方案详情
文
The composition of the essential oil of Uruguayan citrus clementine hort....
方案详情

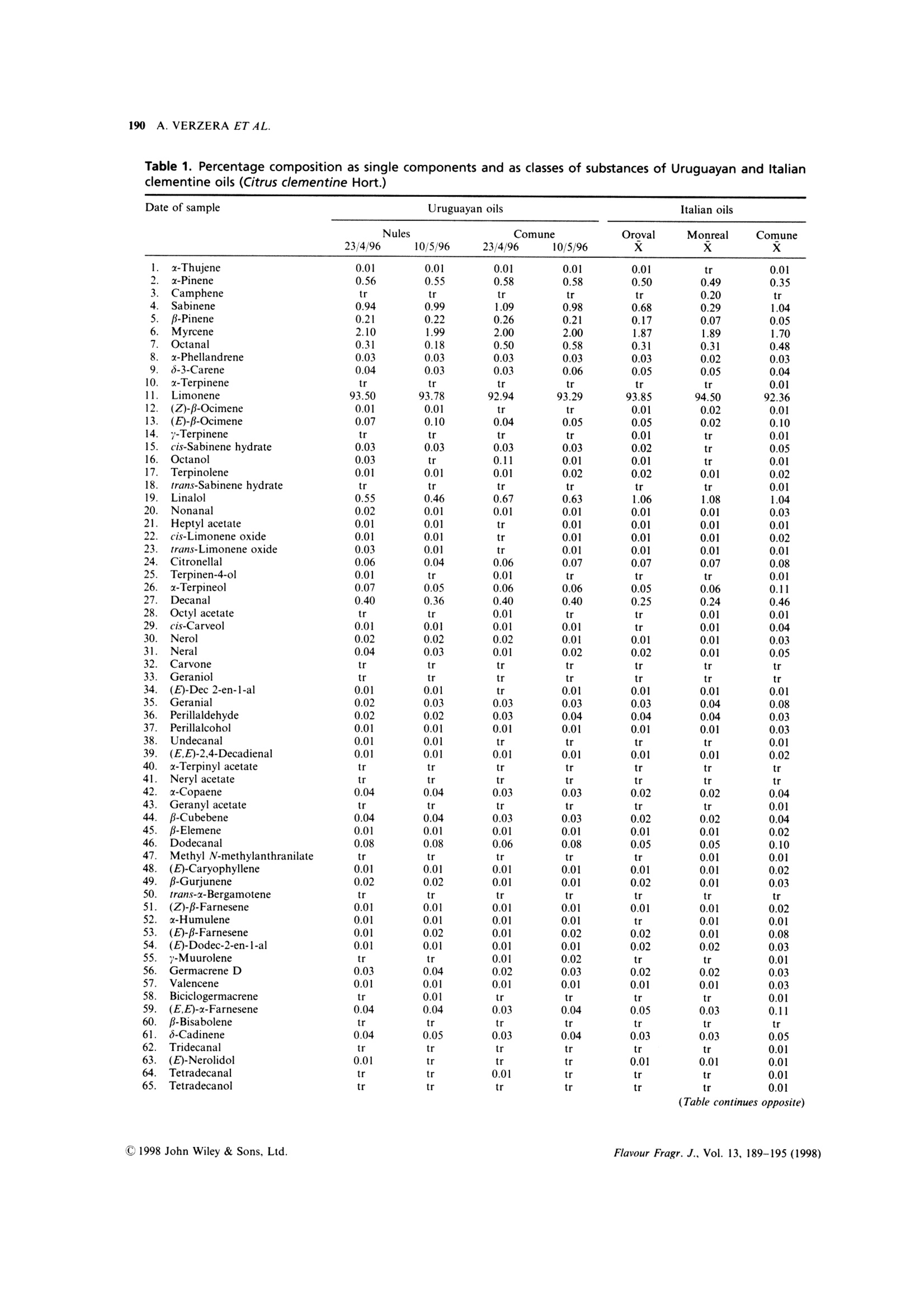
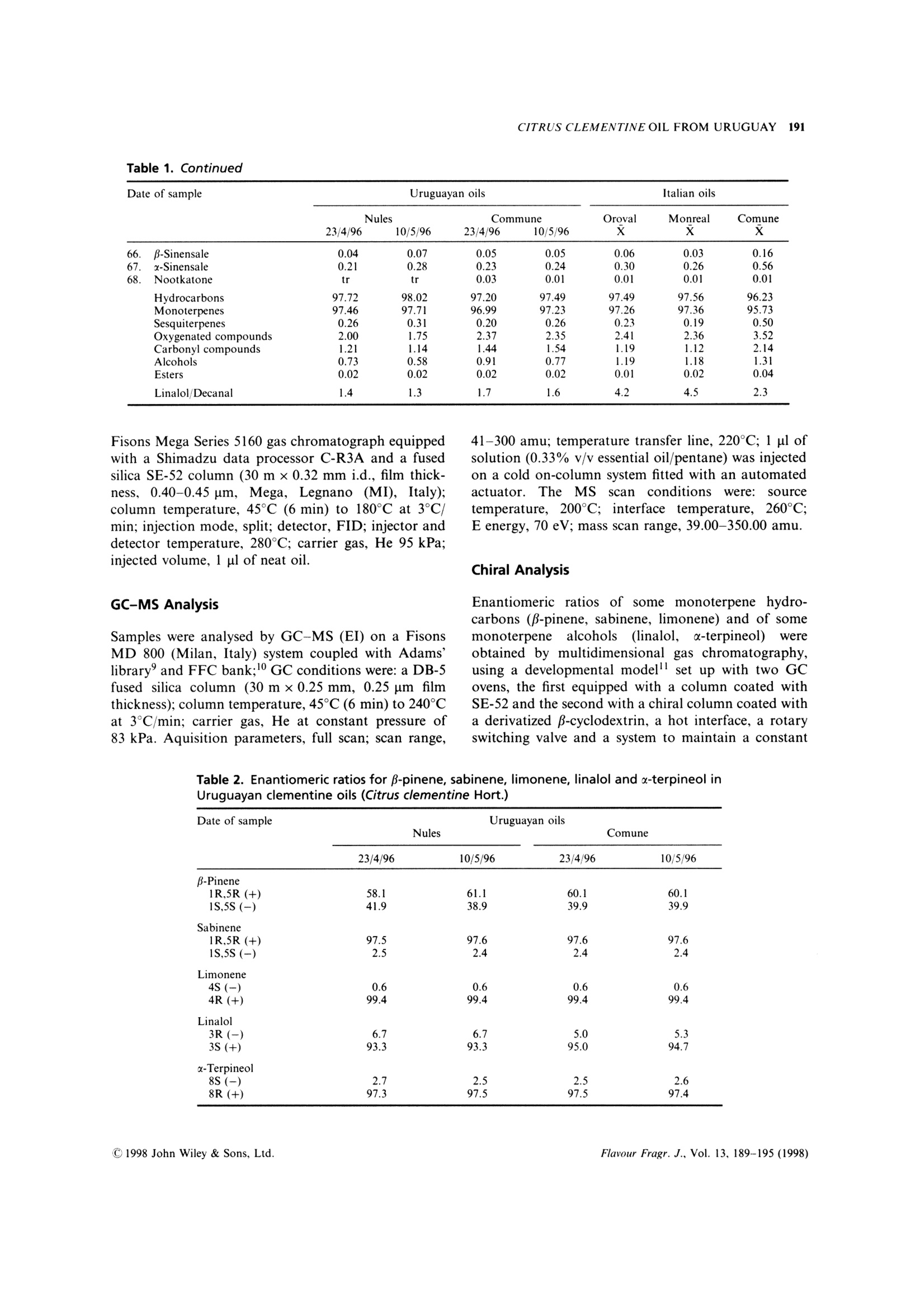
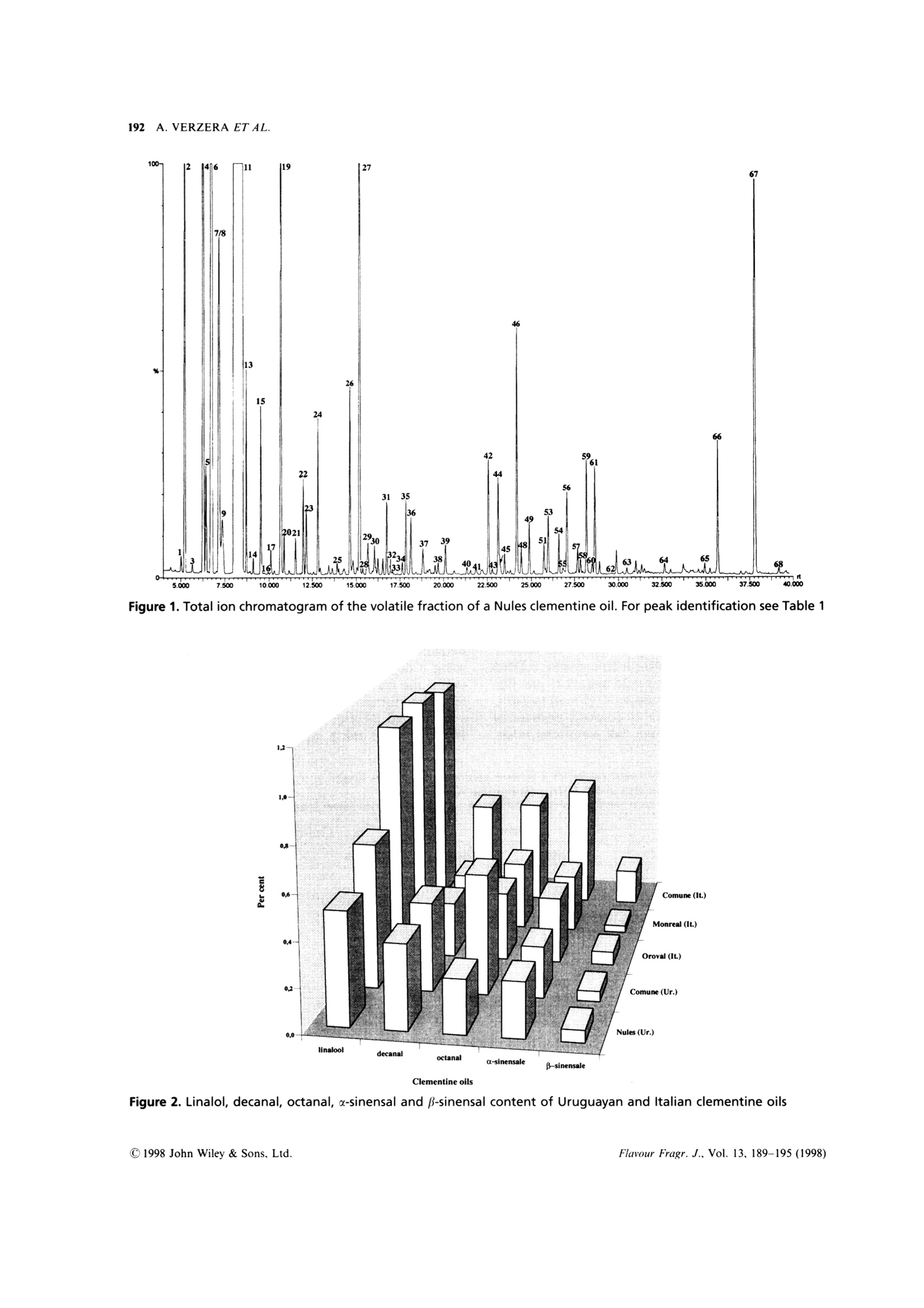
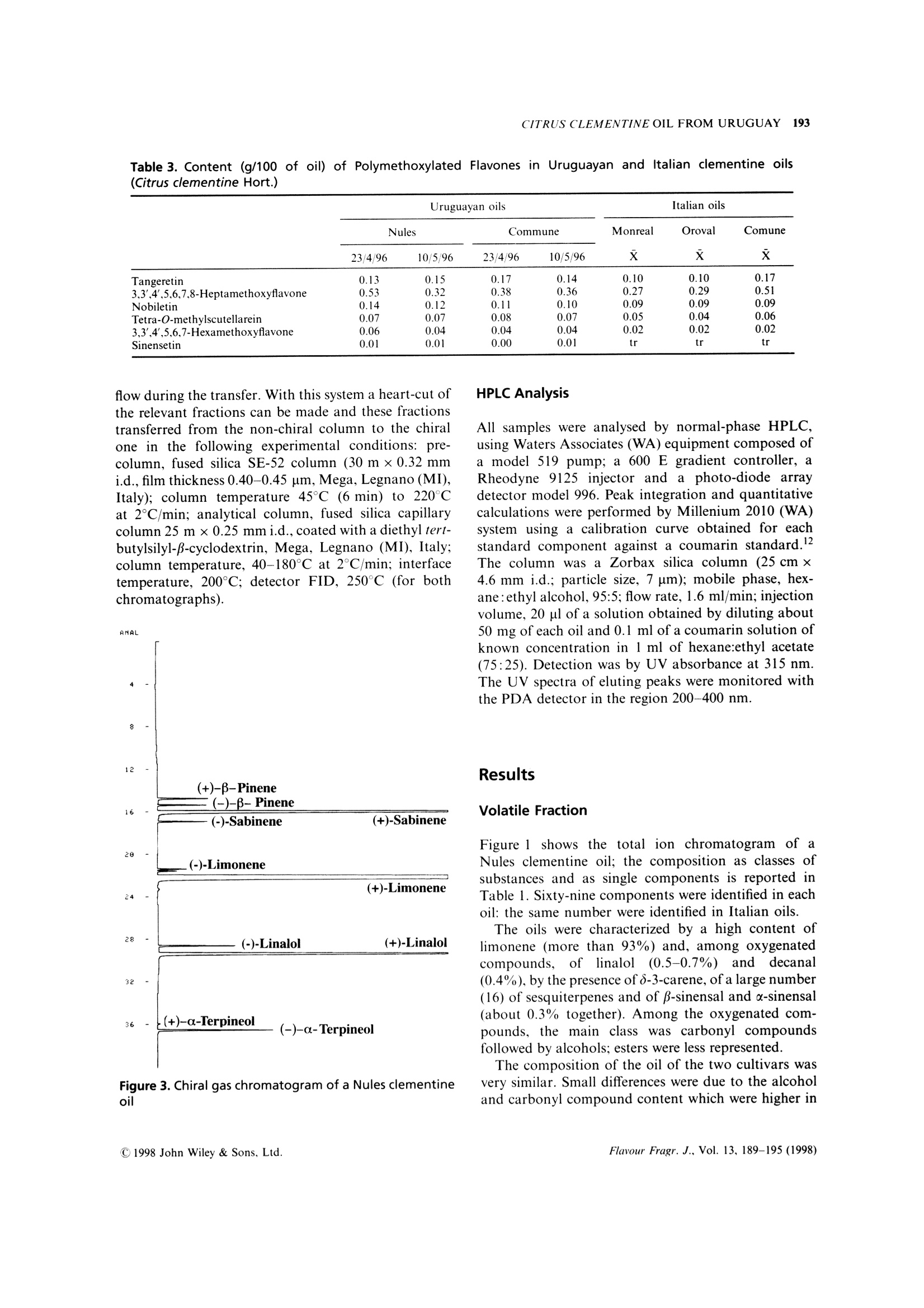
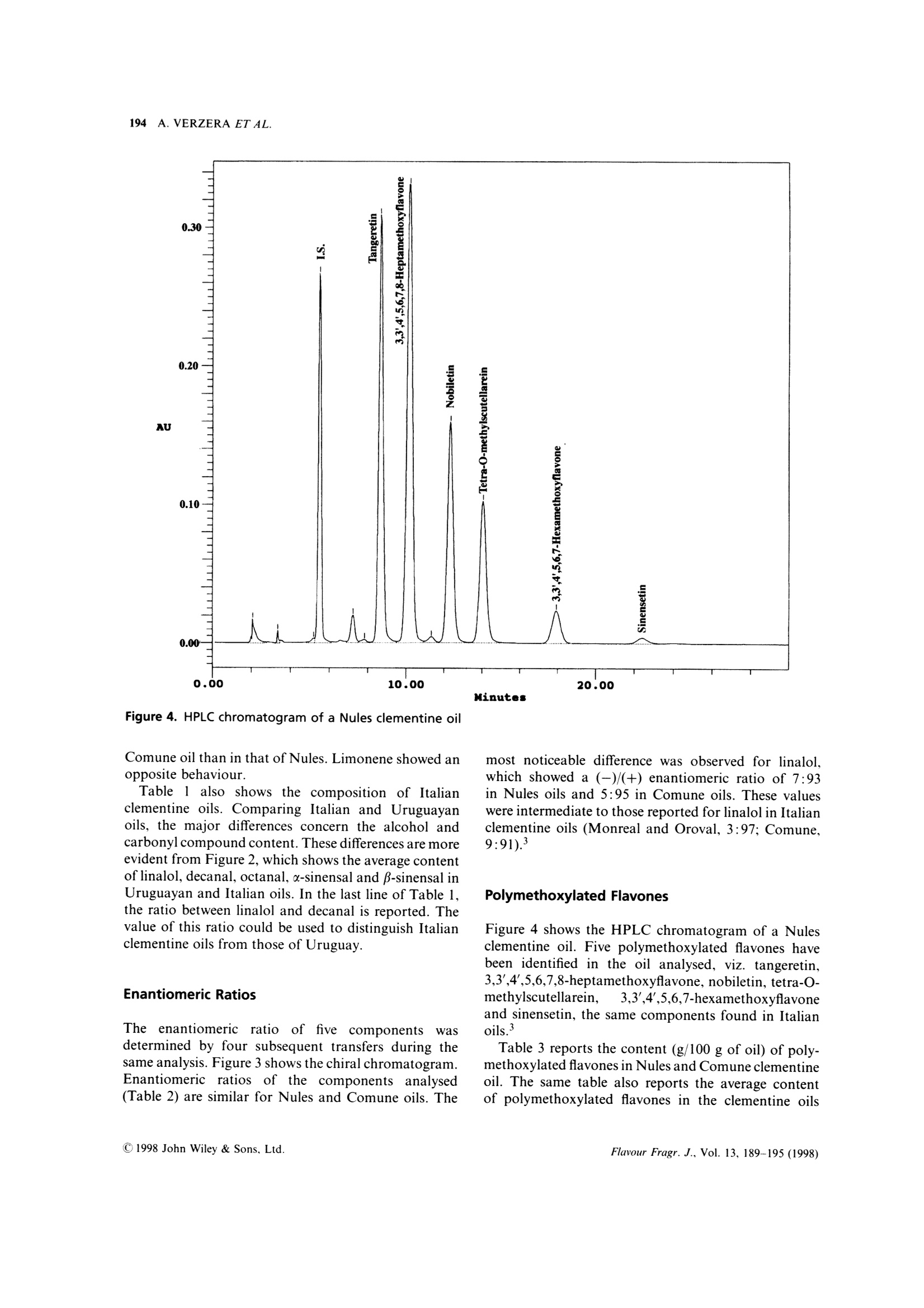
FLAVOUR AND FRAGRANCE JOURNALFlavour Fragr.J., 13, 189-195 (1998) 190A. VERZERA ET AL. Uruguayan essential oils. Part X. Composition of the oilof Citrus clementine Hort.t Antonella Verzera,1* Alessandra Trozzi,2 Luigi Mondello,1Eduardo Dellacassa and Daniel Lorenzo 1Dipartimento Farmaco-chimico, Facolta di Farmacia, Universita di Messina, Viale Annunziata, I-98168 Messina, Italy 2Facolta di Farmacia, Universita di Reggio Calabria, I-88021 Catanzaro, Italy 3Catedra de Farmacognosia, Facultad de Quimica, Universitad de la Republica, Avda. General Flores 2124, UR-11800 Montevideo,Uruguay Received 29 April 1997Accepted 10 July 1997 ABSTRACT: The composition of the essential oil of Uruguayan Citrus clementine Hort., prepared in thelaboratory from the fruit of Nules and Comune cultivars, has been studied. The volatile fraction was analysedby HRGC and HRGC-MS (quadrupole); 69 components were identified; the enantiomeric distribution ofB-pinene, sabinene, limonene, linalol and a-terpineol was studied by multidimensional HRGC-HRGC. Poly-methoxylated flavones present in the non-volatile residue were analysed by normal-phase HPLC. The results werecompared with those of Italian clementine oil. C 1998 John Wiley & Sons, Ltd. KEY WORDS: Citrus clementineHort.; clementine; Rutaceae; Nules;CComune;volatile fractioncomposition;:eenantioselective gas chromatography; polymethoxylated flavones Introduction In recent years, Citrus clementine Hort. has become themost popular mandarin variety in the Mediterraneanregion, with production expanding particularly inMorocco and Spain. Clementines have been developedespecially in Spain, and many new mutations have beendiscovered recently. This has extended the clementineseason in Europe from its former 2-month period, fromNovember to December, to cover the period frommid-October to mid-February.2 In a previous paper weanalysed the composition of Citrus clementine oil fromthe cultivars Monreal, Oroval and Comune grown inItaly. The composition of the volatile fraction and non-volatile residue was reported. This composition wasthen compared with that of Italian sweet orange andmandarin oil. In the context of research on Uruguayan citrusessential oils, we report here the results relative to thecomposition of the oil of two clementine cultivars oilgrown in Uruguay: Nules and Comune. The cultivarNules was discovered in Casrellon Province, Spain, in1953 where it constitutes around half of the currentplantings. In recent years Uruguay clementine production hasincreased and the export of clementine fruits grew from ( *Correspondence to: A . V e rzera, D i partimento Fa r m a co-chimico, Facol t a diFarmacia, Universita di M essina, V iale A n nunziata, 1-98168 Messina. Italy+ For p art I X s ee r ef. 1 . ) 71,420 g in 1995 to 86,340 g in 1996. The productionperiod for clementine fruit in Uruguay extends fromApril to July. The extraction of this essential oil isnot an industrial process but we like to check thecomposition of these oils which could provide new andinteresting material for the food industries. In theliterature there arenoreportsaboutUruguayan clementine oil, while only a few papersdeal with Italian4-7 and Algerian oils. Experimental Research was carried out on two samples of Nulesclementine oil and two samples of Comune clementineoil. Fruity were picked from April to May 1996 inEstacion Experimental INIA-Salto Grande, Departe-mento de Salto in North Uruguay. Extraction of theessential oil was carried out in the laboratory byapplying manual pressure on the rind so as to cause thebreaking of the utricles and the release of the oil itself,which was collected on a watch glass, transferred to atest tube, centrifuged and analysed. GC Analysis The volatile fraction was analysed by HRGC, under thesame conditions used for Italian clementine oils,’ on a Table 1. Percentage composition as single components and as classes of substances of Uruguayan and Italianclementine oils (Citrus clementine Hort.) (Table continues opposite) Date of sample Uruguayan oils Italian oils Nules Commune Oroval Monreal Comune 23/4/96 10/5/96 23/4/96 10/5/96 x x x 66. B-Sinensale 0.04 0.07 0.05 0.05 0.06 0.03 0.16 67. x-Sinensale 0.21 0.28 0.23 0.24 0.30 0.26 0.56 68. Nootkatone tr tr 0.03 0.01 0.01 0.01 0.01 Hydrocarbons 97.72 98.02 97.20 97.49 97.49 97.56 96.23 Monoterpenes 97.46 97.71 96.99 97.23 97.26 97.36 95.73 Sesquiterpenes 0.26 0.31 0.20 0.26 0.23 0.19 0.50 Oxygenated compounds 2.00 1.75 2.37 2.35 2.41 2.36 3.52 Carbonyl compounds 1.21 1.14 1.44 1.54 1.19 1.12 2.14 Alcohols 0.73 0.58 0.91 0.77 1.19 1.18 1.31 Esters 0.02 0.02 0.02 0.02 0.01 0.02 0.04 Linalol/Decanal 1.4 1.3 1.7 1.6 4.2 4.5 2.3 Fisons Mega Series 5160 gas chromatograph equippedwith a Shimadzu data processor C-R3A and a fusedsilica SE-52 column (30 m x0.32 mm i.d., film thick-ness, 0.40-0.45 um, Mega, Legnano (MI), Italy);column temperature, 45℃ (6 min) to 180°C at 3C/min; injection mode, split; detector, FID; injector anddetector temperature, 280℃; carrier gas, He 95 kPa;injected volume, 1 pl of neat oil. GC-MS Analysis Samples were analysed by GC-MS (EI) on a FisonsMD 800 (Milan,Italy) system coupled with Adams’library’ and FFC bank;1 GC conditions were: a DB-5fused silica column (30 m×0.25 mm, 0.25 um filmthickness); column temperature, 45℃ (6 min) to 240°℃at 3°C/min; carrier gas, He at constant pressure of83 kPa. Aquisition parameters, full scan; scan range, 41-300 amu; temperature transfer line, 220°C; 1 pl ofsolution (0.33% v/v essential oil/pentane) was injectedon a cold on-column system fitted with an automatedactuator. The MS scan conditions were: sourcetemperature, 200C; interface temperature, 260℃;E energy, 70 eV; mass scan range, 39.00-350.00 amu. Chiral Analysis Enantiomeric ratios of some monoterpene hydro-carbons (B-pinene, sabinene, limonene) and of somemonoterpene alcohols (linalol, a-terpineol)wereobtained by multidimensionalgas chromatography,using a developmental modell set up with two GCovens, the first equipped with a column coated withSE-52 and the second with a chiral column coated witha derivatized β-cyclodextrin, a hot interface, a rotaryswitching valve and a system to maintain a constant Table 2. Enantiomeric ratios for B-pinene, sabinene, limonene, linalol and x-terpineol inUruguayan clementine oils (Citrus clementine Hort.) Date of sample Uruguayan oils Nules Comune 23/4/96 10/5/96 23/4/96 10/5/96 B-Pinene 1R,5R (+) 58.1 61.1 60.1 60.1 1S,5S (-) 41.9 38.9 39.9 39.9 Sabinene 1R,5R (+) 97.5 97.6 97.6 97.6 1S,5S(-) 2.5 2.4 2.4 2.4 Limonene 4S (-) 0.6 0.6 0.6 0.6 4R(+) 99.4 99.4 99.4 99.4 Linalol 3R(-) 6.7 6.7 5.0 5.3 3S (+) 93.3 93.3 95.0 94.7 x-Terpineol 8S (-) 2.7 2.5 2.5 2.6 8R (+) 97.3 97.5 97.5 97.4 98 35.000 nt 37.500 5009 40.000 7.500 10.000 175m 15.000 17.500 20.000 22.500o 25.000 30.000 Figure 1. Total ion chromatogram of the volatile fraction of a Nules clementine oil. For peak identification see Table 1 Figure 2. Linalol, decanal, octanal, u-sinensal and B-sinensal content of Uruguayan and Italian clementine oils Table 3. Content (q/100 of oil) of Polymethoxylated Flavones in Uruguayan and Italian clementine oils(Citrus clementine Hort.) Uruguayan oils Italian oils Nules Commune Monreal Oroval Comune 23/4/96 10/5/96 23/4/96 10/5/96 x x x Tangeretin 0.13 0.15 0.17 0.14 0.10 0.10 0.17 3,3',4',5,6,7,8-Heptamethoxyflavone 0.53 0.32 0.38 0.36 0.27 0.29 0.51 Nobiletin 0.14 0.12 0.11 0.10 0.09 0.09 0.09 Tetra-O-methylscutellarein 0.07 0.07 0.08 0.07 0.05 0.04 0.06 3,3',4',5,6,7-Hexamethoxyflavone 0.06 0.04 0.04 0.04 0.02 0.02 0.02 Sinensetin 0.01 0.01 0.00 0.0 tr tr tr flow during the transfer. With this system a heart-cut ofthe relevant fractions can be made and these fractionstransferred from the non-chiral column to the chiralone in the following experimental conditions: pre-column, fused silica SE-52 column (30 m x0.32 mmi.d., film thickness 0.40-0.45 um, Mega, Legnano(MI),Italy); column temperature 45℃ (6 min) to 220℃at 2℃/min; analytical column, fused silica capillarycolumn 25 m ×0.25 mmi.d., coated with a diethyl tert-butylsilyl-B-cyclodextrin, Mega, Legnano (MI), Italy;column temperature, 40-180°℃ at 2°C/min; interfacetemperature, 200℃; detector FID, 250℃ (for bothchromatographs). Figure 3. Chiral gas chromatogram of a Nules clementineoil HPLC Analysis All samples were analysed by normal-phase HPLC,using Waters Associates (WA) equipment composed ofa model 519 pump; a 600 E gradient controller, aRheodyne 9125 injector and a photo-diode arraydetector model 996. Peak integration and quantitativecalculations were performed by Millenium 2010 (WA)system using a calibration curve obtained for eachstandard component against a coumarin standard.The column was a Zorbax silica column (25 cmx4.6 mm i.d.; particle size, 7 um); mobile phase, hex-ane:ethyl alcohol, 95:5; flow rate, 1.6 ml/min; injectionvolume, 20 ul of a solution obtained by diluting about50 mg of each oil and 0.1 ml of a coumarin solution ofknown concentration in 1 ml of hexane:ethyl acetate(75:25). Detection was by UV absorbance at 315 nm.The UV spectra of eluting peaks were monitored withthe PDA detector in the region 200-400 nm. Results Volatile Fraction Figure 1 shows the total ion chromatogram of aNules clementine oil; the composition as classes ofsubstances and as single components is reported inTable 1. Sixty-nine components were identified in eachoil: the same number were identified in Italian oils. The oils were characterized by a high content oflimonene (more than 93%) and, among oxygenatedcompounds,s, oflinalol(0.5-0.7%))and(decanal(0.4%), by the presence of 8-3-carene, of a large number(16) of sesquiterpenes and of β-sinensal and a-sinensal(about 0.3% together). Among the oxygenated com-pounds, the main class was carbonyl compoundsfollowed by alcohols; esters were less represented. The composition of the oil of the two cultivars wasvery similar. Small differences were due to the alcoholand carbonyl compound content which were higher in AU Figure 4. HPLC chromatogram of a Nules clementine oil Comune oil than in that of Nules. Limonene showed anopposite behaviour. Table 1 also shows the composition of Italianclementine oils. Comparing Italian and Uruguayanoils, the major differences concern the alcohol andcarbonyl compound content. These differences are moreevident from Figure 2, which shows the average contentof linalol, decanal, octanal, u-sinensal and p-sinensal inUruguayan and Italian oils. In the last line of Table 1,the ratio between linalol and decanal is reported. Thevalue of this ratio could be used to distinguish Italianclementine oils from those of Uruguay. Enantiomeric Ratios The enantiomeric ratio of ffive componentsWwasdetermined by four subsequent transfers during thesame analysis. Figure 3 shows the chiral chromatogram.Enantiomeric ratios of the componentsanalysed(Table 2) are similar for Nules and Comune oils. The most noticeable difference was observed for linalol.which showed a (-)/(+) enantiomeric ratio of 7:93in Nules oils and 5:95 in Comune oils. These valueswere intermediate to those reported for linalol in Italianclementine oils (Monreal and Oroval, 3:97; Comune,9:91). Polymethoxylated Flavones Figure 4 shows the HPLC chromatogram of a Nulesclementine oil. Five polymethoxylated flavones havebeen identified in the oil analysed, viz. tangeretin,3,3',4',5,6,7,8-heptamethoxyflavone, nobiletin, tetra-O-methylscutellarein, 3,3',4',5,6,7-hexamethoxyflavoneand sinensetin, the same components found in Italianoils.3 Table 3 reports the content (g/100 g of oil) of poly-methoxylated flavones in Nules and Comune clementineoil. The same table also reports the average contentof polymethoxylated flavones in the clementine oils of the three Italian cultivars previously analysed.As in the Italian oils, the main componentwas3,3',4',5,6,7,8-heptamethoxyflavone which ranges from0.32 to 0.53 g/100 in the oils analysed. As regardpolymethoxyflavones, Italian and Uruguayan clemen-tines appear very similar. References 1. E. Dellacassa, D. Lorenzo, L. Mondello and P. Dugo, J. Essent.Oil Res., 9, 673 (1997). 2. J. Saunt, editor, Citrus Varieties of the World, SinclairInternational Ltd., Norwich (1990). 3. A. Verzera, L. Mondello, A. Trozzi and P. Dugo, Flavour Fragr.J., 12,163(1997). 4. I. Calvarano, F. Bovalo and A. Di Giacomo, Essenz. Deriv.Agrum., 44, 117 (1974). 5. G. Dugo, A. Cotroneo, A. Trozzi, M. BarbeniandA. Di Giacomo, Flavour Fragr. J., 3, 161 (1988)). 6.(G. Ruberto, D. Biondi, M. Piattelli, P. Rapisarda an. A. Starrantino, J. Essent. Oil Res., 6, 1 (1994). 7. L. Mondello, P. Dugo, K. D. Bartle, G. Dugo and A. Cotroneo,Flavour Fragr. J., 10, 33(1995). 8. A. Baaliouamer, B.-Y. Meklati, D. Fraisse and C. Scahrff,J. Essent. Oil Res., 4, 251 (1992). 9. R. Adams, Identification of Essential Oil Components by GasChromatography/Mass Spectroscopy, Allured Publishing Corpor-ation, Carol Stream, IL (1995). 1o. L]. Mondello, P. Dugo, A. Basile and G. Dugo, J. MicrocolumnSep., 7, 58 (1995). 11. L. Mondello, M. Catalfamo, P. Dugo, G. Dugo,J. Chromatogr.Sci., 36,201(1998). 12. P. Dugo, L. Mondello, E. Cogliandro, I. Stagno d'Alcontres andA. Cotroneo, Flavour Fragr. J., 9, 105 (1994). CCC C John Wiley & Sons, Ltd.Flavour Fragr. J., Vol. (
确定


还剩5页未读,是否继续阅读?
扬州华明仪器设备有限公司为您提供《乌拉圭柑橘克莱门园艺精油中成分分析检测方案 》,该方案主要用于日用化学品/香精香料中成分分析检测,参考标准--,《乌拉圭柑橘克莱门园艺精油中成分分析检测方案 》用到的仪器有
相关方案
更多








