
方案详情
文
the hydrodistilled essential oil from aerial parts of cymbopogon winterianus Jowitt....
方案详情

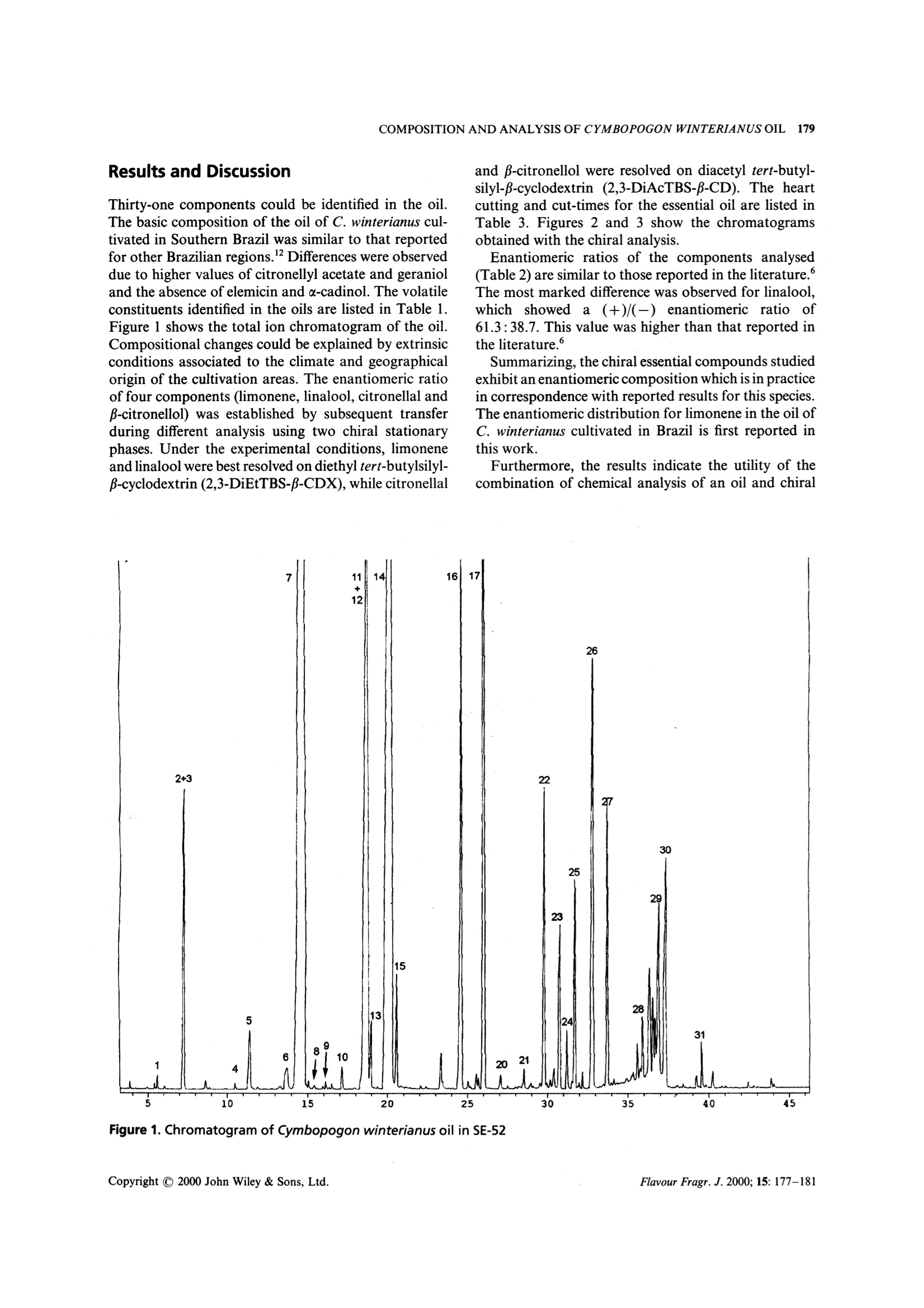
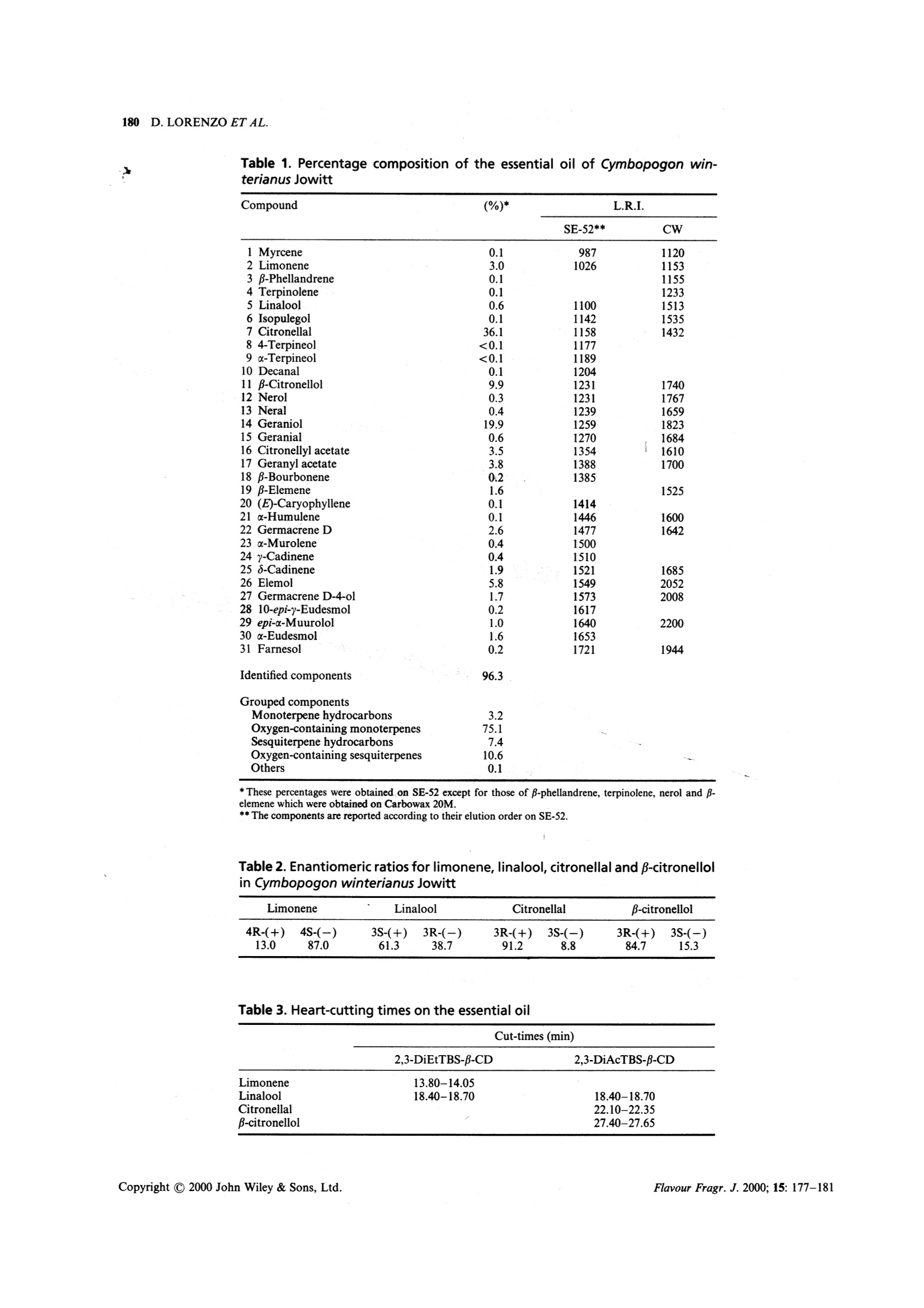
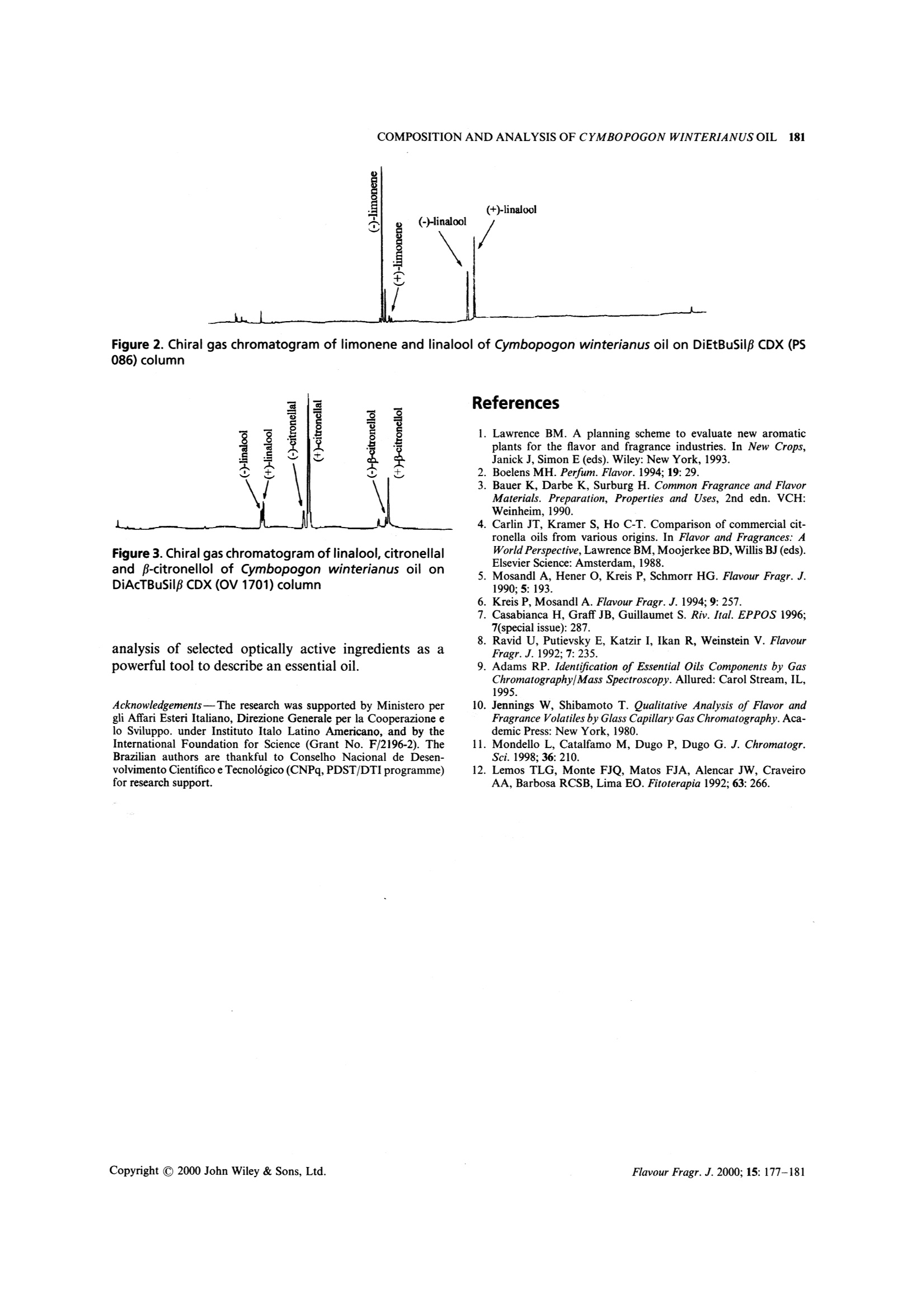
FLAVOUR AND FRAGRANCE JOURNALFlavour Fragr.J. 2000; 15: 177-181 178D.LORENZO ETAL. Composition and Stereoanalysis of Cymbopogonwinterianus Jowitt Oil from Southern Brazil D. Lorenzo,E.Dellacassa,*L. Atti-Serafini,’A. C. Santos,’C. Frizzo,N. Paroul,’P. Moyna,12L. Mondelloand G. Dugo 1Catedra de Farmacognosia, Facultad de Quimica, Universidad de la Republica, Avda. General Flores 2124, UR-11800 Montevideo,Uruguay "Instituto de Biotecnologia Universidade de Caxias do Sul, Rua Fco. Getulio Vargas 1130 Caxias do Sul, RS, Brazil Dipartimento Farmaco-chimico, Facolta di Farmacia, Universita di Messina, Via SS. Annunziata, 98168 Messina, Italy Received 1 May 1999 Revised 10 December 1999 Accepted 16 December 1999 ABSTRACT: The hydrodistilled essential oil from aerial parts of Cymbopogon winterianus Jowitt, cultivated inSouthern Brazil, was analysed by GC-MS. Thirty-one components, representing 96% of the oil, were char-acterized. Enantiomeric ratios of limonene, linalool, citronellal and p-citronellol were obtained by mul-tidimensional gas chromatography, using a developmental model set up with two GC ovens. The enantiomericdistributions are discussed as indicators of origin authenticity and quality of this oil. Copyright C 2000 JohnWiley & Sons, Ltd. KEY WORDS: Cymbopogon winterianus Jowitt; citronella; volatile fraction composition; enantioselective gaschromatography Introduction Citronella oils are produced from two distinct types ofcitronella grass: Cymbopogon nardus L. Rendle, knownas lenabatu or Ceylon citronella, and C. winterianusJowitt, known as maha pengiri or Java-type citronella.The Ceylon type is the original cultivated variety andthe Java type seems to have arisen as a distinct form ofthe Ceylon type. Until the early part of this century the Ceylon typecitronella was the most widely produced oil. Graduallythe Java type has come to dominate the market, due toits higher yield of oil. It is grown commercially on aworldwide scale in places like Haiti, Central America,the South Pacific and tropical Africa. More recentlyother countries, such as Brazil, have contributed to thecitronella oil production. Java citronella oil is used directly in perfumery, but ( * Correspondence to : E. Dellacassa, C a tedra de Farmacognosia, Fac u ltad deQuimica, Universidad d e la Republica, Avda.General F lores 2124, UR-11800 M ontevideo, Uruguay. ) ( Contract/grant sponsor: Min i stero per gli Affa r i Esteri Italiano, Italy. C ontract/grant sponsor: Direzione Generale per la Cooperazione e lo Sviluppo, I nstituto Italo-Latino-Americano. Contract/grant sponsor: I nternational Foundation f o r Science.Contract/grant number: F/2196-2. ) ( Contract/grant sponsor: Conselho N acional de Desenvolvimento Cientifico e Tecnologico. ) Contract/grant number: CNPq, PSDT/DTI. is also one of the most important sources of naturalcitronellal, this component being present at higher con-centration than in Ceylon oil.2.3 Before the advent of chemical sprays, Ceylon cit-ronella was used in combination with Virginian redcedarwood oil in commercial insect repellents. Probablydue to its association with insect repellency, citronellais usually overlooked as an aromatherapy oil ingredient. A comprehensive survey on the composition of cit-ronella oils has been published,4 showing considerabledifferences in the components of the Java oils in relationto genetic, climatic and geographical origins, as well ason the stage of development of the plants used for theoil distillation. Given the wide use of citronella oils and their frequentuse as a basis of blends with other oils, as well as theirvariability, chemical evaluation of their monoterpenoidconstituents is of fundamental interest. Furthermore,the enantiomeric distribution of the different mono-terpenic components has been reported as criteria forgenuinity in citronella oils.5-8 As part of our research on Brazilian essential oils, wereport here the results relative to the composition andenantiomeric distribution of four monoterpenes (lim-onene, linalool, citronella and p-citronellol) of Cym-bopogon winterianus Jowitt cultivated in the South ofBrazil. Experimental Materials The aerial parts of Java citronella (C. winterianus Jow-itt) were harvested from a small-scale experimental areacultivated at the Prefeitura de Santo Antonio daPatrulha in Rio Grande do Sul State (Brazil), in April1997. The essential oil was obtained from the aerialparts after 2 h of hydrodistillation in the pilot plant ofthe Instituto de Biotecnologia, Universidade de Caxiasdo Sul. The average yield was 0.74 (w/w). GC Analysis The composition of the oil was carried out by GC on aShimadzu 14 B gas chromatograph equipped with aFID and a Shimadzu data processor software EZ-Chrom, using two capillary columns. The first was aSE-52 (Mega, Legnano, Italy) cross-linked fused-silicacapillary column (25 mx0.32 mm i.d.), coated with 5%phenyl-polymethylsiloxane (0.40-0.45 um phase thick-ness); column temperature, 60C (8 min) to 180℃ at3℃/min, 180-250℃ at 20℃/min,250℃ (10 min).Injector temperature 250℃; detector temperature280℃; injection mode, split; split ratio 1:30; volumeinjected, 0.2 pl of the oil. Carrier gas was hydrogen, 55kPa. The second was a Carbowax 20M (Ohio Valley,USA)bonded fused-silica capillary column (25 mx0.32 mmi.d.), coated with polyethylene glycol (0.25 pm phasethickness); column temperature, 40℃ (8 min) to 180Cat 3C/min, to 230°℃ at 20°C/min. Injector temperature250°C; detector temperature 250℃; injection mode,split; split ratio 1:30; volume injected, 0.2 pl of the oil.Carrier gas was hydrogen, 30 kPa. GC-MS Analysis GC-MS analysis were conducted using a Shimadzu QP5050 equipped with Adams library, using two capillarycolumns. The first was a SE-52 (Mega, Legnano, Italy)cross-linked fused-silica capillary column (25 mx0.25mm i.d.), coated with 5% phenyl-polymethylsiloxane(0.25 um phase thickness); column temperature, 60℃(8 min) to 180℃ at 3℃/min, to 230℃ at 20℃/min.Injector temperature 250°℃; injection mode, split; splitratio 1.40; volume injected,0.2pl of the oil. Helium wasused as a carrier, using 122.2 kPa (51.6 cm/s); interfacetemperature 250°℃; acquisition mass range 40-400;solvent cut, 2 min. The second was a BP 20 (SGE, Australia) bondedfused-silica capillary column (25 mx0.25 mm i.d.),coated with polyethylene glycol (0.25 um phase thick-ness); column temperature, 40℃ (8 min) to 180℃ at3℃/min, to 230℃ at 20℃/min. Injector temperature 250℃; injection mode, split; split ratio 1.40; volumeinjected, 0.2 pl of the oil. Carrier gas was He, 92.6 kPa(55.9 cm/s); interface temperature 250℃; acquisitionmass range 40-400; solvent cut, 2 min. Individual components were identified by com-parison of both mass spectrum and their GC retentiondata with those of authentic compounds previously ana-lysed and stored in the data system. Otheridentificationswere made by comparison of mass spectra with those inthe data system libraries and cited in the literature.9,10The linear retention index (LRI) of individual com-ponents were measured on both columns (SE 52 andCarbowax 20M). Chiral Analysis Enantiomeric ratios of limonene, linalool, citronellaland p-citronellol were obtained by multidimensionalgas chromatography, using a model under devel-opment set up with two GC ovens, the first equippedwith a column coated with SE-52 and the second with achiral column coated with a derivatized -cyclodextrin,and a hot interface, a rotary switching valve and asystem to maintain a constant flow during the transfer.With this system a heart-cut of the relevant fractionscould be made and these fractions transferred from thenon-chiral column to the chiral one in the followingexperimental conditions: precolumn, SE-52 (Mega,Legnano, Italy) cross-linked fused-silica capillary col-umn (30 mx0.32 mm i.d.), coated with 5% phenyl-polymethylsiloxane (0.40-0.45 pm phase thickness);column temperature; 45℃(6min) to 280℃ at 2℃/min;280℃ (15 min). Two different analytical columns wereused, the separation of limonene and linalool enanti-omers was achieved using a fused-silica capillary col-umn (25 m×0.25 mm i.d., 0.25 um phase thickness),coated with 2,3-di-O-ethyl 6-O-t-butyldimethylsilyl-cyclodextrin in PS 086(13% phenylmethyl-poly-siloxane) (Mega, Legnano, Italy) while citronellal andB-citronellol enantiomers were separated with a fused-silica capillary column (25 mx0.25 mm i.d., 0.25 pmphase thickness), coated 2,3-di-O-acetyl 6-0-t-butyl-dimethylsilyl B-cyclodextrin in OV 1701 (14% cyano-propylphenyl-methylpolysiloxane) (Mega, Legnano,italy); injection temperature, 250°C; column tempera-ture, 45℃ (6 min) to 90℃ at 2℃/min, 90℃ (20 min);90℃ to 180℃ at 2°℃/min, 180℃ (10 min); interfacetemperature, 200℃; detector FID, 280℃ (for bothchromatographs). Volume injected, 1 pl of an oildilution 1:10; injection mode, split; split ratio 1:15.Carrier gas was Ar, 90 kPa (precolumn), 110 kPa (ana-lytical column). Evaluation of the elution order of theenantiomers was achieved using monoterpene mixtureswith known enantiomeric ratio. Results and Discussion Thirty-one components could be identified in the oil.The basic composition of the oil of C. winterianus cul-tivated in Southern Brazil was similar to that reportedfor other Brazilian regions.Differences were observeddue to higher values of citronellyl acetate and geranioland the absence of elemicin and a-cadinol. The volatileconstituents identified in the oils are listed in Table 1.Figure 1 shows the total ion chromatogram of the oil.Compositional changes could be explained by extrinsicconditions associated to the climate and geographicalorigin of the cultivation areas. The enantiomeric ratioof four components (limonene, linalool, citronellal andβ-citronellol) was established by subsequent transferduring different analysis using two chiral stationaryphases. Under the experimental conditions, limoneneand linalool were best resolved on diethyl tert-butylsilyl-β-cyclodextrin (2,3-DiEtTBS-B-CDX), while citronellal and β-citronellol were resolved on diacetyl tert-butyl-silyl-β-cyclodextrin (2,3-DiAcTBS-B-CD). The heartcutting and cut-times for the essential oil are listed inTable 3. Figures 2 and 3 show the chromatogramsobtained with the chiral analysis. Enantiomeric ratios of the components analysed(Table 2) are similar to those reported in the literature.°The most marked difference was observed for linalool,which showed a (+)/(-) enantiomeric ratio of61.3:38.7. This value was higher than that reported inthe literature.° Summarizing, the chiral essential compounds studiedexhibit an enantiomeric composition which is in practicein correspondence with reported results for this speciesThe enantiomeric distribution for limonene in the oil ofC. winterianus cultivated in Brazil is first reported inthis work. Furthermore, the results indicate the utility of thecombination of chemical analysis of an oil and chiral 7 2+3 5 6 4 人 11+12 910 14 13 16 15 17 22 20 21 23 Figure 1. Chromatogram of Cymbopogon winterianus oil in SE-52 Table 1. Percentage composition of the essential oil of Cymbopogon win-terianus Jowitt *These percentages were obtained on SE-52 except for those of p-phellandrene, terpinolene, nerol and β-elemene which were obtained on Carbowax 20M. ** The components are reported according to their elution order on SE-52. Table 2. Enantiomeric ratios for limonene, linalool, citronellal and p-citronellolin Cymbopogon winterianus Jowitt Limonene Linalool Citronellal B-citronellol 4R-(+) 4S-(-) 3S-(+) 3R-(-) 3R-(+) 3S-(-) 3R-(+) 3S-(-) 13.0 87.0 61.3 38.7 91.2 8.8 84.7 15.3 Table 3. Heart-cutting times on the essential oil 2,3-DiEtTBS-B-CD 2,3-DiAcTBS-B-CD Limonene Cut-times (min) 13.80-14.05 Linalool 18.40-18.70 18.40-18.70 Citronellal 22.10-22.35 B-citronellol 27.40-27.65 Figure 2. Chiral gas chromatogram of limonene and linalool of Cymbopogon winterianus oil on DiEtBuSilf CDX (PS086) column Figure 3. Chiral gas chromatogram of linalool, citronellaland p-citronellol of Cymbopogon winterianus oil onDiAcTBuSil CDX (OV 1701) column analysis of selected optically active ingredients as apowerful tool to describe an essential oil. Acknowledgements-The research was supported by Ministero pergli Affari Esteri Italiano, Direzione Generale per la Cooperazione elo Sviluppo. under Instituto Italo Latino Americano, and by theInternational Foundation for Science (Grant No. F/2196-2). TheBrazilian authors are thankful to Conselho Nacional de Desen-volvimento Cientifico e Tecnologico (CNPq, PDST/DTI programme)for research support. References 1. Lawrence BM. A planning scheme to evaluate new aromaticplants for the flavor and fragrance industries. In New Crops,Janick J, Simon E (eds). Wiley: New York, 1993. 2. Boelens MH. Perfum. Flavor. 1994;19: 29. 3. Bauer K, Darbe K, Surburg H. Common Fragrance and FlavorMaterials. Preparation, Properties and Uses, 2nd edn. VCH:Weinheim, 1990. 4. Carlin JT, Kramer S, Ho C-T. Comparison of commercial cit-ronella oils from various origins. In Flavor and Fragrances: AWorld Perspective, Lawrence BM, Moojerkee BD, Willis BJ (eds).Elsevier Science: Amsterdam, 1988. 5. Mosandl A, Hener O, Kreis P, Schmorr HG. Flavour Fragr. J.1990;5:193. 6. Kreis P, Mosandl A. Flavour Fragr. J. 1994;9: 257. 7. Casabianca H, Graff JB, Guillaumet S. Riv. Ital. EPPOS 1996;7(special issue): 287. 8. Ravid U, Putievsky E, Katzir I, Ikan R, Weinstein V. FlavourFragr. J.1992; 7: 235. 9. Adams RP. Identification of Essential Oils Components by GasChromatography/Mass Spectroscopy. Allured: Carol Stream, IL,1995. 10. Jennings W, Shibamoto T. Qualitative Analysis of Flavor andFragrance Volatiles by Glass Capillary Gas Chromatography.Aca-demic Press: New York, 1980. 11. Mondello L, Catalfamo M, Dugo P, Dugo G. J. Chromatogr.Sci. 1998;36: 210. 12. Lemos TLG,Monte FJQ, Matos FJA, Alencar JW, CraveiroAA, Barbosa RCSB, Lima EO. Fitoterapia 1992; 63: 266. Flavour Fragr. J. opyright C John Wiley & Sons, Ltd. Copyright C John Wiley & Sons, Ltd.Flavour Fragr. J.
确定


还剩3页未读,是否继续阅读?
扬州华明仪器设备有限公司为您提供《水蒸馏精油中化学成分检测方案 》,该方案主要用于日用化学品/香精香料中化学成分检测,参考标准--,《水蒸馏精油中化学成分检测方案 》用到的仪器有
相关方案
更多







