.29 Alcohols 1.43 13.41 . Esters 0.33 0.16 Ethers and oxides tr 11.09 Ketones 0.03 0.08 “See reference 24. See Table 1. distilled lime oil. From the data reported it is seen thatthe samples analysed differ little in their qualitativecomposition. Their quantitative composition,withregard to the major components, is also very similar. Monoterpene hydrocarbons represent 68-72% ofthe whole oil, while the sesquiterpenes vary between3.1% and 5.5%. Aldehydes and esters only account for0.2-0.6% and 0.1-0.3% respectively. Alcohols andethers are the most abundant oxygenated components;alcohols were around 13% of the whole oil; ethers andoxides were between 9% and 14%, respectively. The composition of distilled lime oil differs greatlyfrom the volatile composition of cold-pressed lime oils.Such differences are seen in Table 2, where the averagecompositions in classes of substances of the two oils arecompared. Our results agree with those of Clark andChamblee.19,20 The mean percentage of monoterpene hydrocarbonsin the distilled lime oils analysed is about 70% whilein cold-pressed Key lime oils, type A, previouslyanalysed,24 it is about 86%. These differences aremainly due to monoterpene bicyclic hydrocarbons suchas sabinene, β-pinene, a-thujene and a-pinene; in factthese compounds, in heated aqueous medium, veryeasily undergo isomerization and hydration reac-tions19.25-28 and therefore tend to disappear in distilledoils. The most abundant monoterpene hydrocarbon,limonene, even if involved in transformation processesthat occur during the distillation, does not change verydrastically in content. Sesquiterpene hydrocarbons, which in cold-pressedoils accounts for 7.6%, drop to 4.4% in the distilledoils. These differences are mostly due to loss of-caryophyllene, trans-a-bergamotene, germacrene-Dand germacrene-B. Aliphatic aldehydes in distilled oils are about 50%less than in cold-pressed oils. Monoterpene aldehydesundergo cyclization and oxidation reactions, there-fore their content in distilled oils is about 30% less thanthe values observed for cold-pressed oils.24 The mainproducts formed by the acidic catalyzed reaction ofhydrocarbons and aldehydes are alcohols and oxides,as is evident from the high content of these componentsin distilled lime oil. Acknowledgements - This research was supported by Ministerodell'Universita e della Ricerca Scientifica e Tecnologica of Italy(60% and 40% research funds). The coordinator of the researchgroups was Professor Giovanni Dugo. References ( 1. P art LVI: A. V erzera, A. Trozzi, G. Licandro and S . Scuderi, E ssenz. Deriv. Agrum., 66,353(1996). ) L. Haro-Guzman, Essenz. Deriv. Agrum. 50, 332(1980) ( N. A nand a n d C . L . G r een, I F EAT/CITRAG InternationalConference on C itrus Oils, Taormina, I taly (November 1987). ) ( )(1943) ) ( . E. Kovats, Helv. Chim. Acta, 46, 2 705(1963). ) ( J. Perez Zayas and R . Tapanes, R e v. C E NIC, Cienc. F i s ., 5 ( 1), 1 (1974). ) ( 9 . R . T a panes, Re v . CENIC, Cien c . Fis . , 5(1) , 13 (1974). ) ( 10. M . A. Azzouz and G. A. R e i neccius,J. F o od. Sci. , 4 1 , 3 24 ( 1 976). ) ( 11. D . McHale, 8th International Congress of Essential Oils, C a nnes, F rance (October 1 980). ) ( 12. 1 M. G. Moshonas and P. E. Shaw, J. Agric. Food. Chem., 28 680 ( 1980). ) ( . J . A . P ino and R . Tapanes, J. Food. Technol., 18, 523 (19 8 3). + A . nalytical M ethods Committee, Analyst, 1 0 9, 1 3 43 ( 19 84). ) ( B4 R .T. Alessandro,J. M. Adams and M. A. M i skiewicz, J. A ssoc. O ff. Anal. Chem., 68, 1 1 54 (1985). ) ( 16. . L. Haro and W. E. Faas, Pe r f. Flav., 10(5), 67 ( 1985). ) ( 17. T. S . Chamblee, B. C. C lark, T. Radford a nd G. A. Iacobucci, J. Chromatogr., 330, 141(1985). ) ( . D. S. Khurdiya a nd M. L . Maheshwari, Pa f ai J., (2), 25 (1988). . B. C. Clark and T . S . Chamblee, i n O f-flavors in Food and B everages, E lsevier, Amsterdam ( 1 992). ) 20T..S.Chamblee and B. C. Clark, J. Essent. Oil Res.,9,267(1997). ( 21. R 1 . P. Adams, Id e ntification of Essential Oils Components by GasChromatography/Mass Spectroscopy, Allured Publishing, CaroB S tream, IL (1995). ) ( 22. 1 F lavor a nd F r agrance Library, C . E. In s truments, Ro d ano, M ilano, Italy (1995). ) ( 23. L 1 . Mondello, P. D ugo, A. B a sile, G. D ugo and K . D . B a rtle,J. Microcol. S ep. 7, 581 (1995). ) ( 24. P 1 . D ugo, L. M ondello,G. Lamonica a n d G . D u go,J. Agric.Food Chem., 45, 3608 (1997). ) ( 25 . C. M. W i lliams and D. Whittaker,J. Chem. Soc. (B), 668 (1971). ) 26.. C. M. Williams and D. Whittaker, J. Chem. Soc.(B),672(1971). ( 27. M . A. Cooper, C. M. H o lden, P . L o ftus and D . W h ittaker, J . C hem. S o c. Perkin Trans., I I , 665 (1971). ) ( 28 A . . V e rzera, I. Stagno d 'Alcontres, M. G. D onato, R. Del Duce a nd C. Gigliotti, Industrie delle Bevande, 119, 379 (1992). ) CJohn Wiley & Sons, Ltd.CCC Flavour Fragr. J., Vol. (C John Wiley & Sons, Ltd.
确定


还剩3页未读,是否继续阅读?
扬州华明仪器设备有限公司为您提供《蒸馏油中组合物分析检测方案 》,该方案主要用于日用化学品/香精香料中组合物分析检测,参考标准--,《蒸馏油中组合物分析检测方案 》用到的仪器有
相关方案
更多







