原位细胞3D切割成像平台-CellSurgeon

|
德国LLS ROWIAK公司推出的CellSurgeon是一款、非接触的3D纳米激光活细胞显微成像切割系统。它具特色的多光子切割技术,能够从细胞内或组织内的任意点开始切割,实现真正意义上的定点操作。并且CellSurgeon还配有MPM成像模块,能够实现实时的荧光标记或无标记成像,定位所需操作的部位和实时观测细胞动态变化。通过CellSurgeon研究者能够进行实时的活细胞、组织操作和观测,帮助研究者更好的研究原位细胞的生理活性。
|
应用领域
■ 染色体切割
■ 亚细胞器的实时观测切割
■ 原位组织的单细胞分离
■ 薄组织的显微切割
■ 基于激光的光转染技术
CellmatchSurgeon切割原理
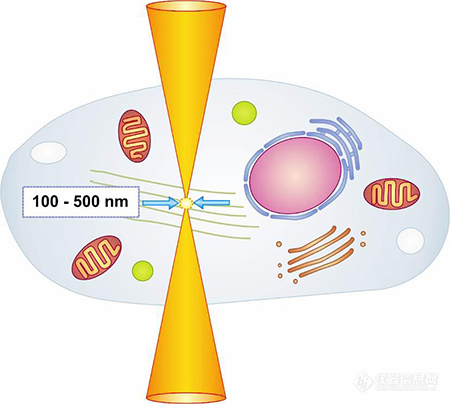
CellSurgeon将近红外超短脉冲激光器耦合到显微镜中,并利用高数值孔径物镜聚焦超短激光脉冲,仅在最小的聚焦体积内产生高强度能量引起多光子吸收,然后以非常精确的方式在活细胞中实现亚细胞水平的细胞结构可视化操作。由于几乎没有热能或机械能传递,靠近激光束紧焦点的细胞结构依旧保持完好无损。
CellSurgeon的切割方式

双光子切割 VS 单光子切割 可从组织中的任意部位开始切割
| 
CellSurgeon的切割方式 |
为何选用CellSurgeon?
■ 多光子实时成像追踪
■ 的3D切割
■ 无需前处理即可直接切割
■ 直接的原位切割
■ 活细胞或组织均可直接切割
■ 大限度保存生物信息的完整
■ 能够兼容多种型号的显微镜
基本参数
■ 激光:飞秒近红外激光,单波长或可变
■ 扫描器:双独立扫描镜
■ 扫描精度:700 X 700 ~ 300 X 100
■ 最大分辨率:700 X 700(1,43 f/s)
■ 最大扫描速度:300 X 100(10 f/s)
■ 切割模式:不同波长的2D或3D精准手动或自动切割
■ 控制器:驱动所有机动单元:显微镜、扫描器、 z驱动器、扫描台以及所有相关配件
数据测试
■ 动脉激光切割和成像
30 fps超短激光脉冲对小鼠血管的损伤

体内激光诱导血栓的三维重建,采用FITC-葡聚糖染色双光子成像监测激光损伤后血栓的形成情况

■ 肌动蛋白丝的切割
用飞秒激光切割肌动蛋白细丝

■ 有丝分裂纺锤体的亚细胞解剖
GBP标记的有丝分裂纺锤体,光漂白(A)和切割消融(B)

■ 细胞器消融
不同功率激光对核的消融,激光消融前(A)和后(B)

线粒体消融,激光消融前(左)和后(右)

■ 从细胞到组织的动态观测与切割
CellSurgeon能够胜任各种类型的切割任务,无论是的染色质还是活体组织,它都能很好地胜任。
该设备可以兼容多种型号的显微镜,并且支持显微操作针等配件,能够在切割后实现对切割部分的转移。

从细胞团中切除的细胞并用微毛细管将提取细胞切出

固定的CHO的Alexa488标记的毒伞素切割
| 
活U2OS细胞的FP635标记的肌动蛋白的切割
|

活GM-7373牛主动脉内皮细胞的 MitoTracker Orange ®的单线粒体消融实验
| 
活GM-7373牛主动脉内皮细胞诱导凋亡实验
|


白蚁的组织切割


小鼠活体血管切割
■ 基于激光的原位细胞转染
无论是电转还是脂质体都需要先将细胞悬浮才能够进行入转染,但是Cellsurgeon能够在原位对细胞进行光穿孔实现细胞的转染,这种技术对于研究原位的细胞转染有着重大意义。

使用CellSurgeon对ZMTH3细胞进行转染pEGFP-C1、pEGFP-HMGA2、pEGFP-HMGB1经过48小时的图像
发表文章
1. Nolte, P.; Brettmacher M.; Gröger, C. J.; Gellhaus, T.; Svetlove A.; Schilling, A. F.; Alves, A.; Rußmann, C.; Dullin, C.; (2023) Spatial correlation of 2D hard�tissue histology with 3D microCT scans through 3D printed phantoms Sci Rep 13, 18479
2. Kevin Janot, Grégoire Boulouis, Géraud Forestier, Fouzi Bala, Jonathan Cortese, Zoltán Szatmáry, Sylvia M. Bardet, Maxime Baudouin, Marie-Laure Perrin, Jérémy Mounier, Claude Couquet, Catherine Yardin, Guillaume Segonds, Nicolas Dubois, Alexandra Martinez, Pierre-Louis Lesage, Yong-Hong Ding, Ramanathan Kadirvel , Daying Dai, Charbel Mounayer, Faraj Terro, Aymeric Rouchaud. (2023) WEB shape modifications: “angiography–histopathology correlations in rabbits” J NeuroIntervent Surg 2023;0:1–7.
3. Géraud FORESTIER, Jonathan CORTESE, Sylvia M. BARDET, Maxime BAUDOUIN, Kévin JANOT, Voahirana RATSIMBAZAFY, Marie-Laure PERRIN, Jérémy MOUNIER, Claude COUQUET, Catherine YARDIN, Yan LARRAGNEGUY, Flavie SOUHAUT, Romain CHAUVET, Alexis BELGACEM, Sonia BRISCHOUX, Julien MAGNE, Charbel MOUNAYER, Faraj TERRO, Aymeric ROUCHAUD. (2023) “Comparison of Arterial Wall Integration of different Flow Diverters in rabbits” the CICAFLOW study Journal of Neuroradiology, In press.
4. Donath, Sören, Leon Angerstein, Lara Gentemann, Dominik Müller, Anna E. Seidler, Christian Jesinghaus, André Bleich, Alexander Heisterkamp, Manuela Buettner, and Stefan Kalies. (2022). “Investigation of Colonic Regeneration via Precise Damage Application Using Femtosecond Laser-Based Nanosurgery” Cells 11, no. 7: 1143. https://doi.org/10.3390/cells11071143
5. Müller, Dominik, Sören Donath, Emanuel G. Brückner, Santoshi Biswanath Devadas, Fiene Daniel, Lara Gentemann, Robert Zweigerdt, Alexander Heisterkamp, and Stefan M.K. Kalies. (2021). “How Localized Z-Disc Damage Affects Force Generation and Gene Expression in Cardiomyocytes” Bioengineering 8, no. 12: 213. https://doi.org/10.3390/bioengineering8120213
6. Müller D, Klamt T, Gentemann L, Heisterkamp A, Kalies SMK (2021) Evaluation of laser induced sarcomere micro-damage: Role of damage extent and location in cardiomyocytes. PLoS ONE 16(6): e0252346. https://doi.org/10.1371/journal.pone.0252346
7. Bouyer M; Garot C; Machillot P; Vollaire J; Fitzpatrick V; Morand S; Boutonnat J; Josserand V; Bettega G; Picart C (2021) 3D-printed scaffold combined to 2D osteoinductive coatings to repair a critical-size mandibular bone defect Materials Today Bio 11 100113
8. Verhaegen C, Kautbally S, Zapareto D C, Brusa D, Courtoy G, Aydin S, Bouzin C, Oury C, Bertrand L, Jacques P J, Beauloye C, Horman S, Kefer J (2020) Early thrombogenicity of coronary stents: comparison of bioresorbable polymer sirolimus-eluting and bare metal stents in an aortic rat model. Am J Cardiovasc Dis. 10(2):72-83
9. Zeller-Plumhoff B, Malicha C, Krüger D, Campbella G, Wiesea B, Galli S, Wennerberg A, Willumeit-Römer R, Wieland F (2020) Analysis of the bone ultrastructure around biodegradable Mg–x Gd implants using small angle X-ray scattering and X-ray diffraction Acta Biomaterialia 101 637–645
10. Rousselle S D , Wicks J R, Tabb B C, Tellez A, O’Brien M (2019) Histology Strategies for Medical Implants and Interventional Device Studies Toxicologic Pathology Vol. 47(3) 235-249
11. Neuerburg C, Mittlmeier L M, Keppler A M, Westphal I, Glass Ä, Saller M M, Herlyn P K E, Richter H, Böcker W, Schieker M, Aszodi A, Fischer D C (2019) Growth factor-mediated augmentation of long bones: evaluation of a BMP-7 loaded thermoresponsive hydrogel in a murine femoral intramedullary injection model. Journal of Orthopaedic Surgery and Research 14 297
12. Kunert-Keil C, Richter H, Zeidler-Rentzsch I, Bleeker I, Gredes T (2019) Histological comparison between laser microtome sections and ground specimens of implant-containing tissues. Annals of Anatomy 222 153–157
13. Gabler C, Saß JO, Gierschner S, Lindner T, Bader R, Tischer T (2018) In Vivo Evaluation of Different Collagen Scaffolds in an Achilles Tendon Defect Model. BioMed Research International 208
14. Wolkers W, Vásquez-Rivera A, Oldenhof H, Dipresa D, Goecke T, Kouvaka A, Will F, Haverich A, Korossis S, Hilfiker A (2018) Use of sucrose to diminish pore formation in freeze-dried heart valves. Scientific Reports 8 12982
15. Albers J, Markus MA, Alves F, Dullin C (2018) X-ray based virtual histology allows guided sectioning of heavy ion stained murine lungs for histological analysis. Scientific Reports 8(1) 7712
16. Boyde A (2018) Evaluation of laser ablation microtomy for correlative microscopy of hard tissues. Journal of Microscopy 271(8) 1-14
17. Pobloth AM, Checa S, Razi H, Petersen A, Weaver JC, Schmidt-Bleek K, Windolf M, Tatai AÁ, Roth CP, Schaser KD, Duda GN, Schwabe P (2018) Mechanobiologically optimized 3D titanium-mesh scaffolds enhance bone regeneration in critical segmental defects in sheep. Science Translational Medicine 10 423
18. Joner M, Nicol P, Rai H, Richter H, Foin N, Ng J, Cuesta J, Rivero F, Serrano R, Alfonso F (2018) Very Late Scaffold Thrombosis: Insights from Optical Coherence Tomography and Histopathology. EuroIntervention 13(18)
19. Boyde A, Staines KA, Javaheri B, Millan JL, Pitsillides AA, Farquharson C (2017) A distinctive patchy osteomalacia characterises Phospho1 deficient mice. Journal of Anatomy 231 298-308
20. Kowtharapu BS, Marfurt C, Hovakimyan M, Will F, Richter H, Wree A, Stachs O, Guthoff RF (2017) Femtosecond laser cutting of human corneas for the subbasal nerve plexus evaluation. Journal of Microscopy 265(1) 21–26
21. Will F, Richter H (2015) Laser-based Preparation of Biological Tissue. Laser Technik Journal 12(5) 44-47
22. Richter H, Ratliff J, Will F, Stolze B (2015) Time- and material saving laser microtomy for hard tissue and implants. European Cells and Materials 29 Suppl.2 4
23. Richter H, Ramirez Ojeda DF, Will F (2014) Lasergesteuerte Probenpräparation von Hartgeweben und Biomaterialien. BIOspektrum 05 14
24. Bourassa D, Gleber S-C, Vogt S, Yi H, Will F, Richter H, Shin CH, Fahrni CJ (2014) 3D Imaging of Transition Metals in the Zebrafish Embryo by X-ray Fluorescence Microtomography. Metallomics 6 1648-1655
25. Schimek K, Busek M, Brincker S, Groth B, Hoffmann S, Lauster R, Lindner G, Lorenz A, Menzel U, Sonntag F, Walles H, Marx U, Horland R. (2013) Integrating biological vasculature into a multi-organ-chip microsystem. Lab Chip 13 3588-3598
26. Richter H, Ratliff J (2012) A Non-Contact Method of Sectioning Cardiovascular Arteries Containing Metallic Stents Using Laser Technology. J Histotechnol 35 (4) 205
27. Richter H, Lubatschowski H, Will F (2011) Laser in Medizin & Biologie: Laser-Mikrotomie mit ultrakurzen Pulsen – Neue Perspektiven für die Gewebe- und Biomaterialbearbeitung. Biophotonik 09 50-52
28. Lubatschowski H, Will F, Przemeck S, Richter H (2011) Laser Microtomy. Handbook of Biophotonics Vol. 2: Photonics for Health Care Wiley-VCH 151-157
29. Kermani O, Will F, Massow O, Oberheide U, Lubatschowski H (2010) Control of Femtosecond Thin-flap LASIK Using OCT in Human Donor Eyes. Journal of Refractive Surgery 26(1) 57-61
30. Baumgart J, Bintig W, Ngezahayo A, Lubatschowski H, Heisterkamp A (2010) Fs-laser-induced Ca2+ concentration change during membrane perforation for cell transfection. Optics Express 18 (3) 2219
31. Kermani O, Will F, Massow O, Oberheide U, Lubatschowski H. (2009) Echtzeitsteuerung einer Femtosekundenlaser Sub-Bowman-Keratomileusis an humanen Spenderaugen mittels optischer Kohärenztomographie. Klin Monatsbl Augenheilkd 226 965-969
32. Kütemeyer K, Baumgart J, Lubatschowski L, Heisterkamp A (2009) Repetition rate dependency of low density plasma effects during femtosecond-laser-based surgery of biological tissue. Appl. Phys. B 97(3) 695
33. Baumgart J, Kuetemeyer K, Bintig W, Ngezahayo A, Ertmer W, Lubatschowski H, Heisterkamp A (2009) Repetition rate dependency of reactive oxygen species formation during femtosecond laser-based cell surgery. J Biomed Opt 14(5) 054040
34. Kermani O, Will F, Lubatschowski H (2008) Real-Time Optical Coherence Tomography-Guided Femtosecond Laser Sub-Bowman Keratomileusis on Human Donor Eyes. Am J Ophthalmol 146 42–45.
35. Kermani O (2008) „Sehendes Skalpell” schon heute realisierbar. Ophthalmologische Nachrichten 09 (Kongressausgabe)
36. Baumgart J, Bintig W, Ngezahayo A, Willenbrock S, Murua Escobar H, Ertmer W, Lubatschowski H, Heisterkamp A (2008) Quantified femtosecond laser based opto-perforation of living GFSHR-17 and MTH53a cells. Opt. Express 16(5) 3021-3031
37. Baumgart J, Kuetemeyer K, Bintig W, Ngezahayo A, Ertmer W, Lubatschowski H, Heisterkamp A (2008) Investigation of reactive oxygen species in living cells during femtosecond laser based cell surgery. Proc. SPIE Optical Interactions with Tissue and Cells XIX Vol 6854
38. Heisterkamp A, Baumgart J, Maxwell IZ, Ngezahayo A, Mazur E, Lubatschowski H (2007) Fs-Laser Scissors for Photobleaching, Ablation in Fixed Samples and Living Cells, and Studies of Cell Mechanics. Laser Manipulation of Cells and Tissues; Elsevier Inc. 293-307
39. Will F, Block T, Menne P, Lubatschowski H (2007) Laser Microtome: all optical preparation of thin tissue samples. Proceedings of SPIE 6460 646007-1
40. Lubatschowski H (2007) Laser Microtomy – Opening a new Feasibility for Tissue Preparation. Optic & Photonic WILEY-VCH 49 – 51
41. Menne P (2007) Microtomy with Femtosecond Lasers. Biophotonics International; Laurin Publishing Co. Inc. May 2007 35 – 37
用户单位
部分用户单位:
Bayer HealthCare, Cardiovascular Research
Leibniz University Hannover, Institute of Biophysics
Leibniz University Hannover, Institute for Quantum Optics-1,-2
University of Rostock, Division of Medicine Clinic III, Hematology, Oncology and Palliative Medicine
Institute for Bioprocessing and Analytical Measurement Techniques (iba)
mfd Diagnostics GmbH
![]()
































