
方案详情
文
the specific character and the intensity of flavor and fragrance....
方案详情

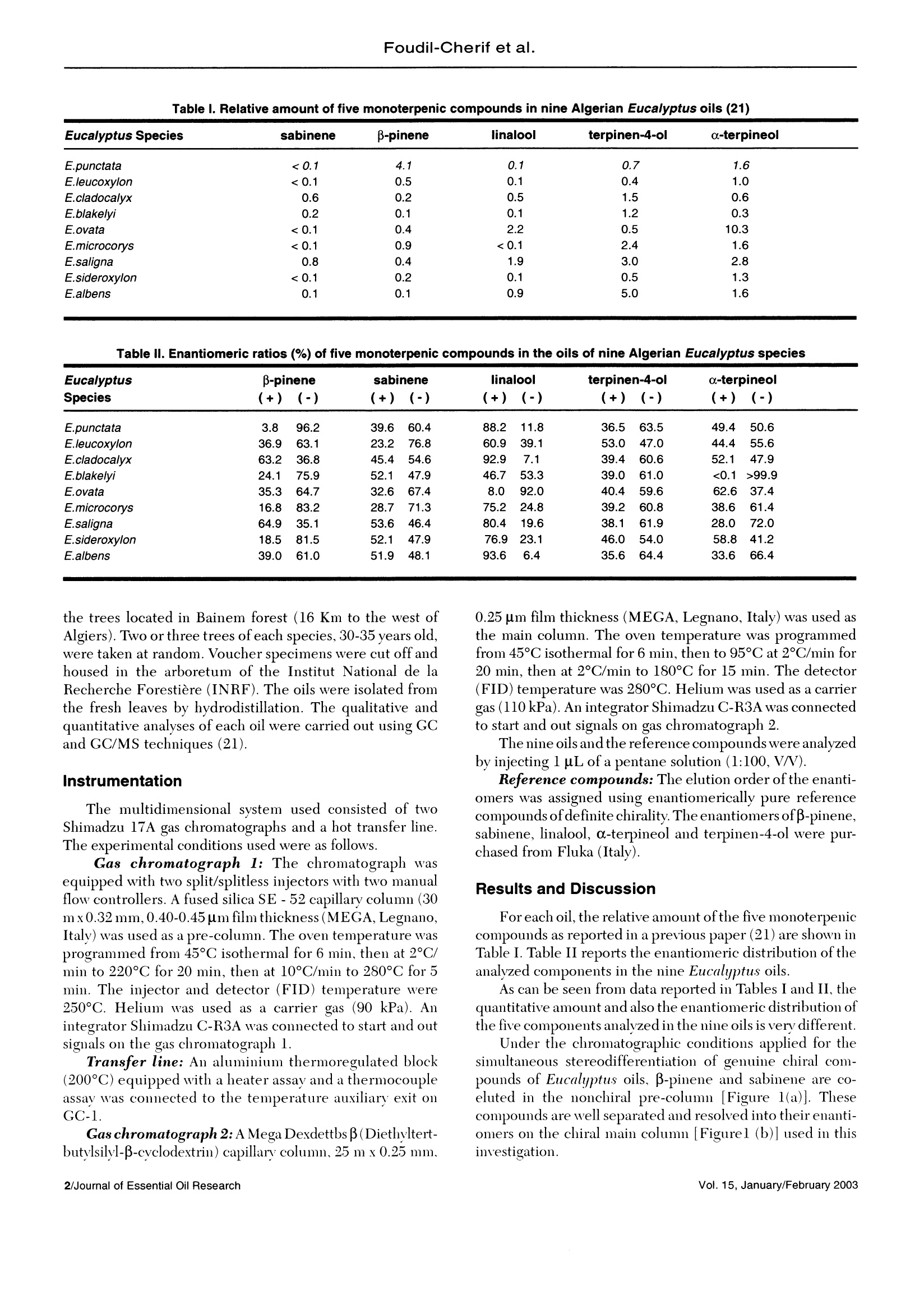
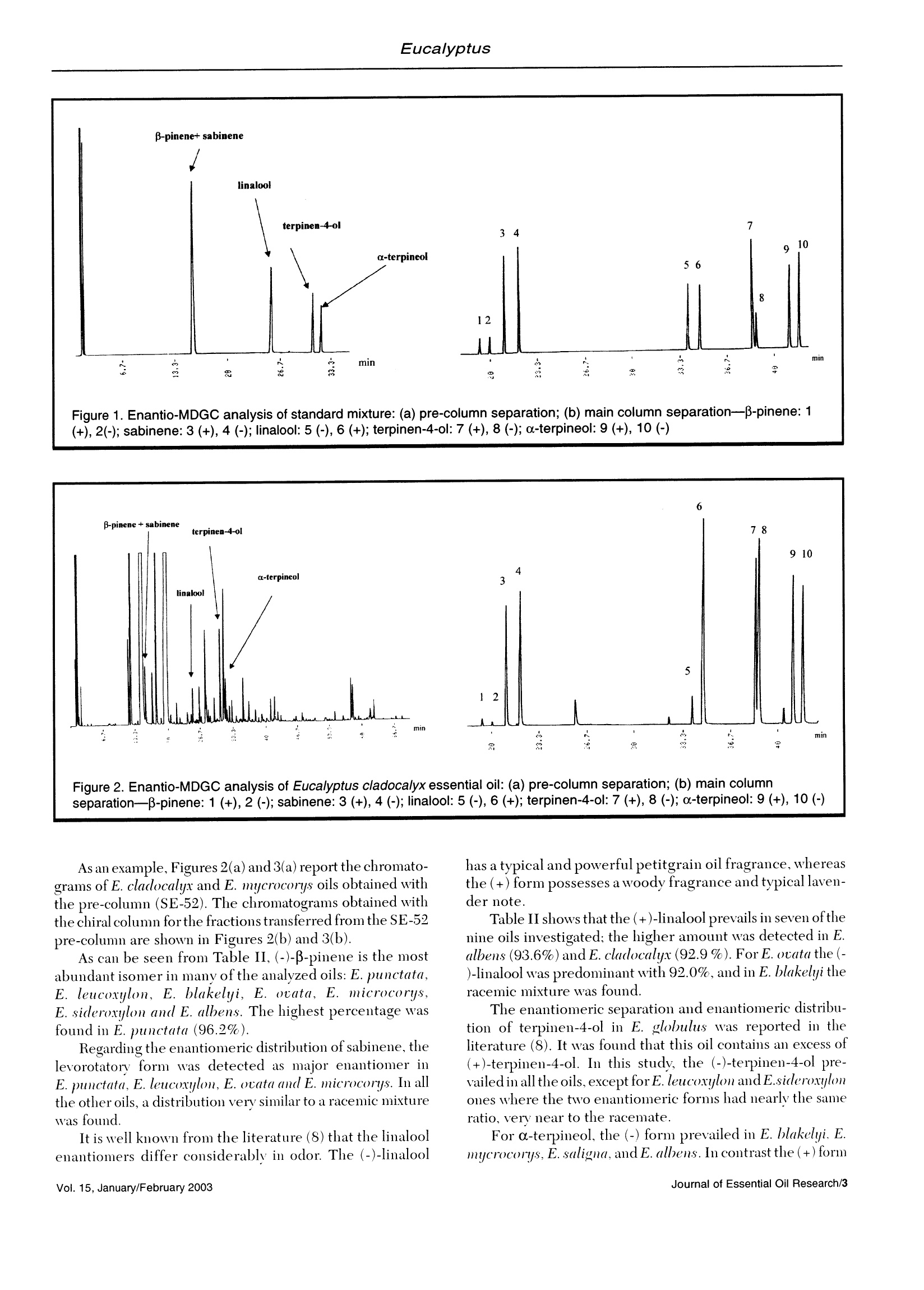
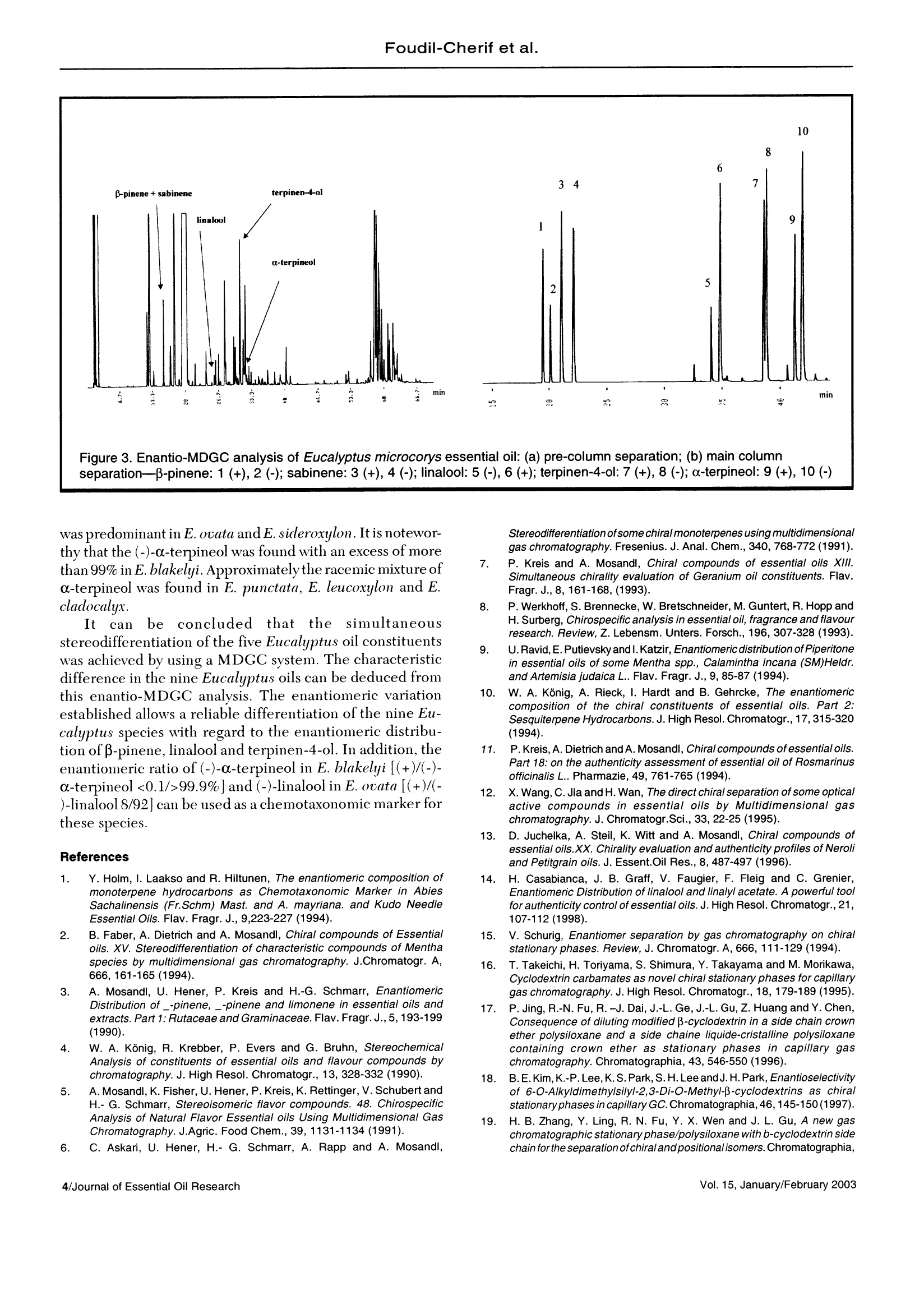
J. Essent. Oil Res., 15, 1-5(January/February 2003) Foudil-Cherif et al. Enantiomeric Distribution of Five MonoterpenicCompounds in Nine Algerian Eucalyptus EssentialOils by Direct Multidimensional Gas Chromatography Y. Foudil-Cherif* Universite des Sciences et de la Technologie Houari Boumediene, Institut de Chimi; El-Alia BP 32, Bab-Exsouar AlgerAlgerie B.Y. Meklati C.R.A.P.C, BP 248 Alger RP 16004, Algerie L. Mondello, G. Zappia and G. Dugo Dipartimento Farmaco-chimico, Facolt di Farmacia, Università di Messina, Italy Abstract The enantiomeric distribution of sabinene,β-pinene,linalool,terpinen-4-ol,and o-terpineol for nine Eucalyptusspecies has been determined by using a fully automated, multidimensional, double-oven(MDGC)system equippedwith a SE-52 precolumn and a B-cyclodextrin main column. This system allows fractions to be multitransferred duringthe same GC analysis, and the use of the two GCs independently when the multitransfer option is rejected. Theresults obtained allowed a reliable differentiation between the nine eucalyptus oils. Key Word Index Eucalyptus punctata, Eucalyptus sideroxylon, Eucalyptus saligna, Eucalyptus cladocalix, Eucalyptus albens,Eucalyptus ovata, Eucalyptus leucoxylon, Eucalyptus blakelyi, Eucalyptus microcorys, Myrtaceae, chiral analysis,enantiomeric distribution. Introduction The specific character and the intensity of flavor andfragrance compounds is often related to their stereochemis-try. In this way, the knowledge of the enantiomeric ratios ofsome constituents can be used as a potent genus chemotaxo-nomic marker(1-2). In addition, it could provide, for example,a better insight on the quality of the oil, on the isolationtechnique employed and on their geographic origin (3-14). During the last decade, investigations dealing with theenantiomeric composition of the essential oils were carried outby gas chromatography(15). The availability andthe increasingnumber of new chiral stationary phases (16-19) offer a meansfor direct, sensitive and accurate analysis of enantiomericdistribution of chiral components in complex mixtures. More recently, the enantiomeric distribution ofmonoterpenic biogenic volatile organic compounds in ambi-ent air around Algerian Eucalyptus globulus, Cedlrus atlanticaand Pinus halepensis were evaluated by gas chromatographyon a B-cyclodextrin chiral capillary column (20). The direct enantiomeric separation ofcompounds usingasimple gas chromatography technique with a single chiral column is often difficult owing to the possible overlapping ofthe peaks. This difficulty can be avoided if a multidimensionalgas chromatography (MDGC) is performed, since it permitsthe prefractionation of the sample, and the consequent chiralanalysis of single components or at least to obtain simplerfractions than the whole sample, without problems of peakoverlapping. In this paper, in order to evaluate the stereoisomericdifferentiation of nine Algerian Eucalyptus oils, the enantio-meric separation of β-pinene, sabinene, linalool, a-terpineol,terpinen-4-ol was carried out by MDGC using a modified β-cyclodextrin stationary phase as main column. This systemwas previously used for the chiral separation of some compo-nents of citrus oils (22-26). Experimental Plant materials: The adult leaves of the nine Eucalyp-tus species (Eucalyptus punctata DC, E. sideroxylon Cumn.,E. saligna Smith, E. cladocalix F. Muell., E. albens Miq.exBenth., E. ocata Labill.,E. leucoxilon F. Muell., E. BlakelyiA. Cunn and E. microcorys F. Muell.) were collected from Revised: June 2001 Accepted: July 2001 Table l. Relative amount of five monoterpenic compounds in nine Algerian Eucalyptus oils (21) Eucalyptus Species sabinene β-pinene linalool terpinen-4-ol a-terpineol E.punctata <0.1 4.1 0.1 0.7 1.6 E.leucoxylon <0.1 0.5 0.1 0.4 1.0 E.cladocalyx 0.6 0.2 0.5 1.5 0.6 E.blakelyi 0.2 0.1 0.1 1.2 0.3 E.ovata <0.1 0.4 2.2 0.5 10.3 E.microcorys <0.1 0.9 <0.1 2.4 1.6 E.saligna 0.8 0.4 1.9 3.0 2.8 E.sideroxylon <0.1 0.2 0.1 0.5 1.3 E.albens 0.1 0.1 0.9 5.0 1.6 Table Il. Enantiomeric ratios (%) of five monoterpenic compounds in the oils of nine Algerian Eucalyptus species Eucalyptus -pinene sabinene linalool terpinen-4-ol a-terpineol Species (+)(-) (+)(-) (+) (-) (+)(-) (+)(-) E.punctata 3.8 96.2 39.6 60.4 88.2 11.8 36.5 63.5 49.4 50.6 E.leucoxylon 36.9 63.1 23.2 76.8 60.9 39.1 53.0) 47.0 44.4 55.6 E.cladocalyx 63.2 36.8 45.4 54.6 92.9 7.1 39.4 60.6 52.1 47.9 E.blakelyi 24.1 75.9 52.1 47.9 46.7 53.3 39.0 61.0 <0.1>99.9 E.ovata 35.3 64.7 32.6 67.4 8.0 92.0 40.4 59.6 62.6 37.4 E.microcorys 16.8 83.2 28.7 71.3 75.2 24.8 39.2 60.8 38.6 61.4 E.saligna 64.9 35.1 53.6 46.4 80.4 19.6 38.1 61.9 28.0 72.0 E.sideroxylon 18.5 81.5 52.1 47.9 76.9 23.1 46.0 54.0 58.8 41.2 E.albens 39.0 61.0 51.9 48.1 93.6 6.4 35.6 64.4 33.6 66.4 the trees located in Bainem forest (16 Km to the west ofAlgiers). Two or three trees of each species, 30-35 years old,were taken at random. Voucher specimens were cut off andhoused in the arboretum of the Institut National de laRecherche Forestiere (INRF). The oils were isolated fromthe fresh leaves by hydrodistillation. The qualitative andquantitative analyses of each oil were carried out using GCand GC/MS techniques (21). Instrumentation The multidimensional svstem used consisted of twoShimadzu 17A gas chromatographs and a hot transfer line.The experimental conditions used were as follows. Gas chromatograph 1: The chromatograph wasequipped with two split/splitless injectors with two manualflow controllers. A fused silica SE -52 capillary column (30m x0.32 mm, 0.40-0.45 um film thickness (MEGA, Legnano,Italy) was used as a pre-column. The oven temperature wasprogrammed from 45°C isothermal for 6 min, then at 2°C/min to 220°C for 20 min, then at 10°C/min to 280°C for 5min. The injector and detector (FID) temperature were250°C. Helium was used as a carrier gas (90 kPa). Anintegrator Shimadzu C-R3A was connected to start and outsignals on the gas chromatograph 1. Transfer line: An aluminium thermoregulated block(200℃) equipped with a heater assay and a thermocoupleassay was connected to the temperature auxiliary exit onGC-1. Gas chromatograph 2: A Mega Dexdettbs B(Diethyltert-butylsilyl-B-cyclodextrin) capillary column, 25 m x0.25 mm. 0.25 um film thickness (MEGA, Legnano, Italy) was used asthe main column. The oven temperature was programmedfrom 45°C isothermal for 6 min, then to 95°C at 2°C/min for20 min, then at 2°C/min to 180°C for 15 min. The detector(FID) temperature was 280°C. Helium was used as a carriergas (110kPa). An integrator Shimadzu C-R3A was connectedto start and out signals on gas chromatograph 2. The nine oils and the reference compounds were analyzedby injecting l uL of a pentane solution (1:100, V/). Reference compounds: The elution order of the enanti-omers was assigned using enantiomerically pure referencecompounds of definite chirality. The enantiomers ofβ-pinene,sabinene, linalool, a-terpineol and terpinen-4-ol were pur-chased from Fluka (Italy). Results and Discussion For each oil, the relative amount ofthe five monoterpeniecompounds as reported in a previous paper (21) are shown inTable I. Table II reports the enantiomeric distribution oftheanalyzed components in the nine Eucalyptus oils. As can be seen from data reported in Tables I and II, thequantitative amount and also the enantiomeric distribution ofthe five components analvzed in the nine oils is verv different. Under the chromatographic conditions applied for thesimultaneous stereodifferentiation of genuine chiral com-pounds of Eucalyptus oils, B-pinene and sabinene are co-eluted in the nonchiral pre-column [Figure 1(a)]. Thesecompounds are well separated and resolved into their enanti-omers on the chiral main column [Figurel (b)] used in thisinvestigation. (+), 2(-); sabinene: 3 (+), 4 (-); linalool: 5 (-), 6 (+); terpinen-4-ol: 7 (+), 8 (-); a-terpineol:9 (+), 10 (-) As an example, Figures 2(a) and 3(a) report the chromato-grams of E. cladocalyx and E. mycrocorys oils obtained withthe pre-column (SE-52). The chromatograms obtained withthe chiral column forthe fractions transferred from the SE-52pre-column are shown in Figures 2(b) and 3(b). As can be seen from Table II, (-)-B-pinene is the mostabundant isomer in many of the analyzed oils: E. punctata,E. leucoxylon, E. blakelyi, E. ovata, E. microcorys,E. sideroxylon and E. albens. The highest percentage wasfound in E. punctata (96.2%). Regarding the enantiomeric distribution of sabinene, thelevorotatory form was detected as major enantiomer inE. punctata, E. leucoxlon, E. ocata and E. microcorys. In allthe other oils, a distribution very similar to a racemic mixturewas found. It is well known from the literature (8) that the linaloolenantiomers differ considerably in odor. The (-)-linalool has a typical and powerful petitgrain oil fragrance, whereasthe (+) form possesses a woody fragrance and typical laven-der note. Table II shows that the (+)-linalool prevails in seven ofthenine oils investigated; the higher amount was detected in E.albens (93.6%)and E. cladocalyx(92.9%). For E. ouatu the (-)-linalool was predominant with 92.0%, and in E. blakelyi theracemic mixture was found. The enantiomeric separation and enantiomeric distribu-tion of terpinen-4-ol in E. globulus was reported in theliterature (8). It was found that this oil contains an excess of(+)-terpinen-4-ol. In this study, the (-)-terpinen-4-ol pre-vailed in all the oils, except for E. leucoxylon and E.sideroxylonones where the two enantiomerie forms had nearly the sameratio, very near to the racemate. For a-terpineol, the (-) form prevailed in E. blakelyi. E.mycrocorys,E. suligna, and E. albens. In contrast the (+) form separation-B-pinene: 1 (+), 2 (-); sabinene: 3 (+), 4 (-); linalool: 5 (-), 6 (+); terpinen-4-ol: 7 (+), 8 (-); a-terpineol: 9 (+), 10 (-) was predominant in E. ovata andE. sideroxylon. It is notewor-thy that the (-)-c-terpineol was found with an excess of morethan 99% in E. blakelyi. Approximatelythe racemic mixture ofa-terpineol was found in E. punctata, E. leucoxylon and E.cladocalyx. ltcann bee concludedthatthe ssiimmuultaneousstereodifferentiation of the five Eucalyptus oil constituentswas achieved by using a MDGC system. The characteristicdifference in the nine Eucalyptus oils can be deduced fromthis enantio-MDGC analysis. The enantiomeric variationestablished allows a reliable differentiation of the nine Eu-calyptus species with regard to the enantiomeric distribu-tion of β-pinene, linalool and terpinen-4-ol. In addition, theenantiomeric ratio of (-)-a-terpineol in E. blakelyi [(+)/(-)-a-terpineol <0.1/>99.9%] and (-)-linalool in E. ovata [(+)/(-)-linalool 8/92] can be used as a chemotaxonomic marker forthese species. References 1. Y. Holm, I. Laakso and R. Hiltunen, The enantiomeric composition ofmonoterpene hydrocarbons as Chemotaxonomic Marker in AbiesSachalinensis (Fr.Schm) Mast. and A. mayriana. and Kudo NeedleEssential Oils. Flav. Fragr.J., 9,223-227 (1994). 2. B. Faber, A. Dietrich and A. Mosandl, Chiral compounds of Essentialoils. XV. Stereodifferentiation of characteristic compounds of Mentha16species by multidimensional gas chromatography. J.Chromatogr. A,666,161-165(1994). 3. A. Mosandl, U. Hener, P. Kreis and H.-G. Schmarr, EnantiomericDistribution of _-pinene, _-pinene and limonene in essential oils andextracts. Part 1:Rutaceae and Graminaceae. Flav. Fragr.J.,5,193-199(1990). 41. W. A. Konig, R. Krebber, P. Evers and G. Bruhn, StereochemicalAnalysis of constituents of essential oils and flavour compounds bychromatography. J. High Resol. Chromatogr.,13,328-332(1990). 5. A. Mosandl,K. Fisher, U. Hener, P. Kreis, K. Rettinger, V. Schubert andH.-G. Schmarr, Stereoisomeric flavor compounds. 48. ChirospecificAnalysis of Natural Flavor Essential oils Using Multidimensional GasChromatography. J.Agric. Food Chem., 39,1131-1134(1991). 6. C. Askari, U. Hener, H.- G. Schmarr, A. Rapp and A. Mosandl, Stereodifferentiation of some chiral monoterpenes using multidimensionalgas chromatography.Fresenius. J. Anal. Chem.,340,768-772(1991). 7. P. Kreis and A. Mosandl, Chiral compounds of essential oils XIl.Simultaneous chirality evaluation of Geranium oil constituents. Flav.Fragr. J.,8, 161-168, (1993). 8. P. Werkhoff, S. Brennecke, W. Bretschneider, M. Guntert, R. Hopp andH. Surberg, Chirospecific analysis in essential oil, fragrance and flavourresearch. Review, Z. Lebensm. Unters. Forsch., 196, 307-328 (1993). 9. U.Ravid,E. Putievsky andl. Katzir, Enantiomeric distribution of Piperitonein essential oils of some Mentha spp., Calamintha incana (SM)Heldr.and Artemisia judaica L.. Flav. Fragr.J.,9,85-87 (1994). 10. W. A. Konig, A. Rieck, I. Hardt and B. Gehrcke, The enantiomericcomposition of the chiral constituents of essential oils. Part 2:Sesquiterpene Hydrocarbons. J. High Resol. Chromatogr.,17,315-320(1994). 11. P. Kreis, A. Dietrich and A. Mosandl, Chiral compounds of essential oils.Part 18: on the authenticity assessment of essential oil of Rosmarinusofficinalis L.. Pharmazie, 49, 761-765(1994). 12. X. Wang,C. Jia and H. Wan, The direct chiral separation of some opticalactive compounds in essential oils by Multidimensional gaschromatography. J. Chromatogr.Sci., 33, 22-25 (1995). 13. D. Juchelka, A. Steil, K. Witt and A. Mosandl, Chiral compounds ofessential oils.XX. Chirality evaluation and authenticity profiles of Neroliand Petitgrain oils. J. Essent.Oil Res., 8, 487-497 (1996). 14 H. Casabianca, J. B. Graff, V. Faugier, F. Fleig and C. Grenier,Enantiomeric Distribution of linalool and linalyl acetate. A powerful too/for authenticity control of essential oils. J. High Resol. Chromatogr.,21,107-112(1998). 15. V. Schurig, Enantiomer separation by gas chromatography on chiralstationary phases. Review, J. Chromatogr. A, 666,111-129(1994). .16. T. Takeichi, H. Toriyama, S. Shimura, Y. Takayama and M. Morikawa,Cyclodextrin carbamates as novel chiral stationary phases for capillarygas chromatography. J. High Resol. Chromatogr.,18, 179-189(1995). .17. P. Jing,R.-N. Fu, R.-J. Dai, J.-L. Ge, J.-L. Gu, Z. Huang and Y.Chen,Consequence of diluting modified β-cyclodextrin in a side chain crownether polysiloxane and a side chaine liquide-cristalline polysiloxanecontaining crown ether as stationary phases in capillary gaschromatography. Chromatographia, 43,546-550(1996). 18. B.E. Kim,K.-P.Lee,K.S.Park,S.H.Leeand J. H.Park,Enantioselectivityof 6-O-Alky/dimethy/silyl-2,3-Di-O-Methyl-B-cyclodextrins as chiralstationary phases in capillary GC. Chromatographia,46,145-150(1997). 19. H. B. Zhang, Y. Ling, R. N. Fu, Y. X. Wen and J. L. Gu, A new gaschromatographic stationary phase/polysiloxane with b-cyclodextrin sidechain for the separation ofchiral andpositional isomers.Chromatographia, ( 46, 40-48 (1997). ) ( 20. N . Yassaa, B. Y. Meklati and A. Cecinato, E v aluation of monoterpenic volatile organic compounds in ambient air around Eucalyptus globulus, Pinus halepensis and Cedrus atlantica trees growing in Algiers city area bychiral and achiral capillary gas chromatography. Atmos.Environ.,34, 2809-2816(2000). ) 21. Y. Foudil-Cherif, B. Y. Meklati,. A. Verzera,L. Mondello and G. Dugo,Chemical Examination of Essential Oils from the Leaves of NineEucalyptus Species Growing in Algeria. J. Essent. Oil Res.,12,186-191(2000). 22. L. Mondello, M. Catalfamo, P. Dugo and G. Dugo, MultidimensionalTandem Capillary Gas Chromatography System for the Analysis of RealComplex Samples. Part I: development of a Fully Automated TandemGas Chromatography System.J. Chromatogr.Sci., 36,201-209(1998). 23. L. Mondello, M. Catalfamo, P. Dugo and G. Dugo, MultidimensionalCapillary GC-GC for the Analysis of Real Complex Samples. Part Il:Enantiomeric Distributionof Monoterpene Hydrocarbons and ( Monoterpene Alcohols of Co l d-Pressed and Dist i lled Lim e Oils. J. M icrocol. Sep., 10, 203-212(1998). ) ( 24. L . Mondello, M. Catalfamo, A . R . P r oteggente, I. Bonaccorsi and G.Dugo, M ultidimensional Capillary G C-GC for the An a lysis of Real C omplex Samples. Part III: Enantiomeric Distribution o f MonoterpeneHydrocarbons and Monoterpene Alcohols of Mandarin Oils. J. A g ric.Food Chem., 46, 54-61(1998). ) ( 25. L . Mondello, M. Catalfamo, A. Cotroneo, G. Dugo, H . McNair and G.Dugo, M u ltidimensional Capillary G C-GC for the An a lysis of Real C omplex Samples. Part IV: Enantiomeric Distribution of Monoterpene H ydrocarbons and Monoterpene Alcohols Lemon Oils. J. H i gh Resolut.Chromatogr., 22,350-356(1999). ) 26. L. Mondello, A. Verzera, P. Previti, F. Crispo and G. Dugo,MultidimensionalCapillary GC-GC for the Analysis of Complex Samples.Part V: Enantiomeric Distribution of Monoterpene Hydrocarbons,Monoterpene Alcohols, and LinalylAcetate of Bergamot (Citrus bergamiaRisso et Poiteau) Oils. J. Agric. Food Chem., 46,4275-4282(1998). Received: December Address for correspondenceC Allured Publishing Corp.Journal of Essential Oil Research/Vol. January/February Vol. January/February Journal of Essential Oil Research
确定


还剩3页未读,是否继续阅读?
扬州华明仪器设备有限公司为您提供《桉树精油中化学成分检测方案 》,该方案主要用于日用化学品/香精香料中化学成分检测,参考标准--,《桉树精油中化学成分检测方案 》用到的仪器有
相关方案
更多







