
方案详情
文
the enantiomeric of the components of essential oils ...
方案详情

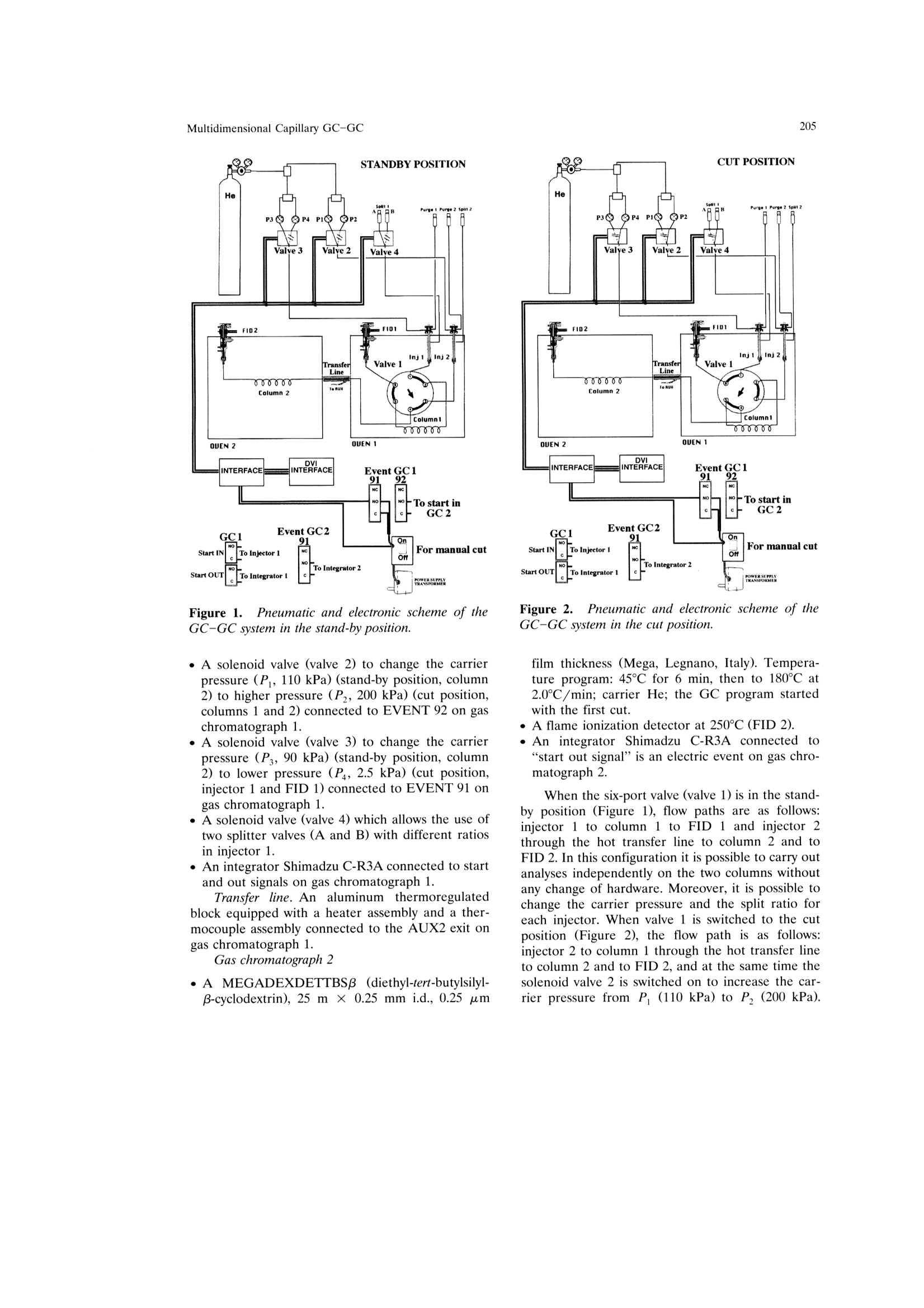
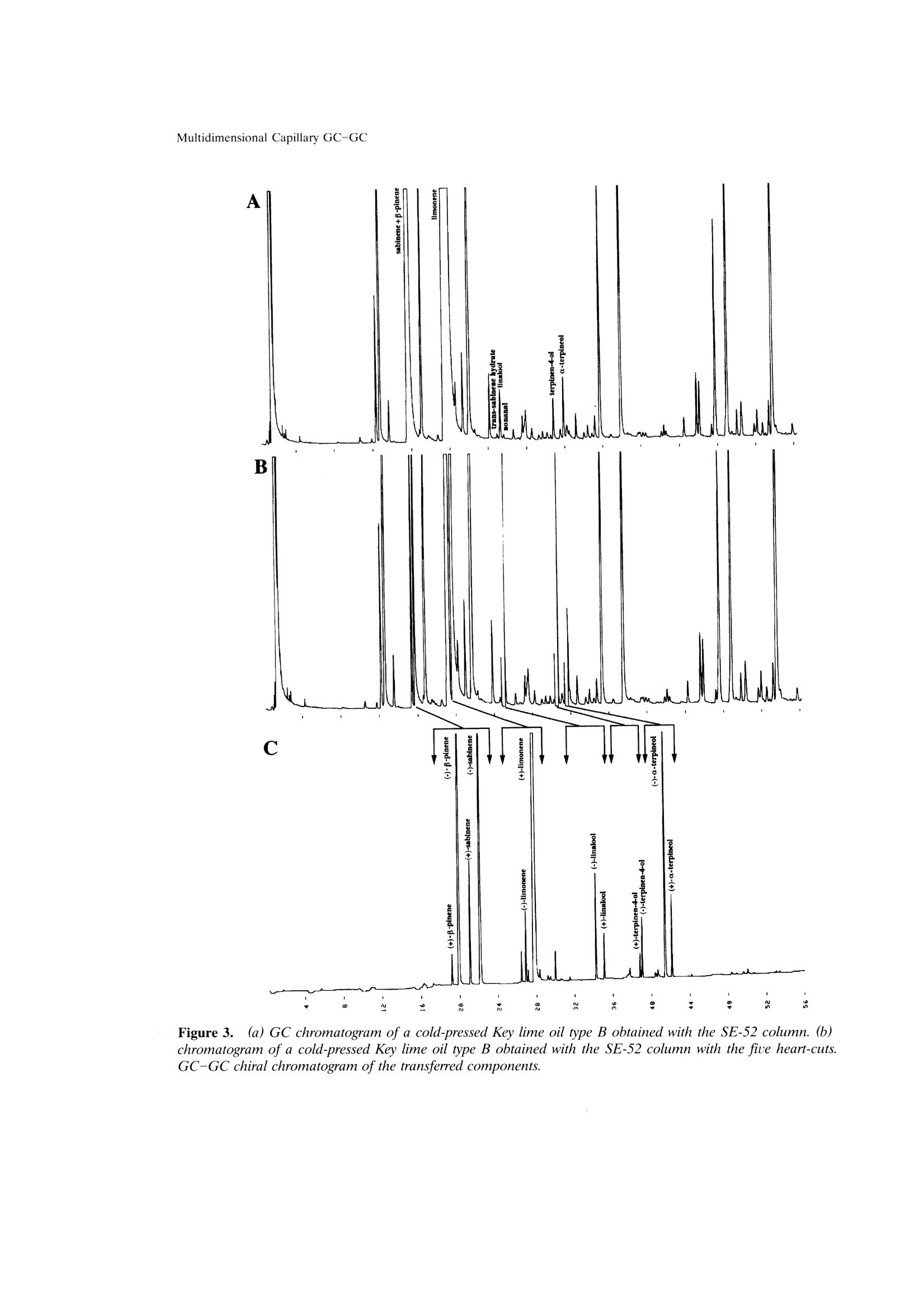
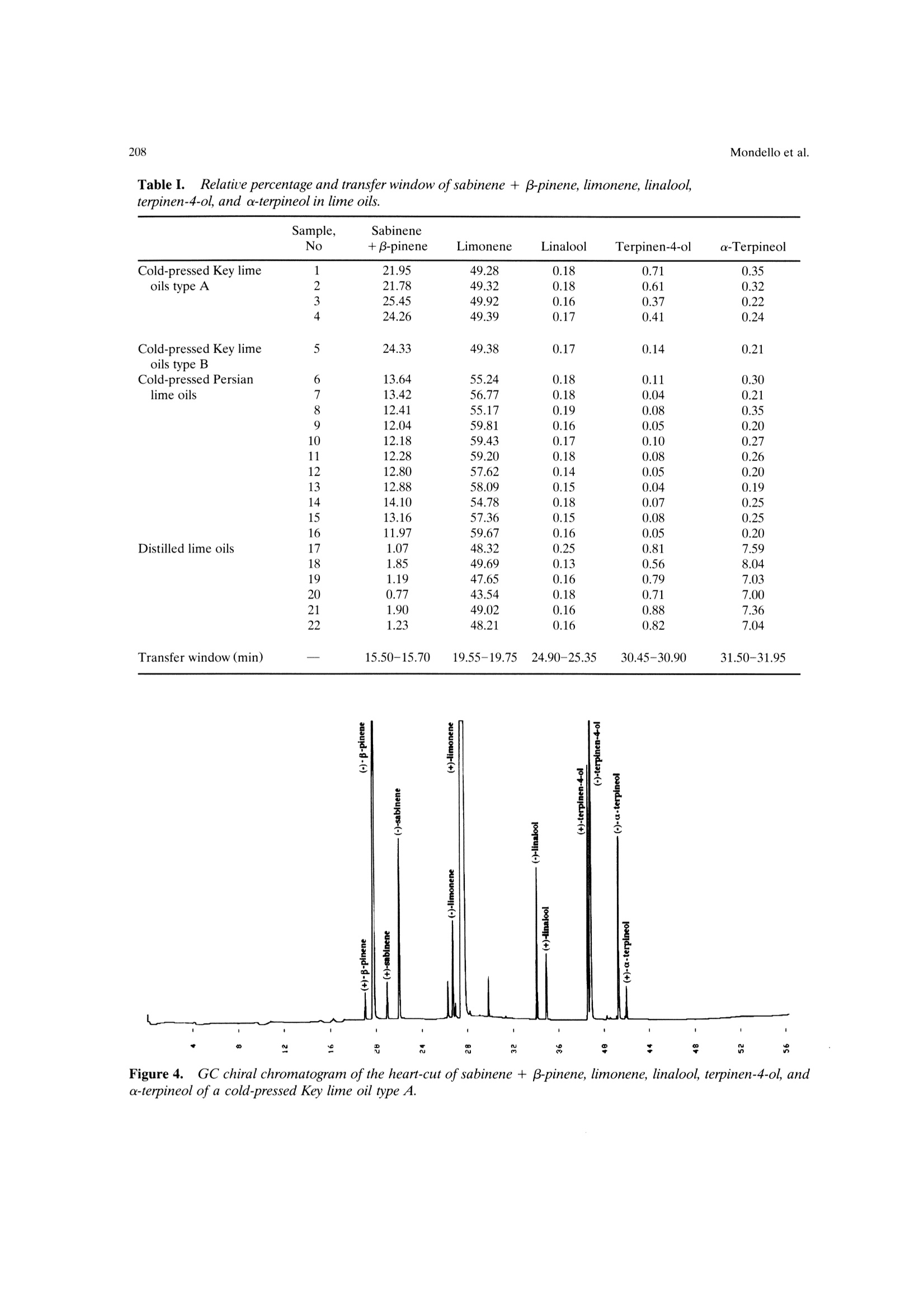
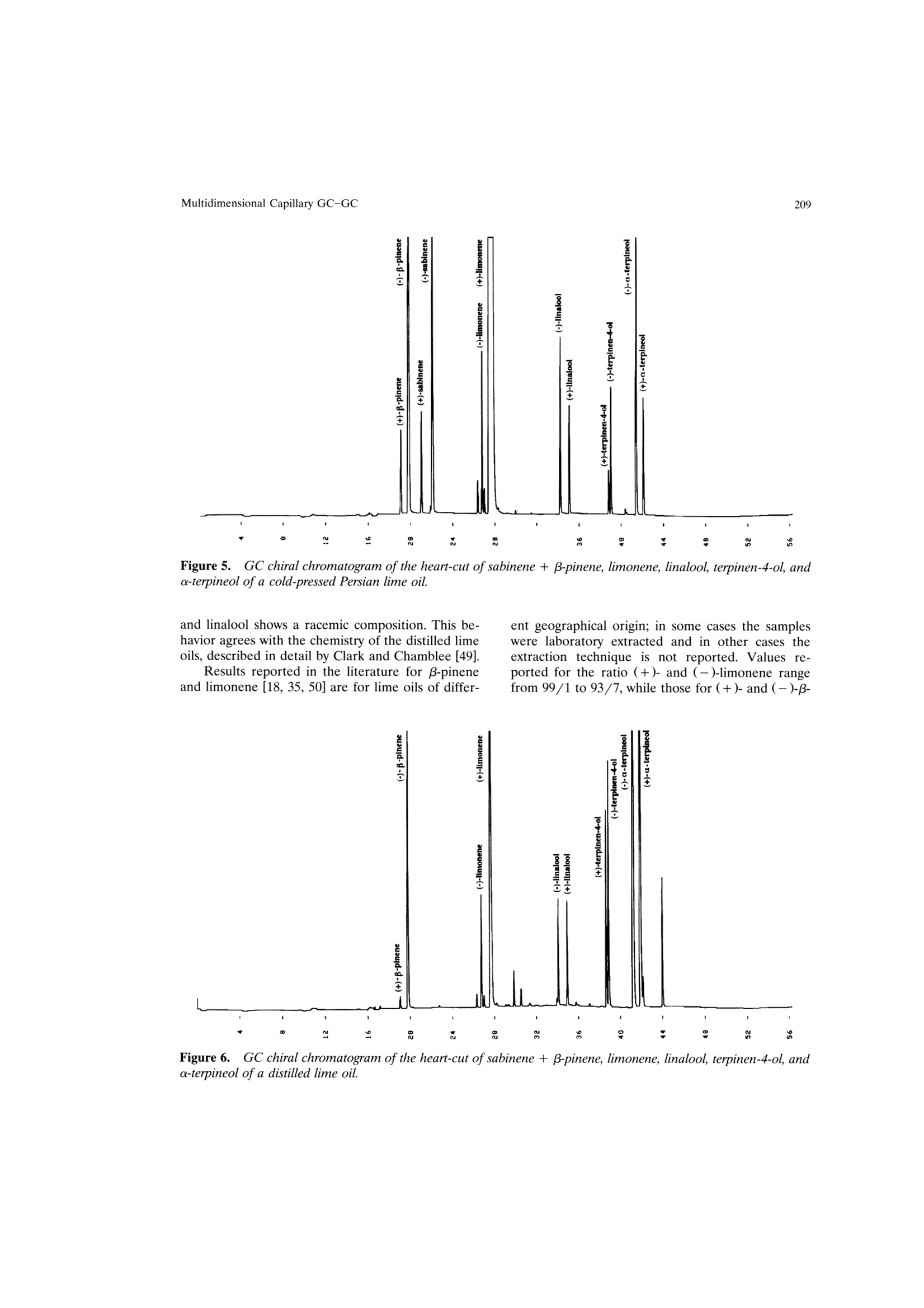
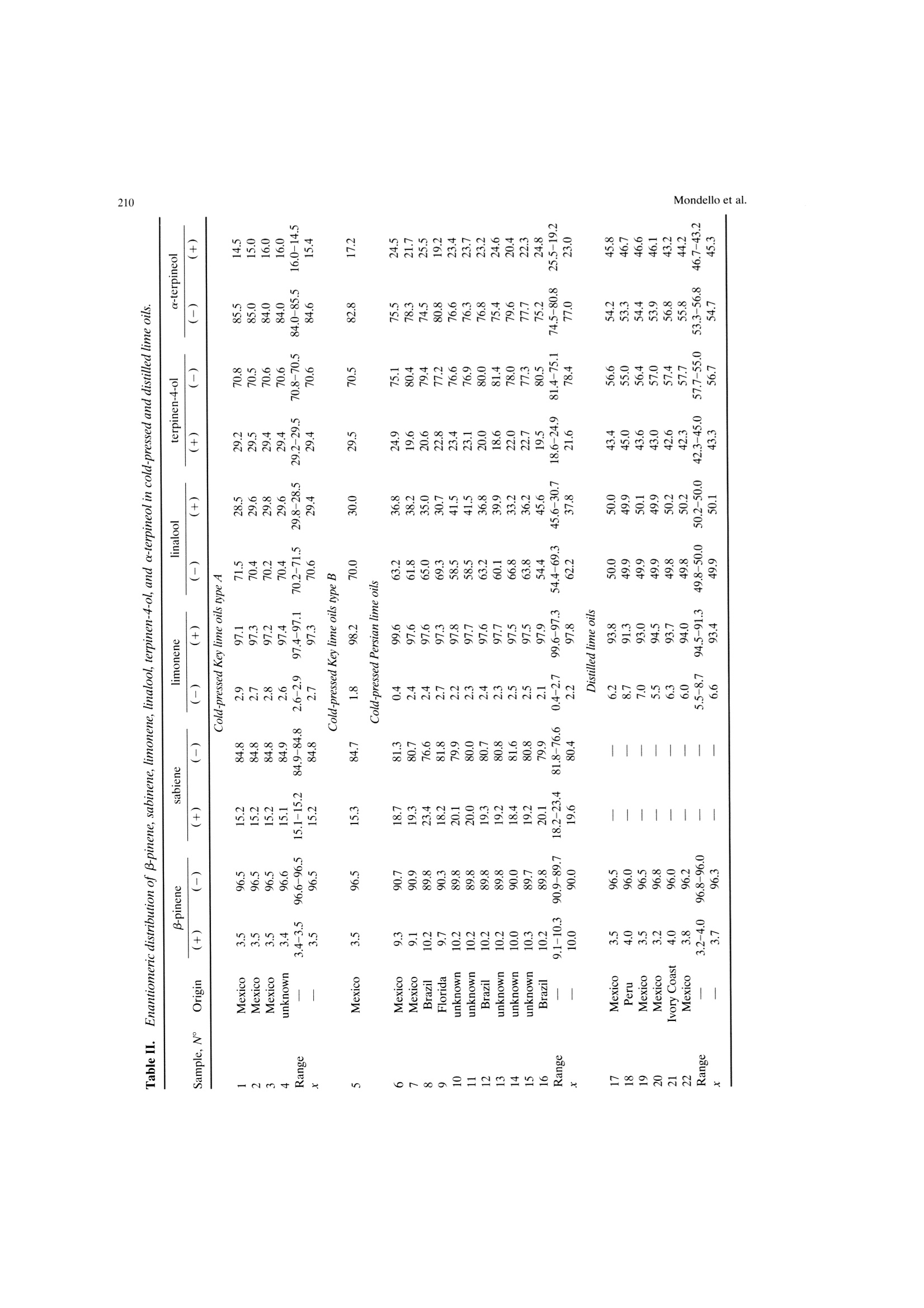
Mondello et al.204 205Multidimensional Capillary GC-GC Multidimensional Capillary GC-GC for the Analysis of Real ComplexSamples.Part II. Enantiomeric Distribution of MonoterpeneHydrocarbons and Monoterpene Alcohols of Cold-Pressed andDistilled Lime Oils Luigi Mondello, Maurizio Catalfamo, Paola Dugo?, Giovanni Dugo 'Dipartimento Farmaco-chimico, Facolta di Farmacia, Universita di Messina, uiale Annuziata 98168,Messina, Italy ‘Dipartimento di Chimica Organica e Biologica Facolta di Scienze MM.FF.NN., Universita di Messina,Salita Sperone, 98165, Messina, Italy Received 14 November 1996; accepted 23 June 1997 Abstract: The enantiomeric distribution of sabinene, B-pinene, limonene, linalool,terpinen-4-ol, and a-terpineol for cold-pressed Key lime oils type A and type B,cold-pressed Persian lime oils, and distilled lime oils has been determined by a fullyautomated, multidimensional, double-o1ven (GC-GC) system using a SE-52 precol-umn and a B-cyclodextrin main column. This system allows fractions to bemultransferred during the same GC analysis and the use of the two GCs indepen-dently when the multitransfer option is not used. The results allow us to distinguishcold-pressed Key lime oils type A and type B, cold-pressed Persian lime oils, anddistilled lime oils.S. 1998 John Wiley & Sons, Inc. J Micro Sep 10: 203-212, 1998 Key words: multidimensional gas chromatography; enantioselective gas chromatogra-phy; lime oils; monoterpene hydrocarbons; monoterpene alcohols INTRODUCTION The enantiomeric distribution of the compo-nents of essential oils can provide useful informationon the genuiness of the oils [1-13], on their qualityand on the extraction techniques employed [14-17],on their geographic origin [18, 19], and on theirbiogenesis [20]. For example, the ratio between (+)-and (-)-limonene has been used to distinguish gen-uine lemon and mandarin oils from reconstitutedones [3, 4]; the presence of synthetic compoundsmixed with genuine gergamot oils [2], with lavenderoils [7], and with some petitgrains oils [8] has beendetected from the enantiomeric ratios of linalooland linalyl acetate, as has the geographic origin ofbitter orange essential oils [19]; the enantiomericratio of terpinen-4-ol is related to the extractiontechnique for mandarin oils [14] while that of a-terpineol is an indicator of the quality of cardamomand Laurus nobilis oils [9]; the ratio of (E)-nerolidolindicates the quality of neroli oils [11], as do theratios of menthol, menthone, and menthyl acetatefor mentha [15] and melissa oils [10]. ( C 1 998 J ohn W iley & Sons, I nc. ) Gas chromatography with chiral columns coatedwith derivatized cyclodextrin is the analytical tech-nique most frequently employed for the determina- tion of the enantiomeric ratio of volatile compounds.Essential oils are usually complex mixtures, sodirect gas chromatographic analysis of the enan-tiomeric ratio of certain components, though some-times possible [21-28], is usually difficult, requiringgreat precision in the choice of experimental condi-tions and of stationary phases in order to avoidoverlaps between the peaks of the enantiomers andthose of other components [3, 4, 29]. On-line cou-pled liquid chromatography-gas chromatography(LC-GC) or multidimensional GC systems permitthe prefractionation of the sample and the conse-quent chiral analysis of single components or at leastof simpler fractions than the whole sample. In thisway problems of peak overlapping are avoided. Data in the literature are scant on the useof (on-line coupled hhigh-performance LC-GC(HPLC-HRGC) systems for the chiral analysis ofcomponents of natural complex mixtures [14, 19, 30],while many examples of the use of multidimensionalgas chromatography for this type of analysis are reported [1, 5, 7,10-13,16,18,25,27, 31-47]. Thislast technique uses the high resolution of gas chro-matography both in the pre-fractionation stage andin the main analysis. The LC-GC systems permit the transfer andthe subsequent GC analysis of LC fractions thatcontain compounds of the same polarity, which showthe same chromatographic behavior in liquid chro-matogrphy, while GC-GC systems allow us to oper-ate transfers of fractions that contain compounds ofthe same volatility but of different chemical class, ifthe two columns are placed in either the same GCoven or two different ovens. The LC-GC systems allow transfers from HPLCto GC of more than one LC fraction. However, eachLC fraction subsequent to the first can be trans-ferred and analyzed by GC only after the end of theprevious GC run [48]. The GC-GC systems, on theother hand, permit several fractions to be trans-ferred from the precolumn to the analytical columnand analyzed in the same GC run; this possibility isenhanced if a cold trap is placed before the inlet ofthe second column. Moreover, when HPLC is cou-pled to HRGC, it is very difficult to transfer differ-ent amounts of components that belong to the sameclass, because these compounds have the same chro-matographic behavior in HPLC and so are coeluted.In multidimensional GC the precolumn works athigh resolution, allowing separation of componentsof the same chemical class, which shonwo ownly smalldifferences in volatility. For this reason, differenttransfer times can be chosen for each componentand different small portions of a component can betransferred. In fact, for the determination in a com-plex matrix (such as an essential oil) of the enan-.tiomeric ratio of two monoterpene hydrocarbonslimonene and β-pinene, present, for example, in arelative percentage of 70 and 10%, the transfer ofdifferent portions of the two components from theLC column to the GC column is impossible, if thecomponents are coeluted in LC. If the two compo-nents are separated in the precolumn by GC analy-sis, the amount then transferred of each can be setso that the concentration analyzed on the maincolumn will give well-resolved peaks; for example,1% of limonene and 10% of B-pinene. Lime essential oils are obtained from Citrusaurantifolia Swingle (Key lime) and C. latifolia Tana-ka (Persian lime). Distilled lime oil is obtained byreacting the flavor compounds of a mixture of essen-tial oil/juice/crushed fruits in acid medium. It ac-counts for about 95% of total lime oil productionworldwide. The other 5% is made up of cold-pressedKey lime oil, type A, obtained by centrifugation ofthe oil/juice emulsion produced by passing the whole fruit through a screw-press which crushes the fruits;of cold-pressed Key lime, type B, obtained by rasp-ing the peel to release the oil; and of cold-pressedPersian lime oil, obtained with the same method asfor cold-pressed Key lime, type B [49]. The enantiomeric distribution of some compo-nents of lime oils has been reported only in threepapers by the Mosandl group [18, 35,50]. In this article the enantiomeric distribution ofsome monoterpene hydrocarbons (sabinene, B-pin-ene, limonene) and of some monoterpene alcohols(linalool, terpinen-4-ol, a-terpineol) of different limeoils is reported.The analyses were carried out withan automated multidimensional GC-GC system de-veloped in our laboratory [51], performing five con-secutive transfers in the same analysis. EXPERIMENTAL The research was carried out on four cold-pressed Key lime oils type A; one cold pressed Keylime oil type B; eleven cold-pressed Persian limeoils; six distilled lime oils. All the samples wereanalyzed by injecting 1 ul of a 10%(v/v) solution ofessential oil in pentane with a split ratio of 1:10. The multidimensional system used in this studywas a developmental model which consisted of twogas chromatographs Shimadzu 17A, a transfer lineand two integrators Shimadzu C-R3A. The instru-mental setup and the experimental conditions usedwere as follows (see Figures 1 and 2): Gas chromatograph 1 ·Two split/splitless injectors at 250℃ with twomanual flow controllers (injectors 1, 2) and aflame ionization detector at 250℃(FID 1). · A SE-52 capillary column 30 m × 0.32 mm i.d.0.40-0.45 um film thickness (Mega, Legnano,Italy); temperature program: 45℃ for 6 min, thento 240℃ at 2.0℃/min; carrier He. A Valco six-ports (-in.) two-position UW typevalve (valve 1) with a right-angle drive (A3RADN6WT) (Valco Europe). The valve has a rotor madeof fluorocarbon-filled crosslinked polyimide and aport diameter of 0.40 mm and can be operated upto 350℃. Moreover, this valve is designed for usewith fused silica columns using a special adapter(Valco FSR.5-5) consisting of a liner which slidesover the fused silica tubing and a ferrule whichmakes up on the liner. A digital valve interface (DVI-220) (Valco Eu-rope) connected to EVENT 91 on gas chromato-graph 1. .· One-sixteenth-inch removable fused silica adapters(FSR.5-5 and FSR.4-5)(Valco Europe) to connectthe valve and the fused silica tubing. Figure 1. Pneumatic and electronic scheme of theGC-GC system in the stand-by position. A solenoid valve (valve 2) to change the carrierpressure (P, 110 kPa) (stand-by position, column2) to higher pressure (P, 200 kPa) (cut position,columns 1 and 2) connected to EVENT 92 on gaschromatograph 1. A solenoid valve (valve 3) to change the carrierpressure (P, 90 kPa) (stand-by position, column2) to lower pressure (P, 2.5 kPa) (cut position,injector 1 and FID 1) connected to EVENT 91 ongas chromatograph 1. A solenoid valve (valve 4) which allows the use oftwo splitter valves (A and B) with different ratiosin injector 1. An integrator Shimadzu C-R3A connected to startand out signals on gas chromatograph 1. Transfer line. An aluminum thermoregulatedoec r_block equipped with a heater assembly and a ther-mocouple assembly connected to the AUX2 exit ongas chromatograph 1. Gas chromatograph 2 e A MEGADEXDETTBSB (diethyl-tert-butylsilyl-B-cyclodextrin), 25m × 0.25 mm i.d., 0.25 um Figure 2.Pneumatic and electronic scheme of theGC-GC system in the cut position. film thickness (Mega, Legnano, Italy). Tempera-ture program: 45℃ for 6 min, then to 180℃ at2.0℃/min; carrier He; the GC program startedwith the first cut. A flame ionization detector at 250℃ (FID 2). An integrator Shimadzu C-R3A connectedd to“start out signal"is an electric event on gas chro-matograph 2. When the six-port valve (valve 1) is in the stand-by position (Figure 1), flow paths are as follows:injector 1 to column 1 to FID 1 and injector 2through the hot transfer line to column 2 and toFID 2. In this configuration it is possible to carry outanalyses independently on the two columns withoutany change of hardware. Moreover, it is possible tochange the carrier pressure and the split ratio foreach injector. When valve 1 is switched to the cutposition (Figure 2), the flow path is as follows:injector 2 to column 1 through the hot transfer lineto column 2 and to FID 2, and at the same time thesolenoid valve 2 is switched on to increase the car-rier pressure from P (110 kPa) to P, (200 kPa). This ensures that the right retention times are ob-tained on columnn 1, even for those componentseluted after more than one transfer. An increase inpressure is especially important in multitransfer op-erations because if the same pressure is maintainedin the cut position as in the stand-by position, a flowdrop occurs when the precolumn(column1) is addedto the main column (column 2). This flow dropcauses a shift of the retention times of the compo-nents eluted after each transfer, and this does notpermit the automatic transfer of more than onefraction during the same analysis. When valve 1 isswitched to the cut position, the flow surge may alsoin some instances extinguish the flame of the detec-tor (FID 1). To prevent this, a solenoid valve (valve3)is added to decrease the carrier pressure from P(90 kPa) to P (2.5 kPa), thus maintaining a constantflow in the detector FID 1 and protecting it fromflow surges due to the absence of column 1 in theflow path. To further regulate the flow at FID 1,another solenoid valve (valve 4) was added. Thisvalve allows splitter 1A to be used in the stand-byposition (split ratio for the sample introduction) andsplitter 1B in the cut position (high split ratio torapidly establish pressure P). When this multidi-mensional system operates in the stand-by position,the two columns work independently and so splitters1A and 2 can be adjusted to optimize the split ratio,while splitter 1B is excluded. When the multidimen-sional system is used, the splitter of injector 2 isadjusted to allow the increased carrier to escapefrom the splitter when valve 1 is switched back tothe stand-by position and the original pressure P isre-established. As shown in Figures 1 and 2, the system iscompletely automated by the use of the externalevents of the gas chromatrograph. The time at whichthe valve should be switched to begin the cuts can bedetermined from a preliminary analysis. After this afully automated analysis is possible by programmingthe valve events. RESULTS AND DISCUSSION A lime essential oil was first analyzed with theSE-52 precolumn to determine the concentrations ofthe components of interest and their retention times,maintaining the multidimensionalsystem in thestand-byposition. Depending on the retention timesand the concentration of each single component,different transfer windows were then chosen andautomatically programmed so that well-resolvedpeaks would be obtained in the chiral column bothfor components present in the oils at high concen-trations and for those present at very low concentra-tions. Figure 3 reports the chromatogram of a cold-pressed Key lime oil type B obtained with the SE-52column and the system in the stand-by position; thechromatogram of the same oil obtained with theSE-52 column and the system in the cut position (onthis chromatogram the cuts are shown); and thechromatogram obtained with the chiral column forthe fractions transferred from the SE-52 precolumn. Limonene in all the oils analyzed, -piene +sabinene in cold-pressed oils, and a-terpineol indistilled oils were only partially transferred becauseof their high concentrations. B-Pinene + sabinenein distilled oils, a-terpineol in cold-pressed oils,linolool and terpinen-4-ol were quantitatively trans-ferred because they were present in lower amounts. The percentage content of the transferred com-ponents in the analyzed oils and the transfer win-dows are shown in Table I. Figures 4-6 show the chiral chromatogram ofB-pinene, sabinene, limonene, linalool, terpinen-4-ol,and a-terpineol for a cold-pressed Key lime oil typeA, a cold-pressed Persian lime oil, and a distilledlime oil, respectively. Table II reports the enan-tiomeric distribution in lime oils of the componentsanalyzed. Cold-pressed Key lime oils type A and cold-pressed Key lime oil type B show practically identi-cal values of the enantiomeric distribution of theanalyzed components, except for a small differencein the enantiomeric distribution of limonene: thetype B oil shows a (-)-limonene content of 1.8%ofthe total amount of limonene, while the type A oilsshow a value (-)-limonene never lower than 2.6%. Cold-pressed Persian lime oils show the samevalue for the enantiomeric distribution of limoneneas Key lime oils but different values for the enan-tiomeric distribution of the other components: (+)-B-pinene, (+)-sabinene, (+)-linalool, and (+)-a-terpineol in Persian lime oils represent a higherpercentage of the total amount of the componentthan in Key lime oils, while for terpinen-4-ol thereverse is true. The differences between Persian andKey lime oils can be attributed to the natural char-acteristics of the two oils and not to the extractiontechnology. The Key lime oil type B analyzed showssimilar values to Key lime oils type A and not toPersian lime oils, although it was obtained with thesame extraction technology used for Persian limeoils. Distilled lime oils, obtained from Key lime fruits,show values of the enantiomeric distribution of theanalyzed components different from those shown bythe cold-pressed oils, except for B-pinene, whichshows the same value as for cold-pressed oils. Theother enantiomeric distributions tend to be racemic, Figure 3.(a) GC chromatogram ofa cold-pressed Key lime oil type B obtained with the SE-52column. (b)chromatogram of a cold-pressed Key lime oil type Bobtained with the SE-52 column with the five heart-cuts.GC-GC chiral chromatogram of the transferred components. Table Il.. Relative percentage and transfer window of sabinene + β-pinene, limonene, linalool,terpinen-4-ol, and a-terpineol in lime oils. Sample, Sabinene No +B-pinene Limonene Linalool Terpinen-4-ol a-Terpineol Cold-pressed Key lime 1 21.95 49.28 0.18 0.71 0.35 oils type A 2 21.78 49.32 0.18 0.61 0.32 3 25.45 49.92 0.16 0.37 0.22 4 24.26 49.39 0.17 0.41 0.24 Cold-pressed Key lime oils type B 5 24.33 49.38 0.17 0.14 0.21 Cold-pressed Persian 6 13.64 55.24 0.18 0.11 0.30 lime oils 7 13.42 56.77 0.18 0.04 0.21 8 12.41 55.17 0.19 0.08 0.35 9 12.04 59.81 0.16 0.05 0.20 10 12.18 59.43 0.17 0.10 0.27 11 12.28 59.20 0.18 0.08 0.26 12 12.80 57.62 0.14 0.05 0.20 13 12.88 58.09 0.15 0.04 0.19 14 14.10 54.78 0.18 0.07 0.25 15 13.16 57.36 0.15 0.08 0.25 16 11.97 59.67 0.16 0.05 0.20 Distilled lime oils 17 1.07 48.32 0.25 0.81 7.59 18 1.85 49.69 0.13 0.56 8.04 19 1.19 47.65 0.16 0.79 7.03 20 0.77 43.54 0.18 0.71 7.00 21 1.90 49.02 0.16 0.88 7.36 22 1.23 48.21 0.16 0.82 7.04 Transfer window (min) — 15.50-15.70 19.55-19.75 24.90-25.35 30.45-30.90 31.50-31.95 Figure 4.. GC chiral chromatogram of the heart-cut of sabinene + β-pinene, limonene, linalool, terpinen-4-ol,anda-terpineol ofa cold-pressed Key lime oil type A. Figure 5. GC chiral chromatogram of the heart-cut of sabinene + B-pinene, limonene, linalool, terpinen-4-ol,anda-terpineol of a cold-pressed Persian lime oil. and linalool shows a racemic composition. This be-havior agrees with the chemistry of the distilled limeoils, described in detail by Clark and Chamblee [49]. Results reported in the literature for B-pineneand limonene [18, 35, 50] are for lime oils of differ- ent geographical origin; in some cases the sampleswere laboratory extracted and in other cases theextraction technique is not reported. Values re-ported for the ratio (+)- and(-)-limonene rangefrom 99/1 to 93/7, while those for (+)- and (-)-B- Figure 6.GC chiral chromatogram of the heart-cut of sabinene + β-pinene, limonene, linalool, terpinen-4-ol, anda-terpineol ofa distilled lime oil. Mondello et al. 寸m oo oN 寸9 寸寸 8988S9s寸-sSES 808-St ks 66t 9OO O N I N N 寸6 6 a 寸 6 1寸 v 7 - 9 7' 8 6 t 7 8寸- 86t 8 99919 6寸6 O6寸 寸9寸1N寸寸 9 9N91 6886166 86 寸 ET6-St6 寸 19 i O寸 0SS-LLS 8L S6I 9986L寸0-868I8 008 寸 w 一十 寸19 一N~~N~ pinene range from 2/98 to 10/90. These rangesagree with the values reported in this work for thedifferent lime oils. As can be seen from the chromatograms re-ported in Figure 3 and from the scheme of theinstrument reported in Figures 1 and 2, the systemmakes it possible to program and carry out fullyautomated multiple transfers, with reproducibility ofthe retention times in the precolumn, even for thosecomponents eluted after numerous transfers. The correct use of the system permits the auto-matic transfer even of those components that eluteon the SE-52 column in a critical zone of the chro-matogram. For example, linalool elutes column be-tween trans-sabinene hydrate and nonanal [Figure3(a)]. It is clear that in such a case the choice oftransfer window is critical as imperfect reproducibil-ity of the retention time could case a partial loss oflinalool or the presence of trans-sabinene hydrateand/or of nonanal in the transferred fraction. When the system is not used as multidimen-sional GC, the simultaneous and independent use ofthe two gas chromatographs is possible without anychange to the hardware configuration. ACKNOWLEDGMENT The authors thank Shimadzu Italia for theircooperation during the development of this work. REFERENCES ( .1 . V . S chubert and A . M o sandl, Phytochem. Anal. 2, 17 1 (1991). ) ( 2. A. C otroneo, A . Verzera, and A . Tr o zzi, F l auourFragr. J. 7, 15 (1992). ) ( 3. G . D ugo, I . Stagno d'Alcontres, A. Cotroneo, and P. Dugo, J . Essent. O il Res. 5 , 589 (1992). ) ( 4. G. D ugo, I. Stagno d'Alcontres, M.G. D onato, and P. Dugo, J. E ssent. O il R es. 5, 21 (1993). ) 5.H. Casabianca and J.-B. Graff, J. High Resolut. Chro-matogr. 17,184 (1994). 6 W.A. Konig, A. Rieck, I. Hard, B. Gehrcke, K.H.Kubeczka, and H. Muhle, J. High Resolut. Chro-matogr. 17, 315(1994). 7.A. Mosandl and V. Schubert,J. Essent. Oil Res. 2,121(1990). 8. U. Ravid, E. Putievsky, and I. Katzir, Flavour Fragr.J. 9,275(1994). 9. U. Ravid, E. Putievsky, and I. Katzir, Flauour Fragr.10,281 (1995). 10. P. Kreis and A. Mosandl, Flauour Fragr. J. 9, 249(1994). 11. W.A. Kong, B. Gehrcke, D. Icheln, P. Evers, J.Donnecke, and W. Wang, J. High Resolut. Chro-matogr. 15, 367 (1992). 12.D. Juchelka, A. Steil, K. Witt, and A. Mosandl, J.Essent. Oil Res. 8, 487 (1996). 13. D. Juchelka, and A. Mosandl, Pharmazie 51,417(1996). 14. G. Dugo, A. Verzera, A. Trozzi, A. Cotroneo, L.Mondello, and K.D. Bartle, Essenz. Deriu. Agrum. 64,35(1994). 15. B. Faber, A. Dietrich, and A. Mosandl, J. Chro-matogr. 666, 161 (1994). 16. P. Kreis, U. Hener, and A. Mosandl, Dtsch. Lebensm.Rundsch.87(1),8(1991). 17. B. Weinreich and S. Nitz, Chem. Mikrobiol.Technol.Lebensm. 14(3-4),117(1992). 18. U. Hener, P. Kreis, and A. Mosandl, Flavour Fragr. J.5,201(1990). 19. G. Dugo, A. Verzera, A. Cotroneo, I. Stagno d'Al-contres, L. Mondello, and K.D. Bartle, Flauour Fragr.J. 9,99 (1994). 20.P. Werkoff, S. Brennecke, W. Bretschneider, M.Gintert, R. Hopp, and H. Surburg, Z. Lebensm.Unters Forsch. 196, 307 (1993). 21.T. Koscielski, D. Sybilska, and J. Jurczak, J. Chro-matogr. 364, 299 (1986). 22. V. Schurig and H.-P. Nowotny, J. Chromatogr. 441,155(1988). 23 V. Schurig and H.-P. Nowotny, Angew. Chem. 102,939(1990). 24. W.A. Konig, Gas Chromatographic Enantiomer Sepa-ration with Modified Cyclodextrins (Huthig, Heidel-berg, 1992). 25. W. Keim, A. Kohnes, and W. Meltzow, J. High Reso-lut. Chromatogr. 14, 507 (1992). 26.C. Bicchi, G. Artuffo, A. D'Amato, C.M. Nano, A.Galli, and M. Galli, J. High Resolut. Chromatogr. 14,301(1991). .A. Mosandl, Food Reu. Int. 11, 597 (1995). 28C. Bicchi.V. Manzin.A. D'Amato, and P. Rubiolo,Flauour Fragr. J. 10, 127 (1995). 29..C. Bicchi, A. D'Amato, V. Manzin, A. Galli, and M.Galli, J. Chromatogr. 666, 137 (1994). 30. H.-G. Schmarr, A. Mosandl, and K. Grob, Chro-matographia 29, 125 (1990). 31. G. Schomburg, H. Husmanm, E. Hubinger, and W.A.Koenig, J. High Resolut. Chromatogr. 7, 404 (1984). 32.4.A1. Bernreuther, N. Christoph, and P. Schreier, J.Chromatogr. 481, 363 (1989). 33. A. Mosandl, U. Hener, U. Hagenauer-Herner, andA. Kustermann, J. High Resolut. Chromatogr. 12, 532(1989). 34. C. Bicchi and A. Pisciotta, J. Chromatogr. 508, 341(1990). 35. A. Mosandl, U. Hener, P. Kreis, and H.-G.Schmarr,Flauour Fragr.J. 5, 193 (1990). ( 36. A . M osandl, K . Fi s cher, U . H ener, K. R e ttinger, V . Schubert, and H.-G. Schmarr, J . Agric. Food Chem. 39, 1131 (1991). ) ( 37. . A. Bernreuther, J . Bank, G. Krammer, a nd P .S chreier, P hytoch e m. Anal. 2, 43 (1991). ) 38. A. Bernreuther and P. Schreier, Phytochem. Anal. 2,167(1991). 39. C. Askari, U. Hener, H.-G. Schmarr, A. Rapp, and A.Mosandl, Fresenius J. Anal. Chem. 340, 768(1991). 40. P. Kreis and A. Mosandl, Flauour Fragr. J. 7, 187(1992). 41.P. Kreis and A. Mosandl, Flauour Fragr. J. 7, 199(1992). 42..A. Mosandl, C. Askari, U. Hener, D. Juchelka, D.Lehmann, P. Kreis, C. Motz, U. Palm, and H.-G.Schmarr, Chirality 4, 50 (1992). 4A. Mosandl, J. Chromatogr. 624, 267 (1992). P. Kreis and A. Mosandl, Flauour Fragr. J. 8, 161(1993). 46..G. Full, P, Winterhalter,G. Schmidt, P. Herion, and P. Schreier, 15th International Symposium on Capil-lary Chromatography, Riva del Garda, Italy, May24-27, 1993. 47. I.H. Hardt, A. Rieck, C. Fricke, and W.A. Konig,Flauour Fragr. J. 10, 165 (1995). 48. F. Munari, G. Dugo, and A. Cotroneo, J. High Reso-lut. Chromatogr. 13, 56 (1990). ( 49. B .C. C lark and T .S. C h amblee,in O f f-flavors in Foodand B euerages, G. Charalembous, E d. (Elsevier Sci-ence, Amsterdam, 1 992). ) ( 50. U . H ener, A. H o llnagel, P . K reis, B. Maas, H .-G. Schmarr,V . S c hubert, K . R e ttinger, B. W eber, a n d A. M osandl, in F lauour Science and Technology, Y . Bessier and A .F. T h omas, Eds. (Wiley, Chichester, 1 990), pp. 25-28. ) ( 51. 1 . L . Mondello, M. Catalfamo, P . Dugo, and G. Dugo,J. C hromatogr. S c i., i n p ress. ) Correspondence to: L. MondelloCC . Microcolumn Separations,
确定





还剩8页未读,是否继续阅读?
扬州华明仪器设备有限公司为您提供《分析单萜烃和单萜醇的冷压和蒸馏油分布》,该方案主要用于其他中--检测,参考标准--,《分析单萜烃和单萜醇的冷压和蒸馏油分布》用到的仪器有
相关方案
更多







