微流成像图像法粒度仪在注射剂中的应用
随着现代医疗技术的飞速发展,注射剂作为一种重要的药物剂型,其质量和安全性受到了广泛关注。在注射剂的生产和使用过程中,颗粒的粒度及形貌是影响其疗效和安全性的关键因素之一。近年来,微流成像图像法粒度仪作为一种先进的粒度分析技术,在注射剂领域的应用日益广泛。 微流成像图像法粒度仪采用动态流式成像原理,通过捕捉流经微流通道的样品颗粒的实时图像,进而分析颗粒的大小、形貌等特征。这一技术的优势在于其直观性和可视化,使得复杂液体制剂、混悬液、微球等颗粒分析变得更为简单、准确。 在注射剂中,微流成像图像法粒度仪的应用主要体现在颗粒的粒度及形貌检测上。通过对注射剂中颗粒的精确测量,可以评估其均一性、分散性以及稳定性,从而确保注射剂的质量符合标准。此外,该技术还能有效检测注射剂中的异物和杂质,进一步保障患者的用药安全。 值得一提的是,微流成像图像法粒度仪在分析球形和短丝状体颗粒时具有独特优势。由于这些颗粒在体内更容易被细胞吸收,因此,对它们的粒度及形貌进行精确测量,有助于优化注射剂的配方和制备工艺,提高药物的生物利用度。 此外,微流成像图像法粒度仪还能为注射剂的临床应用提供有力支持。通过对不同批次、不同生产工艺的注射剂进行粒度及形貌分析,可以评估其批次间的差异和稳定性,为临床用药提供科学依据。 微流成像图像法粒度仪在注射剂领域的应用具有广阔的前景和重要的实际意义。随着技术的不断进步和完善,相信这一技术将为注射剂的质量控制和安全性评估提供更加可靠、高效的手段。
企业动态
2024.03.25
美国MAS毛细管高分辨纳米粒度仪产品优势科普
在现代科研与工业领域,纳米颗粒的粒度测量变得日益重要。美国MAS毛细管高分辨纳米粒度仪凭借其卓越的性能和独特的技术优势,成为这一领域的佼佼者。 首先,MAS毛细管高分辨纳米粒度仪采用先进的毛细管流体动力分馏技术(CHDF)。这一技术能够实现对纳米颗粒的精确分离和测量,提供高分辨率的粒度数据。无论是对于均匀还是非均匀样品,MAS粒度仪都能提供准确、可靠的测量结果,为科研人员提供了强有力的数据支持。 其次,该仪器具有宽广的粒径检测范围,从几纳米到数百微米不等。这意味着MAS粒度仪能够适用于多种不同类型的纳米材料测量,满足不同领域的研究需求。无论是化妆品、医药、还是环保领域,MAS粒度仪都能发挥其独特的优势。 此外,MAS粒度仪还具有出色的操作简便性和稳定性。仪器界面友好,用户只需按照提示进行操作即可完成测量。同时,设备还具备自动校准和温度控制功能,确保了测量结果的准确性和可靠性。这种高度的自动化和智能化设计,大大减轻了用户的工作负担,提高了工作效率。 值得一提的是,MAS粒度仪还具备多种附加功能,如Zeta电位测量、电导率检测等。这些功能使得该仪器能够更全面地了解纳米颗粒的性质和行为,为科研工作者提供更为丰富的研究手段。 美国MAS毛细管高分辨纳米粒度仪凭借其先进的技术、宽广的检测范围、操作简便性和稳定性以及丰富的附加功能,成为了纳米粒度测量领域的领先产品。它将为科研工作者提供更为准确、可靠的数据支持,推动纳米科技的快速发展。
企业动态
2024.03.21
CHDF4000纳米粒度仪测试范围科普
CHDF4000纳米粒度仪是一款基于光散原理的高分辨率仪器,其测试范围广泛且精准,对于纳米级别粒度的测量尤为出色。该仪器使用最新的技术,可以对粒子的大小、形状以及分布进行精确的测量,从而为用户提供全面的粒度分析数据。 首先,CHDF4000纳米粒度仪的测试范围覆盖了从5纳米到3微米的粒子。这一范围涵盖了众多领域中的关键粒度尺寸,如纳米材料、生物医药、食品化工等。无论是研究纳米颗粒的性质,还是监控生产过程中的粒度变化,CHDF4000都能提供准确的数据支持。 其次,CHDF4000纳米粒度仪具有出色的测试精度和分辨率。其高分辨率能力可以确保测量结果的准确性和可靠性,从而帮助用户更好地理解和控制样品的粒度特性。同时,该仪器还具备高粒子检测灵敏度,即使对于浓度较低的样品,也能进行有效的测量。 此外,CHDF4000纳米粒度仪还具备广泛的动态范围检测能力。在主要粒子群中,该仪器能够对次要粒子群进行大动态范围检测,从而捕捉到更多关于样品粒度分布的信息。这一特点使得CHDF4000在复杂样品的分析中表现出色,能够提供更加全面和准确的粒度数据。 值得一提的是,CHDF4000纳米粒度仪的操作简便且自动化程度高。用户只需将样品放入仪器中,即可自动进行粒度分析,无需复杂的设置和操作。这一特点不仅降低了用户的操作难度,还提高了测量效率和数据可靠性。 总之,CHDF4000纳米粒度仪以其广泛的测试范围、出色的测试精度和分辨率、广泛的动态范围检测能力以及简便的操作方式,成为了纳米粒度测量领域的佼佼者。无论是科研实验还是工业生产,该仪器都能为用户提供准确、可靠的粒度分析数据,助力科研和生产的进步。
企业动态
2024.03.20
分馏法原液高分辨纳米粒度仪优势及技术参数
分馏法原液高分辨纳米粒度仪是一种基于毛细管流体分离(CHDF)技术的先进粒度分析设备,其在纳米科技、材料科学、生物医药等领域具有广泛的应用。胤煌将详细介绍该设备的优势及技术参数,帮助读者更好地了解和使用这一设备。 一、分馏法原液高分辨纳米粒度仪优势 高分辨率测量:该设备采用毛细管流体分离技术,能够精确测量粒径在5nm至3μm范围内的胶体粒子,分辨率高,测量准确。 真实PSD数据:通过粒子分离技术,设备能够提供真实、完整的PSD数据,无需对粒子大小分布的形状进行假设,从而更准确地反映样品的粒度特性。 高灵敏度探测:设备使用新的光散原理技术,具有高灵敏度探测能力,能够检测出微量的粒子,确保测量结果的可靠性。 自动化操作:设备操作简便,通过个性化界面即可完成全部操作,大大减轻了实验人员的工作负担。 广泛的应用领域:该设备可用于质量控制、研发以及在线控制等多个方面,适用于多种行业的需求。 二、分馏法原液高分辨纳米粒度仪技术参数 样品瓶数量与尺寸:设备配备72个样品瓶,每个样品瓶尺寸为12mm,容量为2ml,满足大量样品的测量需求。 注射次数与数据保存:每个样品可以重复运行至少15次,确保测量结果的稳定性和可靠性。数据可以自动保存和打印,方便后续分析和处理。 自动样品混合与针冲洗:设备具有自动样品混合功能,有效防止粒子沉降;同时配备针冲洗瓶,保持针外表面清洁,提高测量精度。 注射器体积与精度:设备采用250μL注射器,确保样品的精确取样。测量精度小于±0.5% RSD,满足高精度测量的要求。 材质与耐用性:设备采用防潮材质如聚四氟乙烯和316不锈钢,确保设备的稳定性和耐用性。 尺寸与重量:设备尺寸为24cm(宽)×59cm(深)×52cm(高),净重为42kg,方便实验室摆放和移动。 分馏法原液高分辨纳米粒度仪具有诸多优势和技术参数上的特点,能够满足不同领域对纳米粒度测量的需求。通过使用该设备,用户可以准确、快速地获取样品的粒度分布信息,为科研和生产提供有力支持。
企业动态
2024.03.19
澄清度检查分析仪特点及技术参数科普
澄清度检查分析仪又名澄清度测定仪,是一种广泛应用于水质监测领域的仪器,其采用先进的光学散射原理,可以快速、准确地测定液体的澄清度。该仪器不仅具备高精度、高稳定性的测量性能,还具备智能化、自动化的操作特点,为水质监测工作提供了极大的便利。 在技术参数方面,澄清度检查分析仪展现了出色的性能。其测定原理基于90°散射光,这种散射光方式可以确保测量结果的准确性和可靠性。同时,仪器采用LED光源,波长为860nm,保证了光源的稳定性和长寿命。 测量范围是仪器的一个重要指标,澄清度检查分析仪具有自动可变量程的特点,可以根据不同样品的澄清度自动选择合适的测量范围,包括0~10 NTU、10~100 NTU、100~1000 NTU三个量程。此外,仪器的分辨率也非常高,可以根据不同的测量范围自动调整,确保测量结果的精确性。 在性能方面,澄清度检查分析仪的示值误差仅为±2%F.S,重复性≤0.5%,零点漂移±0.5%F.S,这些指标均优于同类产品,显示出其卓越的测量稳定性。 仪器校准是确保测量结果准确性的重要环节,澄清度检查分析仪支持1~5点自动校准,可以方便地根据需要进行校准操作。同时,仪器还具备数据完整性保障措施,具有4级权限管理功能,可以确保数据的安全性和可靠性。 此外,澄清度检查分析仪还具备强大的数据存储和传输能力。其存储容量高达128G,可以无限制地存储测量数据。同时,仪器支持多种接口方式,包括USB、RS232、RS485、CAN等,方便与计算机或其他设备连接,实现数据的快速传输和共享。 在环境适应性方面,澄清度检查分析仪也表现出色。其可以在室温至65℃的广泛温度范围内正常工作,且电源功率为220V,50Hz,符合一般实验室和现场使用的电源要求。 澄清度检查分析仪以其高精度、高稳定性、智能化和自动化的特点,以及强大的数据存储和传输能力,成为了水质监测领域不可或缺的重要工具。
企业动态
2024.03.18
静态图像法粒度仪:设备技术优势一览
在粒度分析领域,静态图像法粒度仪作为一种先进的设备,为研究者提供了诸多便利。下面我们将详细探讨这种设备的几项主要技术优势。 1. 配合自动制样装置,操作简单,数据稳定 静态图像法粒度仪通常配备自动制样装置,能够快速、准确地制备样品。这种自动制样方式不仅简化了操作流程,还大大提高了数据的稳定性和可靠性。通过自动化技术,避免了人为操作可能带来的误差,确保了实验结果的准确性。 2. 手动选择区域,区域范围可选择,方便快捷 静态图像法粒度仪提供了手动选择区域的功能,使用户能够根据实际需求灵活地调整分析区域。这一设计使得用户能够快速定位到感兴趣的区域,进行有针对性的粒度分析。同时,区域范围的灵活性也增强了该设备的实用性,适用于各种不同类型的粒度分析任务。 3. 视野范围内颗粒自动计数,且可多视野叠加 静态图像法粒度仪具备自动计数的功能,能够快速准确地统计视野范围内的颗粒数量。这一功能极大地提高了分析效率,减少了人工计数带来的误差。此外,该设备还支持多视野叠加,通过将多个视野的图像进行叠加处理,进一步提高了分析的准确性。这种叠加技术能够有效地减小误差,提高数据的可靠性。 4. 节约分析时间,减少人为操作误差 静态图像法粒度仪通过自动化和智能化的设计,大大缩短了分析时间。这不仅提高了工作效率,还降低了长时间观察对实验人员造成的疲劳和误差的可能性。这种高效的分析方式为企业和研究机构节省了宝贵的时间和资源。 5. 丰富的数据展现形式、数据报告模板可选择 静态图像法粒度仪提供了多样化的数据展现形式,包括图表、曲线、表格等。这些形式直观地展示了粒度分布情况,使得用户能够更快速地理解和分析数据。此外,该设备还配备了多种数据报告模板可供选择,使用户能够根据需要生成专业、规范的数据报告。这种灵活的数据处理方式满足了不同领域的实际需求,为用户提供了便捷的数据分析和展示工具。 6. 兼容黑白/彩色模式、偏光模式,观察药物晶型 静态图像法粒度仪具有出色的兼容性,支持黑白、彩色和偏光模式下的观察和分析。这种多模式支持使得该设备在药物晶型观察等领域具有广泛的应用价值。通过不同的观察模式,用户能够更全面地了解药物晶型的特征和变化,为药物研发和质量控制提供有力支持。 7. 可选配固体分散/半固体分散制样装置 为了满足不同固体分散物和半固体分散物的制样需求,静态图像法粒度仪还提供了一系列可选配的制样装置。这些装置能够根据样品的特点进行定制化处理,确保制样过程的准确性和可靠性。这种灵活性使得该设备在固体分散物和半固体分散物的粒度分析中具有独特优势,为用户提供了全面的解决方案。
企业动态
2024.01.29
不溶性微粒检查 你需要这样一本书
不溶性微粒检查,是一个很大的应用场景,一般我们指的都是大医药行业里面质量检查的一个项目,特别是各种类型的注射液。对于不溶性微粒检查,我们首先想到的就是法规是如何规定的,测试方法是什么,如何测试,判定标准是什么等等。所以不溶性微粒检查,有这样一本书你值得拥有。中美欧日四大药典不溶性微粒检查法规的汇整版,外加中英文双语对照版,对您更好的理解法规,了解各国法规之间的区别起到一定的帮助。不溶性微粒检查药典法规分享的这本书里面,包含了中国药典2020版CP0903章节、美国药典USP787、USP788、USP789、USP1787、USP1788、欧洲药典EP2.9.19、日本药典JP6.07、JP6.08 全部章节的原本及中英文对照内容,欢迎大家联系我们领取和沟通。不溶性微粒检查药典法规分享相关书籍及目录如下:
应用实例
2023.08.25
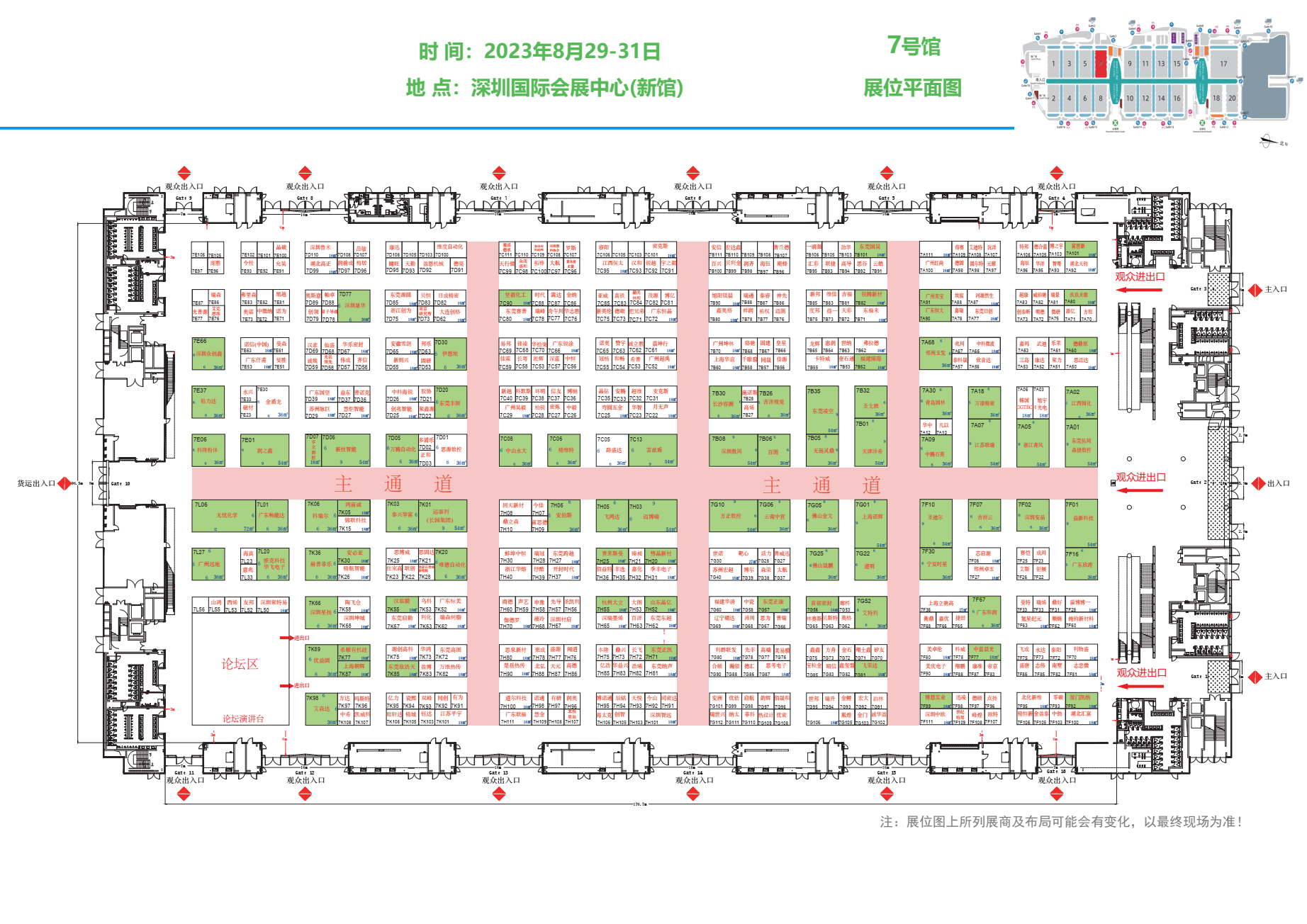
胤煌科技YinHuang Technology 诚邀您参加2023中国(深圳)国际半导体展览会
2023年中国(深圳)国际半导体展览会将于2023年8月29日-8月31日召开,胤煌科技YinHuang Technology作为受邀展商将亮相展会,欢迎各位朋友来我们展位莅临指导。展会相关信息如下:展会时间:2023年8月29日-31日地点:深圳国际会展中心(新馆)展位号:7G105胤煌科技(YinHuang Technology)是一家专注于为医药、半导体及化工材料等行业提供检测分析设备及技术服务的高科技公司,致力于为客户提供全面、准确的检测分析和解决方案。主营产品包括不溶性微粒分析仪,可见异物检查分析仪,原液粒度及Zeta电位分析仪,CHDF高精度纳米粒度仪,高分辨纳米粒度仪,溶液颜色测定仪,澄清度测定仪等,公司自主研发的YH-MIP系列显微计数法不溶性微粒仪、YH-FIPS系列流式动态图像法粒度仪,YH-FIPS系列微流成像颗粒分析仪已经在生物医药、半导体及材料化工领域得到广泛应用.
企业动态
2023.08.09
胤煌科技不溶性微粒-药典新规解读(专题三)将于2022年6月29日晚19:00与您线上相约
胤煌科技不溶性微粒-药典新规解读(专题三)将于2022年6月29日晚19:00与您线上相约大家期待已久的胤煌科技不溶性微粒-药典新规解读(专题三)来啦,此次会议将由胤煌科技创始人、时任总经理柳钟丽为大家做汇报交流。届时将跟大家分享我司为不同客户测样过程中发现的问题、总结的经验及技术的更新,欢迎一同沟通探讨!学无止境,希望大家广邀好友参与我司沟通交流会,邀请排行榜前10名在会后将获得我司寄出的京东购物卡作为奖励。2022年6月29日晚19:00,我们线上等您!
企业动态
2022.06.24
胤煌科技 5W带您浅析显微计数法不溶性微粒分析仪
胤煌科技 5W带您浅析显微计数法不溶性微粒分析仪一、WHAT:什么是显微计数法不溶性微粒分析仪?名词解析,“显微计数法”取自中国药典0903不溶性微粒检查法第二法,即显微计数法,是不溶性微粒检查的一种一直存在的方法,但由于其操作麻烦而不被人喜欢(但其结果准确),“不溶性微粒”是指药品在生产或使用过程中经由各种途径产生或混入的微粒性杂质,粒径在1~50 μm、肉眼不可见,但因其可随血液流动却不能被代谢而可能对人体造成难以发现和潜在的严重危害,“分析仪”即检查注射液中不溶性微粒的仪器。二、WHO:什么人需要用显微计数法不溶性微粒分析仪? 首先我们了解下哪些人更关心注射剂不溶性微粒的安全:药物研发企业的研发人员、质量控制人员、药物审批单位人员、药物监察单位人员等。对于注射剂中不溶性微粒的数量,药典中有硬性标准,微粒超标就会增加对人体造成伤害的可能,这在研发、报批、生产等环节是不被允许的,所以相关人员是显微计数法不溶性微粒分析仪的直接使用者。三、WHY:为何要用显微计数法不溶性微粒分析仪检查注射剂中的不溶性微粒? 中国药典2020版0903不溶性微粒检查法包括光阻法和显微计数法。当光阻法测定结果不符合规定或供试品不适于用光阻法测定时,应采用显微计数法进行测定,并以显微计数法的测定结果作为判定依据。光阻法的测定原理是通过微粒对光的阻挡被传感器进行接收而判定微粒的大小及数量,因此光阻法不适用于黏度过高和易析出结晶的制剂,也不适用于进入传感器时容易产生气泡的注射剂,此时就需要用显微计数法不溶性微粒分析仪进行不溶性微粒检查。 显微计数法不溶性微粒仪计数环节无需人眼观察,计算机直接按照药典规定出具检测分析报告;计数环节全部由计算机完成,测试结果重复性高,且可以自动进行数据比较;仪器全自动进行滤膜全扫描,并进行颗粒图片分析,让颗粒物无处遁行;可以区分颗粒形貌,判定该不溶性微粒是金属还是纤维或是其他,鉴别不溶性微粒的来源(是外源还是内生?),并在实验或生产过程中加以控制或避免。四、WHICH:哪些药物需要用显微计数法不溶性微粒分析仪检查?中国药典2020版0903不溶性微粒检查法具体要求:本法系用以检查静脉用注射剂(溶液型注射液、注射用无菌粉末、注射用浓溶液)及供静脉注射用无菌原料药中不溶性微粒的大小及数量。上文说明一个事实,那就是静脉用注射液如传统的打吊瓶、屁股针、打疫苗及特殊病症用药如胰岛素等注射液最终会进入静脉血管的,都需进行不溶性微粒检查。五、WHERE:显微计数法不溶性微粒分析仪被哪些单位所使用?当前显微计数法不溶性微粒分析仪在研发端头部眼药企业、脂质体企业及mrna企业所广泛欢迎,在放行端也进入了多个省份及省会城市的药品检验所。相信在未来会有越来越多关注注射剂不溶性微粒安全的企业会对我们的显微计数法不溶性微粒分析仪敞开怀抱。胤煌科技显微计数法不溶性微粒分析仪具体简介如下:1、显微计数法不溶性微粒仪(型号:YH-MIP-0103): 1、计数环节无需人眼观察,计算机直接按照药典规定出具检测分析报告;2、计数环节全部由计算机完成,测试结果重复性高,且可以自动进行数据比较;3、全自动进行滤膜全扫描,并进行颗粒图片分析,让颗粒物无处遁行;4、可以区分颗粒形貌,判定该不溶性微粒是金属还是纤维或是其他,鉴别不溶性微粒的来源(是外源还是内生?),并在实验或生产过程中加以控制或避免。2、全自动显微计数法不溶性微粒仪(型号YH-MIP-0205 Pro): 在上一代仪器已有的技术优势下,进行了全新升级,新的产品在原有分步式自动扫描、自动测试、自动计数的基础上,将自动过滤、自动干燥、自动上样等功能进行了集成,真正意义上做到了如光阻法检测一样的简单且可无人值守,同时在原有超分辨算法的基础上,加入AI智能算法,方便客户更好的对不溶性微粒的来源进行判断,以便对不溶性微粒的来源进行判断和避免。
应用实例
2022.06.11
胤煌科技 显微计数法不溶性微粒仪 ——观察注射剂中不溶性微粒世界的“眼睛”
注射剂(injection)系指药物制成的供注入体内的无菌溶液(包括乳浊液和混悬液)以及供临用前配成溶液或混悬液的无菌粉末或浓溶液。 注射剂作用迅速可靠,不受pH、酶、食物等影响,无首过效应,可发挥全身或局部定位作用,适用于不宜口服药物和不能口服的病人,但注射剂研制和生产过程复杂,安全性及机体适应性差,成本较高,而在研发生产过程中其中的不溶性微粒超标对人体健康是个很大危害。 注射剂中的不溶性微粒是指药品在生产或使用过程中经由各种途径产生或混入的微粒性杂质,粒径在1~50 μm、肉眼不可见,但因其可随血液流动却不能被代谢而可能对人体造成难以发现和潜在的严重危害。20世纪30年代起研究人员开始认识到不溶性微粒的危害,并于60、70年代间对此开展了大量的实验及临床研究,随后不溶性微粒控制被纳入注射剂质量标准,且其检测方法得到不断改进。现在,有关注射剂中不溶性微粒可能对人体造成危害的观念已为临床广泛接受,过敏反应、静脉炎、血管栓塞、微循环堵塞、动脉硬化、热原反应、肉芽肿、肺栓塞等多种与不溶性微粒有关的不良反应都会引起医护人员的重视。不溶性微粒的伤害 《中国药典2020版》对注射剂中不溶性微粒的控制要求仅限于粒径≥10和25 μm的微粒数,对粒径<10μm的却没有具体要求,实际上,人体最小的毛细血管内径仅有4~7 μm,婴、幼儿的毛细血管更细,只有粒径在2 μm以下的微粒才可能通过肾交换被排出体外,而粒径为2~10 μm的微粒无法被排出。微粒进入体内造成危害的部位一般多在肺、脑、肾、眼等处,较大的微粒会直接造成局部循环障碍、引起血管栓塞或导致肉芽肿,且有短期内可见的特点,而粒径为2~10 μm的微粒则可能造成潜伏性的更大危害。颗粒的千奇百态下面我将为大家呈现显微计数法不溶性微粒仪下注射剂中微粒的聚集现象:颗粒的聚集过程聚集的颗粒无法洗去 由图可见,注射用乳剂随着时间的流逝其中不溶性微粒已经发生了聚集现象,2-10μm的粒子已经长大到10μm以上,甚至25μm以上,足以堵塞人体的静脉血管。显微镜下的颗粒形貌 如上图,是某在研注射液在显微计数法不溶性微粒仪下的真实照片,可见其中有金属颗粒和橡胶颗粒,颗粒粒径<50μm,为肉眼不可见。所以我们认为的澄清注射液中却大有乾坤。这些不溶性微粒经过注射器就会进入我们的血管,然后通过血液流动而流经全身,且无法被人体溶解和肾脏排出,从而造成血管壁的聚集和堵塞,造成血栓,是药三分段,用错药错用药很容易治病不成而造成二次伤害。 针对药物研发生产过程中的不溶性微粒检查,胤煌科技显微计数法不溶性微粒仪系列产品已经有两种提供给客户,具体如下:一、显微计数法不溶性微粒仪(型号:YH-MIP-0103):1、计数环节无需人眼观察,计算机直接按照药典规定出具检测分析报告;2、计数环节全部由计算机完成,测试结果重复性高,且可以自动进行数据比较;3、全自动进行滤膜全扫描,并进行颗粒图片分析,让颗粒物无处遁行;4、可以区分颗粒形貌,判定该不溶性微粒是金属还是纤维或是其他,鉴别不溶性微粒的来源(是外源还是内生?),并在实验或生产过程中加以控制或避免。二、全自动显微计数法不溶性微粒仪(型号YH-MIP-0205 Pro):在上一代仪器已有的技术优势下,进行了全新升级,新的产品在原有分步式自动扫描、自动测试、自动计数的基础上,将自动过滤、自动干燥、自动上样等功能进行了集成,真正意义上做到了如光阻法检测一样的简单且可无人值守,同时在原有超分辨算法的基础上,加入AI智能算法,方便客户更好的对不溶性微粒的来源进行判断,以便对不溶性微粒的来源进行判断和避免。
应用实例
2022.05.18
显微计数法不溶性微粒仪带您走进注射剂中的微小世界
显微计数法不溶性微粒仪带您走进注射剂中的微小世界 注射剂中的不溶性微粒是指药品在生产或使用过程中经由各种途径产生或混入的微粒性杂质,粒径在1~50 μm、肉眼不可见,但因其可随血液流动却不能被代谢而可能对人体造成难以发现和潜在的严重危害。20世纪30年代起研究人员开始认识到不溶性微粒的危害,并于60、70年代间对此开展了大量的实验及临床研究,随后不溶性微粒控制被纳入注射剂质量标准,且其检测方法得到不断改进。现在,有关注射剂中不溶性微粒可能对人体造成危害的观念已为临床广泛接受,过敏反应、静脉炎、血管栓塞、微循环堵塞、动脉硬化、热原反应、肉芽肿、肺栓塞等多种与不溶性微粒有关的不良反应都会引起医护人员的重视。 《中国药典》对注射剂中不溶性微粒的控制仅限于粒径≥10和25 μm的微粒数,对粒径<10μm的却没有具体要求,实际上,人体最小的毛细血管内径仅有4~7 μm,婴、幼儿的毛细血管更细,只有粒径在2 μm以下的微粒才可能通过肾交换被排出体外,而粒径为2~10 μm的微粒无法被排出。微粒进入体内造成危害的部位一般多在肺、脑、肾、眼等处,较大的微粒会直接造成局部循环障碍、引起血管栓塞或导致肉芽肿,且有短期内可见的特点,而粒径为2~10 μm的微粒则可能造成潜伏性的更大危害。 已有多项研究表明,粒径>10 μm的微粒只占注射剂中微粒的极小部分。 我选取了两组用显微计数法不溶性微粒仪(YH-MIP-0103)测试某企业生产的两批注射蛋白药中不溶性微粒按粒径的数量分布报告如下: 由上图计算可知,粒径<10μm的颗粒数分别占比88.69%和96.95%,而此类颗粒在进行配伍或复溶过程极有可能产生>10μm的颗粒物;并且2-10μm不溶性微粒本身就可以对人体毛细血管造成巨大伤害。 显微计数法不溶性微粒仪(YH-MIP-0103/YH-MIP-0205PRO)是由胤煌科技自主研发生产的符合药典标准的不溶性微粒检查设备,外观及特点如下:技术优势:√全自动的测试系统,自动扫描、计数、出具报告,减少人为操作的误差;√全自动检测系统,减少在使用过程中对测试人员眼睛的伤害;√可以区分颗粒性质,鉴别不溶性微粒的来源,是金属还是纤维;√符合中国药典、美国药典、欧洲药典、日本药典等各国药典对于不溶性微粒检查的要求。 全新一代YH-MIP-0205 Pro型全自动显微计数法不溶性微粒分析仪在上一代仪器已有的技术优势下,进行了全新升级,新产品在原有分步式自动扫描、自动测试、自动计数的基础上,进行了自动过滤、自动干燥、自动上样等功能的集成,真正做到了无人值守即可做到原有显微计数法的复杂操作流程;同时在原有超分辨算法的基础上,加入AI智能算法,方便客户更好的对不溶性的来源进行分类和整理,在今后的实验生产过程中加以避免。如下图,为该试剂在显微计数法不溶性微粒仪下的粒子分布图的一角: 见微知著,对于我们肉眼难见的注射剂不溶性微粒,却可以对我们人类的毛细血管造成灾难性的破坏。今年以来,药监局多次发文重视用药安全和安全用药,特别是大量被使用的疫苗安全更是多次被提及,相信随着人民生活水平及人们认知的不断提高,对于药物不溶性微粒的关注会越来越多,而相应管控也会越来越严。这就要求药企在研发和生产过程中更要了解每支、每批次样品中不溶性微粒的可能来源及大小数量,并加以控制和改进。
应用实例
2022.05.16
【新品发布】流式动态图像法粒度仪YH-FIPS-10隆重登场!
【新品发布】流式动态图像法粒度仪YH-FIPS-10隆重登场! 随着科技不断进步,产品质量快速提升,各行各业对颗粒检测的要求也越来越高。而在实际生产过程中,往往颗粒呈现出不规则形状。颗粒的形貌会影响到产品的稳定性,溶解性,流动性等。因此,颗粒分析不仅仅要做粒度分布的检测,还需要得到颗粒的形貌这一关键信息。 FIPS 10流式动态图像法粒度仪,是采用高速相机实时采集图片,再进行颗粒分析的一种粒度仪。样品在流动过程中实时拍摄,具有足够多的颗粒图片被采集到,使得测试结果具有代表性和统计学意义。技术优势√ 宽广的检测范围(0.3 μm-3 mm)、检测浓度可高达1*107个/mL;√ 专业远心变倍镜头,兼容不同类型粒子测试,杜绝形貌畸变;√ FIPS专利的超分辨算法及AI智能算法相结合,确保数据准确性;√ 数据同时给出粒子形貌、尺寸分布等信息,以达到最“真”统计;√ 符合21 CFR part 11及GMP对数据完整性的要求;测试结果·颗粒的直观形貌&不同尺寸粒子的粒径分布 应用领域YH-FIPS-10流式动态图像法粒度仪不仅可以得到样品中的粒子浓度、不同尺寸粒子的比例分布等详细的PSD信息,实现亚微米到微米级颗粒的计数功能,还可以得到样品的实际颗粒形貌信息,以达样品颗粒的最真实统计。可广泛应用在生物医药、半导体、材料化工、食品卫生等多个领域。企业简介胤煌科技是一家专注于为医药、半导体、面板及材料行业提供粒度及Zeta电位分析设备及技术服务的高科技公司,公司拥有检测平台可以为客户提供专业的第三方检测服务。目前公司自主研发的产品有光阻法不溶性微粒分析仪、显微计数法不溶性微粒分析仪、流式动态图像法粒度粒形分析仪等;还有代理产品伞棚灯、粒度及Zeta电位分析仪、高分辨率纳米粒度仪、原液纳米粒度及Zeta电位分析仪等检测分析设备,可以为mRNA/核酸疫苗、脂质体、乳剂、蛋白注射液等相关领域提供专业的检测分析设备。胤煌科技期待与您合作!
新品
2022.05.11
为什么一定要用显微计数法不溶性微粒仪进行注射剂不溶性微粒检查?
为什么一定要用显微计数法不溶性微粒仪进行注射剂不溶性微粒检查?一、我们先来了解什么是注射剂中的不溶性微粒及其对人体的危害:注射剂中的不溶性微粒是指药品在生产或使用过程中经由各种途径产生或混入的微粒性杂质,粒径在1~50 μm、肉眼不可见,但因其可随血液流动却不能被代谢而可能对人体造成难以发现和潜在的严重危害。具体表现为:过敏反应、静脉炎、血管栓塞、微循环堵塞、动脉硬化、热原反应、肉芽肿、肺栓塞等多种与不溶性微粒有关的不良反应。二、注射剂中的不溶性微粒的具体来源有哪些:注射剂中的不溶性微粒来源于其生产和使用过程,而目前在药品生产和使用的管理方面对此两环节均有相应的规范和制度来保证药品的安全。例如,生产过程中的GMP、《中国药典》对注射剂的检测标准、国家标准化管理委员会关于一次性输液器的国家标准等都对控制不溶性微粒的危害起着重要作用。 三、中国药典关于注射剂中不溶性微粒检测的质量标准: 对注射剂中不溶性微粒的检测标准及方法,《中国药典》已从最初的仅通过目视法检查澄明度发展到目前使用两种方法检测并限定粒径≥10和25 μm的微粒数,且其2010年版中还增加了对供生产注射用的无菌原料药的微粒检查项目。换言之,随着人们对不溶性微粒造成的危害的认识不断深入,《中国药典》对注射剂中微粒的控制也越来越严格。 四、《中国药典》对注射剂中不溶性微粒检查的规定中有两点应予以注意: 1)《中国药典》对注射剂中微粒的控制仅限于粒径≥10和25 μm的微粒数,对粒径<10μm的却没有相应控制。实际上,人体最小的毛细血管内径仅有4~7 μm,婴、幼儿的毛细血管更细,只有粒径在2 μm以下的微粒才可能通过肾交换被排出体外,而粒径为2~10 μm的微粒无法被排出。微粒进入体内造成危害的部位一般多在肺、脑、肾、眼等处,较大的微粒会直接造成局部循环障碍、引起血管栓塞或导致肉芽肿,且有短期内可见的特点,而粒径为2~10 μm的微粒则可能造成潜伏性的更大危害。 2)《中国药典》仅对注射剂成品进行微粒控制,而未对注射剂使用过程中的复溶(复配)及稀释后的输液作出相似规定。绝大多数注射剂在使用时都需要复配或稀释,而复配在操作环境、操作方法和配伍输液等方面均与药典对注射剂中不溶性微粒的检测过程不同,是注射剂在使用过程中引入或形成不溶性微粒的关键环节。有关研究表明,在输液中添加药物、尤其是粉针剂和中草药制剂后会产生明显数量的微粒。 五、《中国药典》2020版对注射剂中不溶性微粒检查具体要求:0903不溶性微粒检查法包括光阻法和显微计数法。当光阻法测定结果不符合规定或供试品不适于用光阻法测定时,应采用显微计数法进行测定,并以显微计数法的测定结果作为判定依据。光阻法的测定原理是通过微粒对光的阻挡被传感器进行接收而判定微粒的大小及数量,因此光阻法不适用于黏度过高和易析出结晶的制剂,也不适用于进入传感器时容易产生气泡的注射剂。两种测定法判定结果仅仅关注于粒径≥10和25 μm的微粒数,对粒径<10μm的却没有相应控制,但实验过程却需进行小粒径个数相应的数量及形貌进行关注。六、为什么一定要用显微计数法不溶性微粒仪进行注射剂不溶性微粒检查:显微计数法不溶性微粒仪计数环节无需人眼观察,计算机直接按照药典规定出具检测分析报告;计数环节全部由计算机完成,测试结果重复性高,且可以自动进行数据比较;仪器全自动进行滤膜全扫描,并进行颗粒图片分析,让颗粒物无处遁行;可以区分颗粒形貌,判定该不溶性微粒是金属还是纤维或是其他,鉴别不溶性微粒的来源(是外源还是内生?),并在实验或生产过程中加以控制或避免。
应用实例
2022.05.09
胤煌科技实现显微镜法检测不溶性微粒仪器设备新突破!
胤煌科技实现显微镜法检测不溶性微粒仪器设备新突破!是否还在为使用传统显微镜法进行制剂中不溶性微粒检测感到苦恼?操作繁琐、人工计数、劳动强度大、主观因素影响大、检测结果不可追溯、验证重复性差…….一切的因素都使得采用显微镜法进行不溶性微粒检测变得困难重重。宝宝心里苦,能怎么办呢?胤煌科技致力于让颗粒检测分析更专业,隆重推出全新一代全自动显微计数法不溶性微粒分析仪! 仪器型号:YH-MIP-0205Pro工作原理:显微计数法/图像法检测范围:1 μm-500 μm 产品创新点介绍·可以完成自动过滤、干燥、测试、出具报告等多项流程;·超分辨算法、AI智能算法等多种图像处理方式的引入,能有效确保测试数据的准确性;·在完美弥补常规显微镜法不溶性微粒检测的缺陷的同时,能准确保留样品中每个粒子的原始形貌,对不溶性微粒的来源或者形成机制都具有警示作用;·符合中国药典、美国药典、欧洲药典及日本药典等各国药典不溶性微粒检查的仪器要求。技术参数仪器型号YH-MIP-0205 Pro测试范围1 μm-500 μm检测原理显微计数法/静态图像法数字摄像头600万彩色高清摄像机放大倍率50-1000倍最大分辨率0.01 μm物镜标配:5X、10X;选配:20X、50X、100X分辨率≥95%照明系统自动LED照明系统电动控制系统XYZ-三轴移动滤膜夹具25 mm/13 mm电源220 V软件运行环境Win 10及以上电脑配置要求CPUI7-11700F/运行内存32G或以上配置; 6G独立显卡,256SSD+2T硬盘; IPS 旋转升降 低蓝光高分辨显示屏;显微计数法不溶性微粒仪让颗粒无处遁形! 企业简介:上海胤煌科技有限公司是一家专注于为医药生物、半导体、面板及材料等行业提供粒度及Zeta电位分析设备及技术服务的高科技公司,公司拥有检测平台可以为客户提供专业的第三方检测服务。目前公司自主研发的产品有光阻法不溶性微粒分析仪、显微计数法不溶性微粒分析仪、流式动态图像法粒度粒形分析仪等;还代理有伞棚灯、粒度及Zeta电位分析仪、高分辨率纳米粒度仪、原液纳米粒度及Zeta电位分析仪等检测分析设备,可以为mRNA、核酸疫苗、药物递送等相关领域提供专业的检测分析设备。 上海胤煌科技有限公司期待与您合作!
新品
2022.05.05
中/美/欧/日四大药典溶液颜色检查规范 --参考与比较
中/美/欧/日四大药典澄清度检查规范-中英双译中国药典20200902 澄清度检查法澄清度检查法系将药品溶液与规定的浊度标准液相比较,用以检查溶液的澄清度。除另有规定外,应采用第一法进行检测。品种项下规定的“澄清”,系指供试品溶液的澄清度与所用溶剂相同,或不超过0.5号浊度标准。“几乎澄清”,系指供试品溶液的浊度介于0.5号至1号浊度标准液的浊度之间。第一法(目视法)除另有规定外,按各品种项下规定的浓度要求,在室温条件下将用水稀释至一定浓度的供试品溶液与等量的浊度标准液分别置于配对的比浊用玻璃管(内径15-16 mm,平底,具塞,以无色、透明、中性硬质玻璃制成)中,在浊度标准液制备5 分钟后,在暗室内垂直置于伞棚灯下,照度为1000 lx,从水平方向观察、比较。除另有规定外外,供试品溶解后应立即检视。第一法无法准确判定两者的澄清度差异时,改用第二法进行测定,并以其测定结果进行判定。浊度标准存贮液的制备 称取于105℃干燥至恒重的硫酸肼1.00 g,置于100 ml量瓶中,加水适量使溶解,必要时可在40℃的水浴中温热溶解,并用水稀释至刻度,摇匀,放置4-6小时;取此溶液于等容量的10%乌洛托品溶液混合,摇匀,于25℃避光静置24小时,即得。该溶液置冷处避光保存,可在2个月内使用,用前摇匀。浊度标准原液的制备 取浊度标准贮备液15.0 ml,置1000 ml量瓶中,加水稀释至刻度,摇匀,取适量,置1 cm吸收池中,照紫外-可见分光光度法(通则0401),在550 nm的波长处测定,其吸光度在0.12-0.15范围内,该溶液应在48小时内使用,用前摇匀。浊度标准液制备 取浊度标准原液与水,按照下表配置,即得。浊度标准液应临用时制备,使用前充分摇匀。 第二法(浊度仪法)供试品的浊度可采用浊度仪测定。溶液中不同大小、不同特性的微粒物质包括有色物质均可使入射光产生散射,通过测定透射光或者散射光的强度,可以检查供试品的浊度。仪器测定模式通常有三种类型,透射光式、散射光式和透射光-散射光比较测量模式(比率浊度模式)。1.仪器的一般要求采用散射光式浊度仪时,光源峰值波长为860 nm;测量范围应包含0.01-100ntu。在0-10ntu范围内分辨率应为0.01ntu;在10-100ntu范围内分辨率应为0.1ntu.2.适用范围及检测原理本法采用散射光式浊度仪,适用于低、中浊度无色供试品溶液的浊度测定(浊度值为100ntu以下的供试品。)因为高浊度的供试品会造成多次散射现象,时散射光强度迅速下降,导致散射光强度不能正确反映供试品的浊度值。0.5-4号浊度标准液的浊度值范围约为0-40ntu。采用散射光式浊度仪测定时,入射光和测定的散射光呈90℃夹角,入射光强度和散射光强度关系式如下。i=k’t i0式中 i为散射光强度,单位为cd; i0 为入射光强度,单位为cd; k’为散射系数; t为供试品溶液的浊度值,单位为ntu(ntu是基于福尔马肼浊度标准液液测定的散射浊度单位,福尔马肼浊度标准液即为第一法中的浊度标准贮备液)。在入射光i0不变的情况下,散射光强度i与浊度值成正比。因此,可以将浊度测量转化为散射光强度的测量。3.系统的适用性试验仪器应定期(一般每月一次)对浊度标准液的线性和重复性进行考察,采用0.5号至4号浊度标准液进行浊度值测定,浊度标准液的测定解果(单位ntu)与浓度间应呈线性关系,线性方程的相关系数应不低于0.999;取0.5号至4号浊度标准液,重复测定5次,0.5号和1号浊度标准液测量浊度值的相对标准偏差应不大于5%,2-4号浊度标准液测量浊度值的相对标准偏差不大于2%。4.测定法按照仪器说明书要求并采用规定的浊度液进行仪器校正。溶液剂直接取样测定;原料药或者其它剂型按照个论项下的标准规定制备供试品溶液,临用时制备。分别取供试品溶液和相应浊度标准液进行测定,测定前应摇匀,并避免产生气泡,读取浊度值。供试品溶液浊度值不得大于相应浊度标准液的浊度值。 美国药典usp44 visual comparison 视觉比较the purpose of this test is to provide the details for the visual comparison of the color and/or turbidance of sample solutions of certain concentration to a standard solution or a series of standard solutions of known concentration. where a color or turbidity comparison is directed, follow the procedures and conditions outlined below for performing these tests.本试验的目的是提供特定浓度的样品溶液与已知浓度的标准溶液或一系列标准溶液的颜色和/或浊度的视觉比较细节。如果需要进行颜色或浊度比较,请遵循以下程序和条件进行这些测试 comparison vessels: color-comparison tubes matched as closely as possible in internal diameter, in depth of sample solution, and in all other respects should be used.对比容器:应使用内径、样品溶液深度和所有其他方面尽可能匹配的颜色对比管。 viewing conditions for turbidity comparison: tubes should be viewed horizontally against a dark background with the aid of a light source directed from the sides of the tubes.浊度比较的观察条件:应在黑暗背景下,借助从管子侧面发出的光源水平观察管子。 viewing conditions for color comparison: tubes should be viewed downward against a white background. most of the time, common room lighting is sufficient to perform the assessment. a light source directed from beneath the bottoms of the tubes may be used if needed and if the practice is consistent between the materials under comparison.颜色比较的观察条件:管子应在白色背景下向下观察。大多数情况下,公共空间照明足以进行评估。如果需要,并且对比材料之间的实践一致,可以使用从管底部下方引导的光源 nephelometry and turbidimetry 散射光浊度法和透射光比浊法1. introduction 介绍nephelometry and turbidimetry are analytical techniques that are based on the principles of light-scattering phenomena. light scattering is the physical phenomenon in which a beam of light changes its direction of propagation (known as deflection) as a result of interaction with sufficiently small matter particles. it has been established from the maxwell electromagnetic theory that a prerequisite for scattering to occur is that the refractive indexes of the suspended particles must be different from those of the suspending liquid. the larger the difference, the more intense the scattering becomes. there are two types of light scattering: 1) elastic scattering, in which the wavelength of the scattered light and incident light are the same; and 2) inelastic light scattering, in which the wavelength of the scattered light and incident light are different. only the first type of light scattering (elastic) is relevant to nephelometry and turbidimetry.散射光浊度法和透射光比浊法是基于光散射现象原理的分析技术。光散射是一种物理现象,其中光束由于与足够小的物质粒子相互作用而改变其传播方向(称为偏转)。根据麦克斯韦电磁理论,散射发生的先决条件是悬浮颗粒的折射率必须不同于悬浮液体的折射率。差异越大,散射越强烈。光散射有两种类型:1)弹性散射,其中散射光和入射光的波长相同;2)非弹性光散射,其中散射光和入射光的波长不同。只有前一种光散射(弹性)与散射光浊度法和透射光比浊法有关。 in turbidimetry, the intensity of the transmitted light is measured and the attenuation of the intensity of incident light as a result of scattering is measured at the direction of incident light (i.e., 0°) and compared to the intensity of incident light (blank measurement). the measured property is an indirect measurement of the scattering effect of the suspended particles and is referred to as turbidance. any absorbance of light by the suspended sample will result in additional attenuation of light intensity (see ultraviolet-visible spectroscopy and ultraviolet-visible spectroscopy—theory and practice ). hence, it is important to ensure that the material being measured does not absorb light at the measurement wavelength. indeed the equations governing absorption and turbidimetry are the same (albeit with different values for the attenuation constants). in nephelometric techniques, the intensity of the scattered light at a 90° angle from the propagation direction of the incident light is measured. therefore, a nephelometric measurement is a direct measurement of the scattering effect of suspended matter.在透射光比浊法中,测量透射光的强度,并在入射光方向(即0°)测量散射导致的入射光强度的衰减,并与入射光强度进行比较(空白测量)。被测特性是悬浮颗粒散射效应的间接测量,称为浊度。悬浮样品对光的任何吸收都会导致光强度的额外衰减(参见 ultraviolet-visible spectroscopy和 ultraviolet-visible spectroscopy—theory and practice)。因此,确保被测材料不会吸收测量波长处的光非常重要。实际上,控制吸收和浊度测定的方程式是相同的(尽管衰减常数的值不同)。在散射光浊度法中,测量与入射光传播方向成90°角的散射光强度。因此,散射光浊度法浊度测量是对悬浮物散射效应的直接测量。 2. terms and definitions 术语和定义terms commonly used in describing turbidimetric and nephelometric techniques are:• turbidance (symbol, s): a measure of the decrease of the transmitted incident light beam intensity as a result of the light-scattering effect of suspended particles. the amount of suspended matter may be measured by observation of either the transmitted light (turbidimetry) or the scattered light (nephelometry).log i0/it = kbc = ti0 = intensity of incident lightit = intensity of transmitted lightk = molar turbidity coefficientb = cell path lengthc = concentrationt = turbidance• turbidity (symbol, τ): in turbidimetric measurements, the turbidity is the measure of the decrease in incident beam intensity/unit length of a given suspension. the international organization for standardization defines turbidity as “the reduction of transparency of a liquid caused by the presence of undissolved matter”.• turbidity measurement units: the turbidity units are stated using a descriptor which indicates the method of measurement.• nephelometric turbidity units (ntus): when the turbidity is measured using a nephelometer, which measures the scattered light at a 90° angle from the direction of propagation of incident light, the units of turbidity are called nephelometric turbidity units (ntus). the magnitude of ntu is defined based on the turbidity generated by primary formazin standard (a suspension made by mixing solutions of hydrazine sulfate and hexamethylenetetramine in water). safer polymer-bead suspensions are now commercially available and are recognized as an acceptable alternative. however, all those standards are traced to formazin. the primary formazin standard solution has been assigned a turbidity of 4000 ntus.other recognized units for turbidity include the formazin turbidity unit (ftu) and the formazin nephelometric unit (fnu). these units are equivalent to ntu for the range from 0–40 ntus.描述浊度法和浊度法的常用术语包括:•浊度(符号s):由于悬浮颗粒的光散射效应,透射入射光束强度降低的一种度量。悬浮物的量可以通过观察透射光(比浊法)或散射光(浊度法)来测量。log i0/it = kbc = ti0=入射光强度it=透射光强度k=摩尔浊度系数b=样品池路径长度c=浓度t=浊度•浊度(符号,τ):在透射光浊度测量中,浊度是给定悬浮液的入射光束强度/单位长度减少的量度。国际标准化组织将浊度定义为“由于存在未溶解物质而导致液体透明度降低”。•浊度测量单位:浑浊度单位用一个描述符表示,该描述符指示测量方法。•散射光浊度计浊度单位(ntu):当使用散射光浊度法测量浊度时,浊度计以与入射光传播方向成90°角的角度测量散射光,浊度单位称为散射光浊度法浊度单位(ntu)。ntu的大小是根据初级福尔马肼标准品(一种将硫酸肼和六亚甲基四胺溶液混合在水中制成的悬浮液)产生的浊度定义的。更安全的聚合物微珠悬浮液现已上市,并被公认为可接受的替代品。然而,所有这些标准都可以追溯到福尔马肼。初级福尔马肼标准溶液的浊度为4000 ntu。其他公认的浊度单位包括福尔马肼比浊法单位(ftu)和福尔马肼浊度法单位(fnu)。这些单位相当于0-40 ntu范围内的ntu。3. applications 应用turbidimetric and nephelometric techniques have applications that include 1) concentration determination of solutions and/or suspensions (determination of several cations and anions by precipitating and suspending the resulting precipitate at well-controlled reaction parameters); 2) measurement of the degree of turbidity of turbid solutions or suspensions; 3) determination of weight-average molecular weights and dimensions of polydisperse systems in the molecular weight range from 1000 to several hundred million; 4) measurement of immunoassays’ reaction kinetics or kinetics of immunoprecipitations (rate nephelometry); 5) monitoring of cell and bacteria growth; and 6) particle size distribution determination of suspended material, particle counting, etc.透射光比浊法和散射光浊度法技术的应用包括1)溶液和/或悬浮液的浓度测定(通过在控制良好的反应参数下沉淀和悬浮产生的沉淀物,来测定几种阳离子和阴离子);2)测量混浊溶液或悬浮液的浊度;3)测定分子量在1000到数亿之间的多分散体系的重均分子量和尺寸;4)测量免疫分析的反应动力学或免疫沉淀动力学(比率散射浊度法);5)监测细胞和细菌的生长;6)悬浮物粒度分布测定、颗粒计数等。 rate nephelometry is widely used for vaccine components assays and/or quantitation of components in blood serum. it is also used for host cell protein qualification in recombinant biopharmaceuticals. when using the technique, the measurement of the change in the light-scattering response by antigen–antiserum or antigen-purified antibody complexes is used to calculate the amount of antigen (ag) or antibody (ab) responsible for the immunological ab-ag precipitation reaction or agglutination reaction. often the antigens under consideration are linked covalently or adsorbed to polymeric microspheres to increase the scattering efficiency; the resulting technique is known as "particle-enhanced immunoassay". although the technique is described as nephelometry, usually both scattered and transmitted light are measured using the ratio instruments.比率散射浊度法广泛用于疫苗成分分析和/或血清成分的定量。它还用于重组生物制药中的宿主细胞蛋白质鉴定。当使用该技术时,通过测量抗原-抗血清或抗原纯化抗体复合物的光散射反应的变化,来计算导致免疫抗体-抗原沉淀反应或凝集反应的抗原(ag)或抗体(ab)的量。通常考虑抗原共价连接或吸附在聚合物微球上,以提高散射效率;由此产生的技术被称为“颗粒增强免疫分析”。虽然这项技术被称为散射光浊度法,但通常散射光和透射光都是用比率仪器测量的。 nephelometric measurements are more reliable in low turbidity ranges (relatively low concentration of the scattering medium). in this range, a linear relationship is observed between the sample concentration and the detector’s signal intensity expressed as ntu. as the concentration increases, so does the incidence of multiple scattering that deviates the response from the linearity. the maximum ntu value, which supports a reliable linearity relationship, is in the range of 1750–2000 ntus. turbidimetry is preferred for higher turbidity ranges (concentrations of the scattering media). to achieve consistent results, all measurement variables must be carefully controlled. where such control is possible, extremely dilute suspensions may be measured.散射光法浊度测量在低浊度范围(散射介质浓度相对较低)更可靠。在该范围内,观察到样品浓度与检测器信号强度(以ntu表示)之间存在线性关系。随着浓度的增加,多次散射的入射角也会增加,从而偏离线性响应。支持可靠线性关系的最大ntu值在1750–2000 ntu范围内。透射光比浊法适用于更高的浊度范围(散射介质的浓度)。为了获得一致的结果,必须仔细控制所有测量变量。在可能的情况下,可以测量极稀的悬浮液。 4. instrumentation 仪器仪表instruments used for turbidimetric and nephelometric measurements are called turbidimeters and nephelometers, respectively. generally, these instruments consist of a mercury lamp with filters for the strong green or blue lines, a shutter, a set of neutral filters with known transmittance, and a sensitive photomultiplier, which can be mounted fixed at 0° or at a 90° angle from the incident light propagation direction, or on an arm that can be rotated around the solution cell and set at any angle from −135° to 0° to +135° by a dial outside of the light-tight housing. solution cells are of various shapes, such as square for measuring 90° scattering; semioctagonal for 45°, 90°, and 135° scattering; and cylindrical for scattering at all angles (see figure 1).用于透射光比浊法和散射光浊度法测量的仪器分别称为透射光浊度计和散射光浊度计。通常,这些仪器包括一个带有滤光器的汞灯(用于强绿线或蓝线)、一个快门、一组具有已知透射率的中性滤光器和一个灵敏的光电倍增管,该光电倍增管可安装在与入射光传播方向成0°或90°角的位置,或者在一个臂上,它可以围绕溶液单元旋转,并通过不透光外壳外的表盘设置为−135°到0°到+135°的任何角度。溶液池的形状多种多样,例如用于测量90°散射的正方形;45°、90°和135°散射为半八角形;圆柱形可适用于所有角度的散射(见图1)。 figure 1. representative nephelometric (turbidimetric) instrument. note that detector 2 may be mounted on a movable arm.图1。代表性浊度仪。注意,探测器2可安装在可移动臂上。 turbidity also can be measured with a standard photoelectric filter photometer or spectrophotometer, preferably with illumination in the blue portion of the spectrum. nephelometric measurements require an instrument with a photocell placed so as to receive scattered, rather than transmitted, light. because this is the same geometry used in fluorometers, they can be used as nephelometers by proper selection of filters. a ratio turbidimeter combines the technology of 90° nephelometry and turbidimetry. it contains photocells that receive and measure scattered light at a 90° angle from the sample as well as receiving and measuring the forward scatter in front of the sample. it also measures light transmitted directly through the sample. linearity is attained by calculating the ratio of the 90° angle scattered light measurement to the sum of the forward scattered light measurement and the transmitted light measurement. the benefit of using a ratio turbidimetric system is that the measurement of stray light becomes negligible. in addition, the determination of turbidity of colored suspensions is done exclusively using turbidimetric or nephelometric instruments with ratio mode because this procedure compensates for the attenuation of light as the result of the suspension color. typically, the light source in these instruments is a tungsten lamp with most of the light intensity at about 550 nm operating at the filament temperature of 2700 k. other suitable light sources are also available. typically, the detectors are ▲silicon diodes▲ (err 1-may-2019) and photomultipliers. an alternative for eliminating the color effect involves using an infrared light-emitting diode as a light source, which yields an emission maximum centered at about 860 nm and a spectral bandwidth of 60 nm. when laser light sources are also used, especially in nephelometric instruments, the technique is commonly known as "laser nephelometry". the advantage of using laser nephelometers is the significant improvement in signal-to-noise ratio at very low detection levels. usually the light source is a laser diode with a working wavelength at 660 nm. the high-power density of the laser beam gives rise to higher scattered intensity from smaller particles. combined with a light trap, which absorbs the unscattered light, the system lowers the stray light significantly. when the use of a nephelometer or turbidimeter is indicated for a procedure in a monograph, instruments working in ratio mode may be used instead.浊度也可以用标准光电滤光光度计或分光光度计测量,最好是在光谱的蓝色部分进行照明。散射光浊度法测量需要一个装有光电管的仪器,以便接收散射光,而不是透射光。由于这与荧光计中使用的几何结构相同,因此可通过适当选择滤光片将其用作浊度计。比率浊度计结合了90°散射光浊度法和透射光比浊法。它包含光电管,接收和测量与样品成90°角的散射光,以及接收和测量样品前面的前向散射光。它还测量直接穿过样品的光。通过90°角散射光测量值,前向散射光测量值和透射光测量值之和,计算两者的比值,可获得线性度。使用比率浊度测量系统的好处是杂散光的测量变得可以忽略不计。此外,彩色悬浮液的浊度测定仅使用透射光比浊法浊度仪或浊度仪(带比率模式)进行,因为该程序补偿了悬浮液颜色导致的光衰减。通常,这些仪器中的光源是钨灯,在2700 k的灯丝温度下工作,大部分光强约为550 nm。也可使用其他合适的光源。通常,探测器是▲硅二极管▲和光电倍增管。另一种消除颜色效应的方法是使用红外发光二极管作为光源,其最大发射中心约为860 nm,光谱带宽为60 nm。当激光光源也被使用时,尤其是在浊度测量仪器中,这种技术通常被称为“激光浊度测量”。使用激光散射光浊度计的优点是,在非常低的检测水平下,信噪比显著提高。通常光源是工作波长为660 nm的激光二极管。激光束的高功率密度使较小粒子产生更高的散射强度。与吸收未散射光的光阱相结合,该系统可显著降低杂散光。当专著中的某个程序指示使用散射光浊度计或透射光浊度计时,可以使用在比率模式下工作的仪器。 5. formazin turbidity standards福尔马肼浊度标准formazin is the only known primary turbidity standard. all other standards are secondary and must be traced to formazin. the primary standard is defined in the ▲iupac compendium of chemical terminology,▲ (err 1-may-2019) 2nd ed. (the gold book) as one that is prepared by the user from traceable materials using well-defined methodologies and conditions.福尔马肼是唯一已知的主要浊度标准。 所有其他标准都是次要的,必须追溯到福尔马肼。 主要标准在▲iupac compendium of chemical terminology▲(err 1-may-2019)第 2 版(金书)中被定义为由用户使用明确定义的方法和条件从可追溯的材料准备的标准。 formazin suspension has many features that ensure its suitability as a primary standard. it can be consistently and accurately prepared from reagent-grade starting materials. the suspension consists of random polymers with different lengths and of random configurations, which result in moieties of varying shapes and sizes ranging from less than 0.1 μm to more than 10 μm. although the polymer chain length distribution has been shown to vary from preparation to preparation, the overall resulting turbidity has been very reproducible.福尔马肼悬浮液有许多特点,以确保其适合作为主要标准。它可以从试剂级的起始材料中始终如一、准确地制备。该悬浮液由不同长度和随机构型的聚合物组成,其组成的聚合物的形状和尺寸从小于0.1 μm到大于10 μm不等。尽管聚合物链长分布已被证明因制备而异,但总的浊度结果是可以很好地重现的。 5.1 preparation of the formazin standards 福尔马肼标准液的制备[note—all procedures described below must be performed at 20 ± 2° (see volumetric apparatus .]• hydrazine sulfate solution: dissolve 1.000 g of acs grade hydrazine sulfate (n2h4·h2so4) in particle-free water in a 100-ml class a volumetric flask and dilute with particle-free water to volume. allow this solution to stand for 4–6 h.•primary formazin standard: dissolve 2.50 g of analytical grade hexamethylenetetramine [(ch2)6n4] in 25.0 ml of particle-free water in a 100-ml flask. add 25.0 ml of hydrazine sulfate solution using a class a 25-ml “to deliver” pipette and mix thoroughly. allow the preparation to stand for 48 h at 25 ± 1° before using. the resulting suspension is stable for 2 months.•formazin stock standard suspension 1: using a 15-ml class a “to deliver” pipette, transfer 15 ml of the primary formazin standard to a 1-l volumetric flask, and dilute with particle-free water to volume and mix. the resulting suspension has a turbidity of 60 ntu.• formazin stock standard suspension 2: using a 50-ml class a “to deliver” pipette, transfer 50 ml of primary formazin standard to a 200-ml volumetric flask, and dilute with particle-free water to volume and mix. the resulting suspension has a turbidity of 1000 ntus.• formazin reference suspensions: prepare by mixing in a 100-ml volumetric flask, portions of the respective formazin stock standard suspension and particle-free water according to table 1.[注:以下所有的程序必须在20±2°的条件下进行(参见)]•硫酸肼溶液:将1.000 g acs级硫酸肼(n2h4·h2so4)溶解在100 ml 的a类容量瓶中中,并用无颗粒水稀释至刻度。让该溶液静置4-6小时。•初级福尔马肼标准液:将2.50 g分析级六亚甲基四胺[(ch2)6n4]溶于25.0 ml无颗粒水中,装入100 ml烧瓶。使用a级25ml移液管加入25.0 ml硫酸肼溶液,并充分混合。使用前,让制剂在25±1°的温度下静置48小时。由此产生的悬浮液可稳定运行2个月。•福尔马肼储备标准悬浮液1:使用15 ml a类移液管,将15 ml福尔马肼初级标准液转移至1 l容量瓶中,并用无颗粒水稀释至刻度并混合。所得悬浮液的浊度为60 ntu。•福尔马肼储备标准悬浮液2:使用50 ml a类移液管,将50 ml福尔马肼初级标准液转移至200 ml容量瓶中,并用无颗粒水稀释至刻度并混合。所得悬浮液的浊度为1000 ntu。•福尔马肼参考悬浮液:根据表1,在100 ml容量瓶中混合各份福尔马肼储备标准悬浮液和无颗粒水,制备福尔马肼参考悬浮液。 6. qualification of turbidimeters and nephelometers 透射光式浊度仪与散射光式浊度仪的鉴定the suitability of a specific instrument for a given procedure is ensured by a stepwise life cycle evaluation for the desired application from selection to instrument retirement. the qualification comprises three components: 1) installation qualification (iq), 2) operational qualification (oq), and 3) performance qualification (pq) (see analytical instrument qualification ).特定仪器对给定程序的适用性由从选择到仪器报废的预期应用的逐步生命周期评估来确保。鉴定包括三个部分:1)安装鉴定(iq)、2)操作鉴定(oq)和3)性能鉴定(pq)(参见analytical instrument qualification章节)。 the purpose of this section is to provide test methods and acceptance criteria to ensure that the instrument is suitable for its intended use (oq), and that it will continue to function properly over extended time periods (pq). as with any spectrometric device, a turbidimetric and nephelometric spectrometer must be qualified for both wavelength (x-axis, if not fixed) and photometric (y-axis, or signal axis) accuracy and precision, and meet the requirements for the stray light. oq is carried out across the operational ranges required within the laboratory for both the absorbance and wavelength scales.本节的目的是提供测试方法和验收标准,以确保仪器适合其预期用途(oq),并在延长的时间段(pq)内继续正常工作。与任何光谱仪一样,透射光式和散射光式浊度光谱仪必须具备波长(x轴,如果不固定)和光度(y轴或信号轴)的准确度及精度,并满足杂散光的要求。oq是在实验室内吸光度和波长标度所需的操作范围内进行的。acceptance criteria for critical instrument parameters that establish “fitness for purpose” are verified during iq and oq. specifications for particular instruments and applications can vary depending on the analytical procedure used and the desired accuracy of the final result. instrument vendors often have samples and test parameters available as part of the iq/oq package.在iq和oq期间,验证确定“用途适用性”的关键仪器参数的验收标准。特定仪器和应用的规格可能因使用的分析程序和最终结果的预期准确度而异。仪器供应商通常将样品和测试参数作为iq/oq包的一部分提供。 wherever possible in the procedures detailed as follows, primary reference standards or certified reference materials (crms) are to be used. formazin is the only primary reference standard used in turbidimetry and nephelometry. all the other standards, including the crms, must be correlated to formazin. the crms should be obtained from a recognized accredited source and include independently verified traceable value assignments with associated calculated uncertainty. crms must be kept clean and free from dust. recertification should be performed periodically to maintain the validity of the certification.在以下详述的程序中,应尽可能使用主要参考标准或认证参考材料(crm)。福尔马肼是比浊法法和浊度法中唯一使用的主要参考标准。所有其他标准,包括crm,必须与福尔马肼相关。crm应从认可的认证来源获得,并包括独立验证的可追溯值分配及相关的计算不确定性。crm必须保持清洁,无灰尘。应定期进行重新认证,以保持认证的有效性。 6.1 calibration校准all of the turbidimetric and nephelometric instruments are calibrated against standards of known turbidity. the instrument must be calibrated using formazin turbidity standards prior to its first time use and at least every 3 months or as specified by the vendor. calibration is performed using at least four formazin turbidity standards whose turbidity proportionally covers the range of interest. many instrument manufactures provide calibration verification standards. they usually consist of sealed sample cells filled with a latex suspension or with metal oxide particles in polymer gel. these standards must be used only for checking the calibration in the time intervals between the instrument recommended calibrations.所有透射光式浊度仪和散射光式浊度仪均根据已知浊度的标准进行校准。在首次使用之前,必须使用福尔马肼浊度标准液对仪器进行校准,至少每3个月或按照供应商的规定进行一次校准。使用至少四种福尔马肼浊度标准液进行校准,其浊度按比例覆盖感兴趣的范围。许多仪器制造商提供校准验证标准。它们通常由其中充满聚合物凝胶中的金属氧化物颗粒的密封样品池或乳胶悬浮液组成。这些标准只能用于检查仪器推荐校准的时间间隔内的校准。 6.2 stray light杂散光stray light (stray radiant energy) is a very significant error source, especially for measurements in the range of the lower turbidity readings. it is defined as external light that reaches the detector without being scattered from the sample. there are several sources of stray light including the inherent cell surface imperfections, reflections from within the cell that are unaccounted for, optical system parts, light sources, and, to a smaller degree, the electronics fluctuations. although there are many design features that instrument vendors use to minimize the stray light, a complete mitigation of the stray light cannot be achieved. unlike spectrophotometric measurements, the stray light cannot be compensated for in turbidimetry. the stray light must be measured and the values should be within the specification range set by the vendor of the particular instrument or 杂散光(杂散光辐射能)是一个非常重要的误差源,特别是在较低的浊度读数范围内的测量。它被定义为到达探测器而不被样品散射的外部光线。杂散光有几种来源,包括电池表面固有缺陷、电池内部未被解释的反射、光学系统部件、光源,以及在较小程度上的电子波动。尽管仪器供应商使用了许多设计功能来最小化杂散光,但无法完全缓解杂散光。与分光光度测量不同,浊度法无法补偿杂散光。必须测量杂散光,其值应在特定仪器供应商设定的规格范围内,或在0-10 ntu范围内测量时小于0.15 ntu,在10-1100 ntu范围内测量时小于0.5 ntu,以较小者为准。 6.3 range of measuring capability测量能力的范围the instrument must be able to measure the turbidity in the range of 0.01–1100 ntus or from 50%–200% of the target turbidity. to demonstrate the linearity for the intended measurements range, choose at least four appropriate reference suspensions from table 1.仪器必须能够测量0.01–1100 ntu范围内或目标浊度50%-200%范围内的浊度。为了证明预期测量范围的线性,从表1中选择至少四种合适的参考悬浮液。 6.4 resolution 解决方案instrument resolution must be 0.01 ntu or less for the measurements range of 0–9.99 ntus; 0.1 ntu or less for the measurements range of 10–99.9 ntus; and 1 ntu for the measurements above 100 ntus.对于0-9.99 ntu的测量范围,仪器分辨率必须小于等于0.01 ntu;测量范围为10-99.9 ntu时,小于等于0.1 ntu;100 ntu以上的测量分辨率值为1 ntu。 6.5 accuracy准确度the instrument reading accuracy must be ±10% of the reading + 0.01 ntu for the measurement range from 0–19.9 ntus, and ±7.5% of the reading for the measurement range from 20–1100 ntus.对于0-19.9 ntu的测量范围,仪器读数准确度必须为读数+0.01 ntu的±10%,对于20-1100 ntu的测量范围,仪器读数准确度必须为读数的±7.5%。 6.6 performance qualification性能鉴定the instrument pq is accomplished periodically or as needed between the calibrations. primary turbidity standards (formazin) or secondary calibration verification standards (latex suspensions or metal oxide particles in polymer gels contained in sealed sample cells) supplied by instrument manufacturers may be used.定期或根据需要在校准期之间完成仪器pq。可使用仪器制造商提供的一级浊度标准(福尔马肼)或二级校准验证标准(乳胶悬浮液或密封样品池中聚合物凝胶中的金属氧化物颗粒)。 7. procedure步骤7.1 turbidimetric procedures 透射光比浊法测试步骤sample cell preparation 样品池准备the sample cells for sample measurements must be clean. follow the sample cell or instrument manufacturer recommendations for cleaning the sample cells appropriately. for low turbidity measurements it is a good practice to use a single-indexed sample cell or a flow cell, which help ensure adequate precision and repeatability of the measurements. using particle-free water, find the sample cell orientation in the sample cell holder that gives the lowest reading. for higher values of turbidity, different sample cells may be used. however, the sample cells must be matched (the difference in readings for a standard prepared at nominal sample concentration from two different sample cells must be within ±0.005 ntu or below the measurement precision requirement, whichever is lower).用于样品测量的样品室必须清洁。按照样品池或仪器制造商的建议适当清洁样品池。对于低浊度测量,最好使用一个单指数样品池或流动池,这有助于确保测量的足够精度和可重复性。使用无颗粒水,在样品池支架中找到读数最低的样品池方向。对于较高的浊度值,可使用不同的样品池。然而,样品池必须匹配(两个不同样品池在标称样品浓度下制备的标准品读数差异必须在±0.005 ntu范围内或低于测量精度要求,以较低者为准)。 sample preparation样品准备prepare the samples as prescribed in the individual monograph. carefully mix the samples thoroughly by swirling or inverting the volumetric flask slowly several times. avoid shaking or stirring since it may introduce bubbles. degassing the samples helps to improve the measurements. for degassing, the samples could stand for several minutes or a vacuum could be applied, or they could be gently sonicated using an ultrasonic bath. after degassing, let the samples stand for several minutes and mix again by carefully inverting two to three times. transfer the sample to the sample cell and take the readings.按照各专题中的规定制备样品。通过缓慢旋转或倒置容量瓶数次,仔细混合样品。避免摇晃或搅拌,因为这可能会产生气泡。对样品进行脱气有助于改进测量。对于脱气,样品可以静置几分钟,或者可以施加真空,或者可以使用超声波浴对其进行轻轻的超声波处理。脱气后,让样品静置几分钟,然后小心地反转两到三次,再次混合。将样品转移至样品池并读取读数。 use of flow cells流动池的使用flow cells are mainly used for low turbidity measurements for samples with small particles. when such cells are used, the sample is introduced by carefully pouring it down the interior edge of the inlet reservoir.in practice, it is advisable to ensure that settling of the particles being measured is negligible. this is usually accomplished by including a protective colloid in the liquid-suspending medium. it is important that results be interpreted by a comparison of readings with those representing known concentrations of suspended matter, produced under precisely the same conditions.流动池主要用于小颗粒样品的低浊度测量。当使用这种样品池时,通过小心地将样品倒入进水仓的内边缘来引入样品。在实际过程中,建议确保被测颗粒的沉降可以忽略不计。这通常通过在液体悬浮介质中加入保护胶体来实现。重要的是,通过将读数与在完全相同的条件下产生的已知悬浮物浓度的读数进行比较来解释结果。 7.2 nephelometric procedures散射光浊度法步骤nephelometric procedures are performed similarly to turbidimetric procedures for both direct measurements and measurements in the ratio mode as described above.散射光浊度法步骤的执行方式与透射光比浊法程序类似,适用于直接测量和上述比率模式下的测量。 rate nephelometric procedures比率模式散射光浊度法步骤the overall procedure for monitoring the progress of the reaction consists of three well-defined steps: 1) record a baseline reading of the turbidity of the medium (blank); 2) record the turbidity after the first reagent (antigen) is added, which results in an increase of the turbidity until a plateau is reached; and 3) add the second reagent (antibody), which results in another turbidity increase and a second plateau followed by a final turbidity increase that continues until a third plateau is reached. the measurement zone is selected from the addition of the antibody until the third plateau, depending on the purpose of the assay and the respective component concentrations. kinetic nephelometry and endpoint nephelometry are two general procedures that are used for quantifying the immune complexes formed in the immunoassay methods (also known as immunonephelometry because the measured turbidity is due to immunocomplexes that are formed). for each procedure, there are several parameters that need to be optimized in each individual application. the main parameters are 1) with or without particle enhancement; 2) particle types, sizes, and respective optimum wavelength, if applicable; 3) monitoring reaction kinetic or endpoint; 4) antibody/antigen under consideration and, related to that, the optimum level of antigen loading; 5) buffers and other ionic species and respective optimal ph; 6) type and concentration of polymers used to modify the solubility of proteins; and 7) temperature and other environmental factors. generally these parameters are optimized during the method development and the values are given in specific monograph(s) and/or chapter(s) as applicable.监测反应进程的总体程序包括三个明确定义的步骤:1)记录介质浊度的基线读数(空白);2) 在添加一种试剂(抗原)后,记录浊度,这会导致浊度增加,直到达到一个稳定期;3)添加第二种试剂(抗体),这会导致另一个浊度增加和第二个稳定期,然后是最终浊度增加,直到达到第三个稳定器。根据分析目的和各自的组分浓度,从添加抗体到第三个稳定期中间选择测量区。动力学散射比浊法和终点散射比浊法是两种通用程序,用于量化免疫分析方法中形成的免疫复合物(也称为免疫散射比浊法,因为测得的浊度是由形成的免疫复合物引起的)。对于每一个步骤,都有几个参数需要在每个单独的应用中进行优化。主要参数为1)有无粒子增强;2) 颗粒类型、尺寸和各自的最佳波长(如适用);3) 监测反应动力学或终点;4) 考虑中的抗体/抗原,以及与之相关的抗原负载的最佳水平;5) 缓冲液和其他离子种类以及各自的最佳ph值;6) 用于改变蛋白质溶解度的聚合物的类型和浓度;7)温度和其他环境因素。通常,这些参数在方法开发过程中进行了优化,具体的专著和/或章节(如适用)中给出了这些值。 kinetic nephelometry: the kinetic nephelometry is advantageous compared to the endpoint nephelometry mainly because of the capability to take a sample blank reading in addition to a reagent blank reading. this procedure assesses the rate of the immunocomplex formation based on the increased intensity response of the scattered light of the chosen wavelength. the reaction kinetic may be monitored continuously or a certain number of data points may be taken, depending on the time response of the instrument used and the type of application. at times it may involve only two data points; however, proper care must be exercised because the choice of point selection can influence the overall accuracy in cases where differences in reaction kinetics exist between samples and calibrating standards. careful consideration should be given to the appropriate choice of specificity control strategy.动力学散射比浊法:与终点散射比浊法相比,动力学散射比浊法具有优势,主要是因为除了试剂空白读数外,还能够读取样品空白读数。该程序基于所选波长的散射光的增强强度响应来评估免疫复合物的形成速率。根据所用仪器的时间响应和应用类型,可连续监测反应动力学,或采集一定数量的数据点。有时它可能只涉及两个数据点;但是在样品和校准标准之间存在反应动力学差异的情况下,选择点可能会影响整体准确度。应仔细考虑特异性控制策略的适当选择。 endpoint nephelometry: in this method, an initial measurement is performed before adding the reagent, which represents the blank reading. a second measurement is performed after the immune complex is formed after approximately 60 min. the difference between these two measurements is proportional to the content of the component being assayed.终点散射比浊法:在该方法中,在添加试剂之前进行初始测量,这代表空白读数。大约60分钟后,在免疫复合物形成后进行第二次测量。这两次测量之间的差异与所分析成分的含量成正比。 8. validation and verification 验证与核查8.1 validation 验证validation is required when a nephelometric/turbidimetric method is intended for use as an alternative to the official procedure for testing an official article. the objective of nephelometric/turbidimetric method validation is to demonstrate that the measurement is suitable for its intended purpose, including quantitative determination of the main component in a drug substance or a drug product (category i assays), quantitative determination of impurities or limit tests (category ii), and identification tests (category iv). depending on the category of the test (see validation of compendial procedures , table 2), the analytical method validation process for nephelometry/turbidimetry requires testing for accuracy, precision, specificity, detection limit (dl), quantitation limit (ql), linearity, range, and robustness. these analytical performance characteristics apply to externally standardized procedures and those that use standard additions.当散射比浊法/透射浊度法拟用作官方物品测试程序的替代方法时,需要进行验证。当散射比浊法/透射浊度法验证的目的是证明测量适用于其预期目的,包括原料药或药品中主要成分的定量测定(i类分析)、杂质的定量测定或限度试验(ii类)以及鉴定试验(iv类)。根据试验的类别(参见validation of compendial procedures,表2),透射浊度法/散射比浊法的分析方法验证过程需要对准确度、精密度、特异性、检测限(dl)、定量限(ql)、线性、范围和稳健性进行试验。这些分析性能特征适用于外部标准化程序和那些使用标准添加的程序。 validation of compendial procedures provides definitions and general guidance on analytical procedures validation without indicating specific validation criteria for each characteristic. the intention of the following sections is to provide the user with specific validation criteria that represent the minimum expectations for this technology. for each particular application, tighter criteria may be needed in order to demonstrate suitability for the intended use.validation of compendial procedures章节提供了分析程序验证的定义和一般指南,但没有说明每个特征的具体验证标准。以下各节的目的是向用户提供具体的验证标准,这些标准代表了对该技术的最低期望。对于每个特定应用,可能需要更严格的标准,以证明其适用于预期用途。 accuracy 准确度for category i, ii, and iii procedures, accuracy can be determined by conducting recovery studies with the appropriate matrix spiked with known concentrations of the analyte. analysts can also compare the assay results obtained using the nephelometric/ turbidimetric procedure under validation to those from an established analytical procedure.validation criteria: 98.0%–102.0% mean recovery for the drug substances, 95.0%–105.0% mean recovery for the drug product assay, and 80.0%–120.0% mean recovery for the impurity analysis. these criteria are met throughout the specified range. 对于i类、ii类和iii类程序,可通过使用加入已知分析物浓度的适当基质进行回收研究来确定准确度。分析员还可以将使用验证中的散射光浊度法/透射光比浊法程序获得的分析结果与已建立的分析程序获得的结果进行比较。验证标准:原料药的平均回收率为98.0%–102.0%,药品分析的平均回收率为95.0%–105.0%,杂质分析的平均回收率为80.0%–120.0%。这些标准在整个规定范围内都得到满足。 precision精度repeatability: the repeatability of the analytical procedure is assessed by measuring the concentrations of six independently prepared sample solutions at 100% of the assay test concentration. alternatively, it can be assessed by measuring the concentrations of three replicates of three separate sample solutions at different concentrations. the three concentrations should be close enough so that the repeatability is constant across the concentration range. if this is done, the repeatability at the three concentrations is pooled for comparison to the acceptance criteria.validation criteria: the relative standard deviation is nmt 1.0% for the drug substance, nmt 2.0% for the drug product assay, and nmt 20.0% for the impurity analysis.重复性:通过测量六种独立制备的样品溶液在100%分析试验浓度下的浓度来评估分析程序的重复性。或者,可以通过测量三种不同浓度的单独样品溶液的三个重复的浓度来评估。三种浓度应足够接近,以便在整个浓度范围内重复性保持恒定。如果这样做,将三种浓度下的重复性汇总,以与验收标准进行比较。验证标准:原料药的相对标准偏差为nmt 1.0%,药品分析的相对标准偏差为nmt 2.0%,杂质分析的相对标准偏差为nmt 20.0%。 intermediate precision: the effect of random events on the analytical precision of the method must be established. typical variables include performing the analysis on different days, using different instrumentation, and/or having the method performed by two or more analysts. at a minimum, any combination of at least two of these factors totaling six experiments will provide an estimation of intermediate precision.validation criteria: the relative standard deviation is nmt 1.5% for the drug substance, nmt 3.0% for the drug product assay, and nmt 25.0% for the impurity analysis.中间精度:必须确定随机事件对方法分析精度的影响。典型的变量包括在不同的日期使用不同的仪器进行分析,和/或由两名或两名以上的分析员进行分析。至少,这些因素中的至少两个的组合,总共6个实验,将提供中等精度的评估。验证标准:原料药的相对标准偏差为nmt 1.5%,药品分析的相对标准偏差为nmt 3.0%,杂质分析的相对标准偏差为nmt 25.0%。 specificity 特异性in nephelometric/turbidimetric measurements, specificity is demonstrated by the lack of interference from other components present in the matrix (other components of the matrix produce a true solution).在散射光浊度法/透射光比浊法的浊度测量中,特异性通过基质中其他成分的干扰(基质的其他成分产生真实溶液)的缺乏来证明。 detection limit 检测限the dl can be estimated by calculating the concentration of a solution that would give the signal-to-noise ratio of ≥3.3. the estimated dl must be confirmed by analyzing samples at the calculated concentration.可以通过计算溶液的浓度来估计检测限dl,该浓度将给出信号的信噪比≥3.3. 必须通过分析计算浓度下的样品来确认估计的dl。 quantitation limit 定量限the ql can be estimated by calculating the concentration of a solution that would give the signal-to-noise ratio of ≥10.0. the estimated ql must be confirmed by analyzing samples at the calculated concentration. measurement of a test solution prepared from a representative sample matrix spiked at the required ql concentration must be performed to confirm sufficient sensitivity and adequate precision. the observed signal-to-noise ratio at the required ql should be >10.validation criteria: for the estimated limit of quantitation to be considered valid, the measured concentration must be accurate and precise at a level ≤50% of the specification.定量限ql可以通过计算溶液的浓度来估算,该浓度将给出信号的信噪比≥10.0. 必须通过分析计算浓度下的样品来确认估算的ql。必须对以所需ql浓度添加的代表性样品基质制备的试液进行测量,以确认其具有足够的灵敏度和精度。在所需ql下观察到的信噪比应大于10。验证标准:估计的定量限被认为是有效的,测量的浓度必须是准确的,并且在≤50%的规格水平上是精确的。 linearity线性a linear relationship between the analyte concentration and measured turbidity response must be demonstrated by preparation of at least four standard solutions at concentrations encompassing the anticipated concentration of the test solution. the standard curve is then evaluated using appropriate statistical methods such as a least-squares regression. deviation from linearity results from instrumental or sample factors, or both, can be reduced to acceptable levels by reducing or increasing the analyte concentration, thereby respectively decreasing or increasing the turbidity readings to within the nephelometer/turbidimeter instrument linearity range.validation criteria: the correlation coefficient (r) must be nlt 0.995 for category i assays and nlt 0.99 for category ii quantitative tests.分析物浓度和测得的浊度响应之间的线性关系必须通过制备至少四种标准溶液来证明,其浓度包括试验溶液的预期浓度。然后使用适当的统计方法(如最小二乘回归)评估标准曲线。通过降低或增加分析物浓度,可将仪器或样品因素或两者的线性偏差降低至可接受水平,从而分别将浊度读数降低或增加至透射光法浊度计/散射光浊度计仪器线性范围内。验证标准:对于i类分析,相关系数(r)必须为nlt 0.995,对于ii类定量测试,相关系数(r)必须为nlt 0.99。 range 范围the operational range of an analytical instrument (and the analytical procedure as a whole) is the interval between the upper and lower concentrations (amounts) of analyte in the sample (including these concentrations) for which it has been demonstrated that the instrumental response function has a suitable level of precision, accuracy, and linearity.validation criteria: for category i tests, the validation range for 100.0% centered acceptance criteria is 80.0%–120.0%. for non-centered acceptance criteria, the validation range is 10.0% below the lower limit to 10.0% above the upper limit. for category ii tests, the validation range covers 50.0%–120.0% of the acceptance criteria.分析仪器(以及整个分析程序)的操作范围是样品中分析物的上下浓度(数量)(包括这些浓度)之间的间隔,已证明仪器响应函数具有适当的精度、准确度和线性水平。验证标准:对于i类试验,100.0%中心验收标准的验证范围为80.0%–120.0%。对于非中心验收标准,验证范围为下限以下10.0%到上限以上10.0%。对于ii类试验,验证范围涵盖验收标准的50.0%–120.0%。 robustness稳健性the reliability of an analytical measurement is demonstrated by deliberate changes to experimental parameters. for nephelometry/turbidimetry this can include, for example, measuring the stability of the analyte under specified storage conditions, varying ph, and adding possible interfering species. robustness is determined concurrently using a suitable design for the experimental procedure.分析测量的可靠性通过有意改变实验参数来证明。 对于散射光浊度法/透射光比浊法,这可以包括:测量分析物在特定储存条件、变化的 ph 值和添加可能的干扰物质下的稳定性。使用适合实验程序的设计,同时确保稳健性。 8.2 verification核查current u.s. good manufacturing practices regulations [21 cfr 211.194(a)(2)] indicate that users of analytical procedures described in the u.s. pharmacopeia and national formulary are not required to validate these procedures if provided in a monograph. instead, they simply must verify their suitability under actual conditions of use.现行的《美国生产规范条例》[21 cfr 211.194(a)(2)]表明,如果专论中提供了这些程序,则美国药典和国家处方集中描述的分析程序的用户无需验证这些程序。相反,他们只需验证其在实际使用条件下的适用性。 the objective of nephelometric/turbidimetric procedure verification is to demonstrate the suitability of a test procedure under actual conditions of use. performance characteristics that verify the suitability of a nephelometric/turbidimetric procedure are similar to those required for any analytical procedure. a discussion of the applicable general principles is found in verification of compendial procedures . verification is usually performed using a reference material and a well-defined matrix. verification of compendial nephelometric/turbidimetric procedures includes, at minimum, the execution of the validation parameters for specificity, accuracy, precision, and ql, when appropriate, as indicated in 8.1 validation.散射光浊度法/透射光比浊法程序验证的目的是证明测试程序在实际使用条件下的适用性。验证散射光浊度法/透射光比浊法程序适用性的性能特征与任何分析程序所需的性能特征相似。适用的一般原则的讨论见verification of compendial procedure 章节。通常使用参考材料和明确定义的基质进行验证。 药典散射光浊度法/透射光比浊法程序的验证至少包括对特异性、准确度、精密度和 ql 的验证参数的执行(如 8.1 验证中所述)。 欧洲药典ep10.02.2.1. clarity and degree of opalescence of liquids 液体的澄清度和乳光度opalescence is the effect of light being absorbed or scattered by submicroscopic particles or optical density inhomogeneities. the absence of any particles or inhomogeneities in a solution results in a clear solution.光被亚微观粒子吸收或散射、或光密度不均匀的产生的效果即为乳光。溶液中不存在任何粒子或不均匀性,就会得到清澈的溶液。 a liquid is considered clear if its clarity is the same as that of water r or of the solvent used, or if its opalescence is not more pronounced than that of reference suspension i (see table 2.2.1.-1), when examined under the conditions described below.在下述条件下检查时,如果液体的透明度与水或所用溶剂的透明度相同,或者其乳光不比参考悬浮液i(见表2.2.1.-1)的乳光更明显,则认为液体是透明的。 requirements in monographs are expressed in terms of the visual method by comparing with the defined reference suspensions (see table 2.2.1.-1). however, instrumental methods may also be used for determining compliance with monograph requirements once the suitability of the instrument has been established as described below and calibration with reference suspensions i-iv and with water r or the solvent used has been performed.通过与规定的参考悬浮液进行比较(见表2.2.1.-1),以目视法表达专著中的要求。然而,一旦仪器的适用性如下所述建立,仪器方法也可用于确定是否符合专论要求,并使用参考悬浮液i-iv和水或所用溶剂进行校准。visual method目视法using identical test-tubes of colourless, transparent, neutral glass with a flat base and an internal diameter of 15-25 mm, compare the liquid to be examined with a reference suspension freshly prepared as described below. ensure that the depths of the layers in the 2 test-tubes are the same (about 40 mm).使用相同的无色透明中性玻璃试管,底座平坦,内径为15-25 mm,将待检液体与下述新制备的参考悬浮液进行比较。确保两个试管中各层的深度相同(约40 mm)。 compare the liquids in diffused daylight 5 min after preparation of the reference suspension, viewing vertically against a black background.制备参考悬浮液5分钟后,在漫射日光下比较液体,在黑色背景下垂直观察。 system suitability. the diffusion of light must be such that reference suspension i can readily be distinguished from water r, and that reference suspension ii can readily be distinguished from reference suspension i (see table 2.2.1.-1).系统适用性。光的扩散必须确保参考悬浮液i可以很容易地与水区分开,并且参考悬浮液ii可以很容易地与参考悬浮液i区分开(见表2.2.1.-1)。instrumental method 仪器法the instrumental assessment of clarity and opalescence provides a more discriminatory test that does not depend on the visual acuity of the analyst. numerical results are more useful for process control and quality monitoring, especially in stability studies. for example, previous numerical data on stability can be extrapolated to determine whether a given batch of a preparation will exceed shelf-life limits prior to the expiry date.仪器法评估给透明度和乳光度的提供了一种更具辨别力的测试,它不依赖于分析人员的视力。 数值结果对于过程控制和质量监控更有用,尤其是在稳定性研究中。 例如,可以从以前关于稳定性的数字数据外推,来确定给定批次的制剂是否会在有效期之前超过保质期限制。 turbidimetry and nephelometry比浊法和浊度法when a suspension is viewed at right angles to the direction of the incident light, the system appears opalescent due to the scattering of light by the particles of the suspension (tyndall effect). a certain portion of the light beam entering a turbid liquid is transmitted, another portion is absorbed and the remaining portion is scattered by the suspended particles. the light-scattering effect of suspended particles can be measured either indirectly by observation of the transmitted light (turbidimetry) or directly by measuring the scattered light (nephelometry). turbidimetry and nephelometry are more reliable in low turbidity ranges, where there is a linear relationship between turbidity values and detector signals. as the degree of turbidity increases, not all the particles are exposed to the incident light and the scattered or the transmitted radiation of other particles is hindered on its way to the detector.当以与入射光方向成直角的角度观察悬浮液时,由于悬浮液颗粒对光的散射(丁达尔效应),系统呈现乳白色。进入混浊液体的光束的一部分被透射,另一部分被吸收,其余部分被悬浮颗粒散射。悬浮颗粒的光散射效应可以通过观察透射光(比浊法)间接测量,也可以通过测量散射光(浊度法)直接测量。比浊法和浊度法在低浊度范围内更可靠,浊度值和检测器信号之间存在线性关系。随着浊度的增加,并非所有粒子都暴露在入射光下,其他粒子的散射或透射辐射在到达探测器的过程中会受到阻碍。 for quantitative measurements, the construction of calibration curves is essential. linearity must be based on at least 4 levels of concentrations. reference suspensions must show a sufficiently stable degree of turbidity and must be produced under well-defined conditions.对于定量测量,校准曲线的构建至关重要。线性必须基于至少4个浓度水平。参考悬浮液必须显示足够稳定的浊度,并且必须在明确的条件下产生。 measurements in ratio mode比率模式下的测量the determination of opalescence of coloured liquids is done using instruments with ratio mode, since colour provides a negative interference, attenuating both incident and scattered light and lowering the turbidity value. the effect is so great, even for moderately coloured samples, that conventional nephelometers cannot be used.由于颜色会产生负干扰,衰减入射光和散射光,降低浊度值,因此使用具有比率模式的仪器测定有色液体的乳光。 这种影响是如此之大,即使是中等颜色的样品,以至于不能使用传统的浊度计。 in turbidimetry or nephelometry with ratio mode, the ratio of the transmission measurement to the 90° scattered light measurement is determined. this procedure compensates for the light that is diminished by the colour of the sample. instruments with ratio mode use as light source a tungsten lamp with spectral sensitivity at about 550 nm operating at a filament colour temperature of 2700 k. other suitable light sources may also be used. silicon photodiodes and photomultipliers are commonly used as detectors and record changes in light scattered or transmitted by the sample. the light scattered at 90 ± 2.5° is measured by the primary detector. other detectors measure back and forward scatter (reflected light) as well as transmitted light. the results are obtained by calculating the ratio of the 90° scattered light measured to the sum of the components of forward scattered and transmitted light values.在比浊法或浊度法中,通过比率模式,确定透射测量与90°散射光测量的比率。该程序补偿因样品颜色而减弱的光线。具有比率模式的仪器使用光谱灵敏度约为550 nm的钨灯作为光源,在2700 k的灯丝色温下工作。也可以使用其他合适的光源。硅光电二极管和光电倍增管常用作探测器,记录样品散射或透射光的变化。由主探测器测量90±2.5°处的散射光。其他探测器测量前后散射(反射光)以及透射光。通过计算测得的90°散射光与前向散射光和透射光值分量之和的比值,可以获得结果。 the instruments used are calibrated against standards of known turbidity and are capable of automatic measurement of turbidity. the test results are obtained directly from the instrument and compared to the specifications in the individual monograph.使用的仪器根据已知浊度标准进行校准,并能够自动测量浊度。测试结果直接从仪器中获得,并与各专著中的规范进行比较。 alternatively, the influence of the colour of the sample may also be eliminated by using an infrared light-emitting diode (ir led) having an emission maximum at 860 nm with a 60 nm spectral bandwidth as the light source of the instrument.或者,也可以通过使用最大发射波长为860nm、光谱带宽为60nm的红外发光二极管(ir led)作为仪器光源来消除样品颜色的影响。 instrument requirements仪器要求instruments complying with the following characteristics and verified using reference suspensions as described below may be used instead of visual examination for determination of compliance with monograph requirements.可使用符合以下特征并使用下述参考悬浮液验证的仪器代替目视检查,以确定是否符合专论要求。 – measuring unit: ntu (nephelometric turbidity units). ntu is based on the turbidity of a primary standard of formazin. ftu (formazin turbidity units) or fnu (formazin nephelometric units) are also used, and are equivalent to ntu in regions of low turbidity (up to 40 ntu). these units are used in all 3 instrumental methods (nephelometry, turbidimetry and in ratio mode).– measuring range: 0.01-1100 ntu.– resolution: 0.01 ntu within the range 0-9.99 ntu; 0.1 ntu within the range 10.0-99.9 ntu; and 1 ntu for the range > 100 ntu.– accuracy: ± (10 per cent of reading + 0.01 ntu) with in the range 0-20 ntu; ± 7.5 per cent within the range 20-1100 ntu.– repeatability: ± 0.05 ntu within the range0-20 ntu; ± 2 per cent of the reading within the range 20-1100 ntu.测量单位:ntu(浊度测量单位)。ntu是基于福尔马肼一级标准品的浊度。也可使用ftu(福尔马肼浊度单位)或fnu(福尔马肼浊度单位),相当于ntu的在低浊度区域(最高40 ntu)。这些单位适用于所有3种仪器方法(比浊法、浊度法和比率模式)。–测量范围:0.01-1100 ntu–分辨率:0-9.99 ntu范围内为0.01 ntu;10.0-99.9 ntu范围内为0.1 ntu;对于大于100 ntu的范围,则为1 ntu–准确度:范围在0-20 ntu之间,读数准确度偏差为±(读数的10%+0.01 ntu);范围在20-1100 ntu时,读数准确偏差为±7.5%。–重复性:在0-20 ntu范围内重复性为±0.05 ntu;在20-1100 ntu范围内读数重复性为±2%。 instruments with measuring range or resolution, accuracy and repeatability capabilities other than those mentioned above may be used provided they are sufficiently validated and are capable for the intended use.测量范围或分辨率、精度和重复性能力不同于上述测量范围或分辨率、精度和重复性能力的仪器经过有效验证,也能够应用于预期用途。 control of instrument performance仪器性能的控制– calibration: performed with at least 4 reference suspensions of formazin covering the measuring range of interest. reference suspensions described in this chapter or suitable reference standards calibrated against the primary reference suspensions may be used.–校准:使用至少4种福尔马肼参考悬浮液进行校准,覆盖感兴趣的测量范围。可使用本章所述的参考悬浮液或根据主要参考悬浮液校准的适当参考标准。 – stray light: 杂散光:在0-10ntu范围内;在10-1100 ntu范围内。杂散光是指到达浊度检测器的光,而不是样品散射的结果。杂散光总是一种正干扰,是低范围浊度测量中的一个重要误差源。杂散光的来源包括:样品池中的缺陷和划痕、光学系统的内部反射、光学元件或样品池被灰尘污染,以及电子噪声。仪器设计也会影响杂散光。在比率模式测量中,杂散光的影响可以忽略不计。 the test methodology for the specific substance/product to be analysed must also be verified to demonstrate its analytical capability. the instrument and methodology shall be consistent with the attributes of the substance to be examined.还必须验证待分析特定物质/产品的试验方法,以证明其分析能力。仪器和方法应与待检物质的属性一致。 measurements of standards and samples should be carried out under the same temperature conditions, preferably between 20 °c and 25 °c.标准品和样品的测量应在相同的温度条件下进行,最好在20 °c和25 °c之间。 reference suspensions 参考悬浮液formazin has several desirable characteristics that make it an excellent turbidity standard. it can be reproducibly prepared from assayed raw materials. the physical characteristics make it a desirable light-scatter calibration standard. the formazin polymer consists of chains of different lengths, which fold into random configurations. this results in a wide variety of particle shapes and sizes, which allows the analysis of different particle sizes and shapes that are found in real samples. stabilised formazin suspensions that can be used to prepare stable, diluted turbidity standards are commercially available and may be used after comparison with the standards prepared as described.福尔马肼有几个理想的特性,使其成为一个优秀的浊度液标准。它可以从经过分析的原材料中重复制备。其物理特性使其成为理想的光散射校准标准。福尔马肼聚合物由不同长度的链组成,这些链折叠成随机构型。这会产生各种各样形状和尺寸的颗粒,从而可以分析真实样品中发现的不同颗粒大小和形状。可用于制备稳定稀释浊度标准品的稳定福尔马肼悬浮液是可商购的,并可在与所述制备的标准品进行比较后使用。 all steps of the preparation of reference suspensions as described below are carried out at 25 ± 3 °c.下述参考悬浮液制备的所有步骤均在25±3°c下进行。 hydrazine sulfate solution. dissolve 1.0 g of hydrazine sulfate r in water r and dilute to 100.0 ml with the same solvent. allow to stand for 4-6 h.硫酸肼溶液。 将 1.0 g 硫酸肼溶解在水中,并用相同的溶剂稀释至100.0 ml。 静置 4-6 小时。 primary opalescent suspension (formazin suspension). in a 100 ml ground-glass-stoppered flask, dissolve 2.5 g of hexamethylenetetramine r in 25.0 ml of water r. add 25.0 ml of the hydrazine sulfate solution. mix and allow to stand for 24 h. this suspension is stable for 2 months, provided it is stored in a glass container free from surface defects. the suspension must not adhere to the glass and must be mixed thoroughly before use.初级乳白色悬浮液(福尔马肼悬浮液)。在100 ml磨砂玻璃塞烧瓶中,将2.5g六亚甲基四胺溶解在25.0 ml水中。添加25.0 ml硫酸肼溶液。混合并静置24小时。如果该悬浮液储存在无表面缺陷的玻璃容器中,则可稳定2个月。悬浮液不得粘附在玻璃上,使用前必须彻底混合。 standard of opalescence. dilute 15.0 ml of the primary opalescent suspension to 1000.0 ml with water r. this suspension is freshly prepared and may be stored for up to 24 h.乳白色的标准浊度液。用水将15.0 ml初级乳白色悬浮液稀释至1000.0 ml。该悬浮液是新制备的,可储存24小时。 reference suspensions. prepare the reference suspensions according to table 2.2.1.-1. mix and shake before use.参考悬浮液。根据表2.2.1-1制备参考悬浮液。使用前混合并摇匀。 measurements of reference suspensions i-iv in ratio mode show a linear relationship between the concentrations and measured ntu values (see table 2.2.1.-2). 在比率模式下,参考悬浮液i-iv的测量结果显示,浓度与测量的ntu值之间存在线性关系(见表2.2.1.-2)。 日本药典17版2.61 turbidity measurement 浊度测量turbidity measurement is used to determine the turbidity (degree of opalescence) for the decision whether the article to be examined complies with the clarity requirement stated in the purity.as a rule, the visual method is specified for the requirement in individual monograph.浊度测量用于确定浊度(乳光度),以决定待检查的物品是否符合纯度中规定的透明度要求。作为一项规则,目视法是针对个别专论中的要求说明的。 1. visual method目视法this is used to determine the degree of opalescence with white (or faintly-colored) fine particles. so the degree of opalescence of a colored sample is liable to be determined lower that it is difficult to compare the degree correctly without using similarly colored reference suspension.这是用来确定乳白色(或淡色)细颗粒的乳光程度。因此,有色样品的乳光度容易被测定得较低,因此,如果不使用类似颜色的参考悬浮液,就很难正确地比较其乳光度。 1.1. reference suspensions参考悬浮液pipet 5 ml, 10 ml, 30 ml and 50 ml of formazin opalescence standard solution, dilute them separately to exactly 100 ml with water, and use these solutions so obtained as reference suspensions i, ii, iii and iv, respectively. shake before use. degrees of opalescence of reference suspensions i, ii, iii and iv are equivalent to 3 ntu, 6 ntu, 18 ntu and 30 ntu, respectively.用移液管分别吸取5 ml、10 ml、30 ml、50 ml福尔马肼标准液,用水分别稀释至100 ml,分别作为参比悬液i、ii、iii、iv。在使用前摇晃。参考悬浮液i、ii、iii和iv的乳光度分别相当于3 ntu、6 ntu、18 ntu和30 ntu。 1.2. procedure步骤place sufficient of the test solution, water or the solvent to prepare the test solution and, where necessary, newly prepared reference suspensions in separate flat-bottomed test tubes, 15 – 25 mm in inside diameter and of colorless and transparent, to a depth of 40 mm, and compare the contents of the tubes against a black background by viewing in diffused light down the vertical axes of the tubes. the diffused light must be such that reference suspension i can be readily distinguished from water, and that reference suspension ii can readily be distinguished from reference suspension i.取足够的待测溶液、水或溶剂,以准备测试溶液,必要时,将新制备的参考悬浮液置于独立的平底试管中,试管内径15 - 25mm,无色透明,深度40 mm。然后在一个黑色的背景下通过漫射光下垂直于管轴进行观察,比较管内的内容。漫射光必须能使参考悬浮体i容易与水区分开来,参考悬浮体ii容易与参考悬浮体i区分开来。 in this test reference suspensions are used when the clarity of the test solution is obscurely and it is not easy to determine that its degree of opalescence is similar or not similar to water or to the solvent used to prepare the test solution.在此测试中,当测试溶液的透明度模糊不清,并且不容易确定其乳光度与水或与用于制备测试溶液的溶剂是否相似时,使用参考悬浮液。 1.3. interpretation注释a liquid is considered “clear” when its clarity is the same as that of water or of the solvent used to prepare the liquid or its turbidity is not more pronounced than that of reference suspension i. if the turbidity of the liquid is more than that of reference suspension i, consider as follows: when the turbidity is more than that of reference suspension i but not more than that of reference suspension ii, express “it is not more than reference suspension ii”. in the same way, when the turbidity is more than that of reference suspension ii but not more than that of reference suspension iii, express “it is not more than reference suspension iii”, and when the turbidity is more than that of reference suspension iii but not more than that of reference suspension iv, express “it is not more than reference suspension iv”. when the turbidity is more than that of reference suspension iv, express “it is more than reference suspension iv”.当液体的澄清度与水或与用于制备液体的溶剂的澄清度相同或其浊度不比参比悬浮液 i 更明显时,该液体被视为“澄清”。如果液体的浊度大于参考悬浮液i,考虑如下:当浊度大于参考悬浮液i但不超过参考悬浮液ii时,表示“不超过参考悬浮液ii”。 同理,当浊度大于参比悬浊液ⅱ但不大于参比悬浊液ⅲ时,表示“不大于参比悬浊液ⅲ”,当浊度大于参比悬浊液ⅲ时 但不超过参考悬浮液iv,表示“不超过参考悬浮液iv”。 当浊度大于参考悬浮液iv时,表示“大于参考悬浮液iv”。 1.4. reagent solutions试剂溶液formazin opalescence standard solution: to exactly 3 ml of formazin stock suspension add water to make exactly 200 ml. use within 24 hours after preparation. shake thoroughly before use. degrees of opalescence of this standard solution is equivalent to 60 ntu.福尔马津乳光标准溶液:准确地取 3 ml福尔马肼储备悬浮液,加水至 200 ml。 配制后24小时内使用。使用前彻底摇匀。此标准溶液的乳光度相当于 60 ntu。 2. photoelectric photometry光电光度法the turbidity can also be estimated by instrumental measurement of the light absorbed or scattered on account of submicroscopic optical density inhomogeneities of opalescent solutions and suspensions. the photoelectric photometry is able to provide more objective determination than the visual method. though they can determine the turbidity by measuring the scattered or transmitted light, the measuring system and light source must be specified in individual test method, and for the comparison of observed data, the same measuring system and light source should be used.由于乳光溶液和悬浮液的亚显微光密度不均匀性,还可以通过仪器测量吸收或散射的光来估计浊度。光电光度法比目测法能够提供更客观的测定。 虽然他们可以通过测量散射光或透射光来确定浊度,但必须在单独的测试方法中标明测量系统和光源,并且为了比较观察数据,应使用相同的测量系统和光源。 in each case, the linear relationship between turbidity and concentration must be demonstrated by constructing a calibration curve using at least 4 concentrations. for colored samples, the turbidity value is liable to be estimated lower because of attenuating both incident and scattered lights due to the absorption by the color, and the transmission-dispersion method is principally used.在每种情况下,浊度和浓度之间的线性关系必须通过使用至少4种浓度构建校准曲线来证明。对于有颜色的样品,由于颜色的吸收,入射光和散射光都被衰减,浊度值容易被估计得较低,主要采用透射-色散法。 2.1. turbidimetry透射光比浊法when a light passes through a turbid liquid the transmitted light is decreased by scattering with the particles dispersed in the liquid. a linear relationship is observed between turbidity and concentration when the particles with a constant size are uniformly dispersed, the size is small and the suspension is not higher concentration. the turbidity can be measured by ultraviolet-visual spectrophotometry using spectrophotometer or photoelectric photometer. the turbidity of the sample in higher concentration can also be measured, however, it is susceptible to the color of the sample, and the measurement is usually performed at around 660 nm to avoid possible disturbance occurred from the absorption by the color.当光通过混浊液体时,透射光通过分散在液体中的颗粒散射而减少。当粒径恒定的颗粒分散均匀、粒径较小且悬浮液浓度不高时,浊度与浓度呈线性关系。浊度可以通过紫外分光光度法使用分光光度计或光电光度计进行测量。较高浓度的样品的浊度也可以测量,但它易受样品颜色的影响,通常在660 nm左右进行测量,以避免颜色吸收可能产生的干扰。 2.2. nephelometry散射光浊度法when a suspension is viewed at right angles to the direction of the incident light, it appears opalescent due to the refraction of light from the particles of the suspension (tyndall effect). a certain portion of the light entering a turbid liquid is transmitted, another portion is absorbed and the remaining portion is scattered by the suspended particles. the scattered light measuring method shows the linear relationship between the nephelometric turbidity units (ntu) values and relative detector signals in a low turbidity range. as the degree of turbidity increases, not all the particles are exposed to the incident light and the scattered radiation of other particles is hindered on its way to the detector.当悬浮物与入射光方向成直角时,由于悬浮物粒子的光线折射(丁达尔效应),悬浮物呈现乳白色。进入混浊液体的光,一部分被透射,一部分被吸收,剩下的部分被悬浮的粒子散射。散射光测量方法显示了低浊度范围内散射浊度单位(ntu)值与相对检测器信号之间的线性关系。随着浊度的增加,并不是所有的粒子都暴露在入射光下,其他粒子的散射辐射在到达探测器的过程中会受到阻碍。 2.3. ratio turbidimetry比率浊度法this method measures both scattered and transmitted light values at the same time, and the turbidity is determined from the ratio of the scattered light value to the transmitted light value. this procedure compensates for the light that is diminished by the color of the sample and eliminates the influence of the color. when the measurement is performed by using an integrating sphere, it is particularly called the integrating sphere method, which measures the total transmitted light value as well as the scattered light value occurred with the suspended particles, and the turbidity can be determined from the ratio of them.该方法同时测量散射光值和透射光值,浊度由散射光值与透射光值的比值确定。此程序可补偿因样品颜色而减弱的光线,并消除颜色的影响。当用积分球进行测量时,特别称为积分球法,它测量悬浮粒子的总透射光值和散射光值,由它们的比值可以确定浊度。 2.4. application of photoelectric photometry for monograph requirements光电光度法在专著要求中的应用the turbidity of the test solution, determined by the photoelectric photometry, can be used as an indicating standard for the conformity to the clarity requirements by converting into ntu by using turbidity known reference solutions such as reference suspensions i – iv, if needed, and water or the solvent used. in an automatically compensable apparatus being calibrated with turbidity known reference solutions, the measuring result is given in ntu and it can be compared directly with required specified value.由光电光度法测定的测试溶液的浊度,可以作为指示标准,通过使用浊度已知的参考溶液,如参考悬浮液 i-iv,如果需要,水和使用的溶剂液也可以,将其以ntu为单位的数据转出。在使用浊度已知参考溶液校准的自动补偿装置中,测量结果以 ntu 为单位给出,并且可以直接与所需的规定值进行比较。 ntu is often used as the unit in the turbidity determinations. it is the unit used in the case when the turbidity is estimated by the instrument which measures the 90 ± 30°scattered light against the incident light intensity, using tungsten lamp, and in the case the estimation is performed by the instrument which measures the 90 ± 2.5°scattered light against the incident light intensity using 860 nm infrared light, fnu is used as the unit. fnu is equivalent to ntu at a range of smaller measurements (less than 40 ntu). for the unit of formazin concentration, ftu is also used, which is defined as a suspension of 1 mg formazin in 1l of purified water is 1 ftu.在浊度测定中经常使用ntu作为单位。它是测量用90±30°散射光对入射光强度的得到浊度信息时使用的单位,使用钨灯,采用860 nm红外光测量90±2.5°的散射光对入射光强度,此时以fnu为单元。在较小的测量范围内(小于40 ntu),fnu相当于ntu。福尔马肼的浓度单位也用ftu,即1l纯净水中1 mg 福尔马肼的悬浮液为1 ftu。 formazin stock suspension. to 25 ml of hexamethylenetetramine ts add 25 ml of hydrazinium sulfate ts, mix, and use after allowing to stand at room temperature for 24 hours. store in a glass container free from surface defects. use within 2 months. shake thoroughly before use. the turbidity of this suspension is equivalent to 4000 ntu.福尔马肼贮备悬浮液:向 25 ml六亚甲基四胺中加入25 ml硫酸肼,混匀,室温静置 24 小时后使用。 储存在没有表面缺陷的玻璃容器中。2个月内使用。使用前彻底摇匀。这种悬浮液的浊度相当于4000 ntu。 formazin opalescence standard solution. to 15 ml of formazin stock suspension add water to make 1000 ml. use within 24 hours after preparation. shake thoroughly before use.福尔马肼标准液:向 15 ml 的福尔马肼贮备悬浮液中加水至 1000 ml。配制后24小时内使用。使用前彻底摇匀。 解决方案:上海胤煌科技针对药剂的澄清度检查推出了以下产品,符合各国药典的溶液澄清度检查规范。1、澄清度检查专用伞棚灯胤煌科技 hn-100a型和hn-200a型澄清度检查专用伞棚灯符合各国药典中目视法检测溶液澄清度的仪器要求,具有光林带型光源,能有效减少目视过程中光对眼睛的刺激,其照度可达5000 lux。其中hn-200a型专用伞棚灯增加了rgb三色光源,可以对有色样品进行澄清度检测。 2、yh-cls-1201澄清度检查分析仪 胤煌科技 此仪器采用全彩液晶触摸屏进行操作控制,可以直接检测注射用原料药和注射剂的澄清度,并具备四级权限管理和审计追踪功能,完全满足gmp的数据完整性要求,是液体一致性评价的有效仪器。
应用实例
2022.04.29
【网络研讨会】如何洞见药品制剂中微粒的千奇百态(4月29日晚7点线上见)
企业动态
2022.04.28
重磅发布!胤煌科技全新一代显微计数法不溶性微粒仪 (YH-MIP-0205PRO))隆重上市!
重磅发布!胤煌科技全新一代显微计数法不溶性微粒仪隆重上市!为什么要选择显微计数法?中国药典2020版规定:第一:当光阻法测定结果不符合规定或供试品不适于用光阻法测定时,应采用显微计数法进行测定,并以显微计数法的测定结果作为判定依据。第二:光阻法不适用于易产生气泡、高粘度的制剂产品,在检测不溶性微粒时要采用第二法(显微计数法)来检测。 上海胤煌科技隆重推出YH-MIP-0205Pro型全自动显微计数法不溶性微粒分析仪仪器型号:YH-MIP-0205Pro工作原理:显微计数法/图像法检测范围:1 μm-500 μm 产品介绍:当第一代YH-MIP-0103显微计数法不溶性微粒分析仪已解决传统显微镜法测试数据准确性不足、操作繁琐、对人眼伤害较大、检测结果不可追溯等问题,实现了自动扫描,自动计数,自动出具报告,软件符合21CFR Part11及GMP对数据完整性的要求。而全新一代YH-MIP-0205 Pro型全自动显微计数法不溶性微粒分析仪在上一代仪器已有的技术优势下,进行了全新升级,新的产品在原有自动扫描,自动测试,自动计数的功能上,新增加了自动过滤,自动干燥,自动上样等功能,同时在原有超分辨算法的基础上,加入AI智能算法,方便客户更好的对不溶性的来源进行分类和整理,实现真正的全自动检查!YH-MIP-0205Pro 全自动显微计数法不溶性微粒分析仪,你值得拥有!创新点:·可以完成自动过滤、自动干燥、自动上样,自动测试、自动出具报告等多项流程;·超分辨算法、AI智能算法等多种算法相结合,能有效确保测试数据的准确性。·在完美弥补常规显微镜法不溶性微粒检测的缺陷的同时,能准确保留样品中每个粒子的原始形貌,对不溶性微粒的来源或者形成机制都具有警示作用。·符合中国药典、美国药典、欧洲药典及日本药典等各国药典不溶性微粒检查的仪器要求;·符合21CFR Part11及GMP对数据完整性的要求。 让颗粒无处遁形! 企业简介:上海胤煌科技有限公司是一家专注于为医药、半导体、面板及材料行业提供粒度及Zeta电位分析设备及技术服务的高科技公司,公司拥有检测平台可以为客户提供专业的第三方检测服务。目前公司自主研发的产品有光阻法不溶性微粒分析仪、显微计数法不溶性微粒分析仪、流式动态图像法粒度粒形分析仪等;还有代理产品伞棚灯、粒度及Zeta电位分析仪、高分辨率纳米粒度仪、原液纳米粒度及Zeta电位分析仪等检测分析设备,可以为mRNA/核酸疫苗、脂质体、乳剂、蛋白注射液等相关领域提供专业的检测分析设备。上海胤煌科技有限公司期待与您合作!
新品
2022.04.28
欧洲药典2.9.19. 颗粒物污染:亚可见颗粒物检查标准
2.9.19. PARTICULATE CONTAMINATION: SUB-VISIBLE PARTICLES2.9.19. 颗粒物污染:亚可见颗粒物 Particulate contamination of injections and infusions consists of extraneous, mobile undissolved particles, other than gas bubbles, unintentionally present in the solutions.注射剂和输液的微粒污染包括在溶液中无意中存在的外来的、可移动的未溶解颗粒,而不是气泡。 For the determination of particulate contamination 2 procedures, Method 1 (Light Obscuration Particle Count Test) and Method 2 (Microscopic Particle Count Test), are specified hereinafter. When examining injections and infusions for sub-visible particles, Method 1 is preferably applied. However, it may be necessary to test some preparations by the light obscuration particle count test followed by the microscopic particle count test to reach a conclusion on conformance to the requirements.对于颗粒物污染的测定包含两种方法,下文进行了详细介绍:方法1(光阻法颗粒计数试验)和方法2(显微颗粒物计数试验)。当检查注射剂和输液中是否存在亚可见颗粒时,最好使用方法1。然而,可能有必要通过光阻法颗粒计数试验和显微颗粒计数试验来测试某些制剂,以得出符合要求的结论。 Not all parenteral preparations can be examined for sub-visible particles by one or both of these methods. When Method 1 is not applicable, e.g. in case of preparations having reduced clarity or increased viscosity, the test is carried out according to Method 2. Emulsions, colloids, and liposomal preparations are examples. Similarly, products that produce air or gas bubbles when drawn into the sensor may also require microscopic particle count testing. If the viscosity of the preparation to be tested is sufficiently high so as to preclude its examination by either test method, a quantitative dilution with an appropriate diluent may be made to decrease viscosity, as necessary, to allow the analysis to be performed.并非所有非肠道制剂都可以通过这些方法中的一种或两种方法检查亚可见颗粒。例如在制剂的透明度降低或粘度增加的情况下,方法 1 不适用时,应根据方法2进行测试,如乳液、胶体和脂质体制剂等。 同样,在吸入传感器时会产生空气或气泡的产品也可能需要进行显微颗粒物计数测试。 如果待测制剂的粘度足够高以至于无法通过任一测试方法对其进行检查,则可以根据需要,用适当的稀释剂进行定量稀释以降低粘度,以便进行分析。 The results obtained in examining a discrete unit or group of units for particulate contamination cannot be extrapolated with certainty to other units that remain untested. Thus, statistically sound sampling plans must be developed if valid inferences are to be drawn from observed data to characterize the level of particulate contamination in a large group of units.在检查一个离散单元或一组单元的微粒污染时获得的结果不能肯定地外推到其他未测试的单元。 因此,如果要从观察到的数据中得出有效的推论,以表征一大组单位中的颗粒污染水平,就必须制定统计上合理的抽样计划。 METHOD 1. LIGHT OBSCURATION PARTICLE COUNTTEST光阻法颗粒计数实验Use a suitable apparatus based on the principle of light blockage which allows an automatic determination of the size of particles and the number of particles according to size. The apparatus is calibrated using suitable certified reference materials consisting of dispersions of spherical particles of known sizes between 10 µm and 25 µm. These standard particles are dispersed in particle-free water R. Care must be taken to avoid aggregation of particles during dispersion.根据光阻原理,使用合适的仪器,可以根据大小自动确定颗粒大小和颗粒数量。使用合适的认证标准物质对仪器进行校准,该标准物质由已知尺寸在10 µm到25 µm之间的球形颗粒的分散体组成。这些标准颗粒分散在无颗粒水中。分散过程中必须注意避免颗粒聚集。 General precautions 一般注意事项The test is carried out under conditions limiting particulate contamination, preferably in a laminar-flow cabinet. Very carefully wash the glassware and filtration equipment used, except for the membrane filters, with a warm detergent solution and rinse with abundant amounts of water to remove all traces of detergent. Immediately before use, rinse the equipment from top to bottom, outside and then inside, with particle-free water R.实验在限制颗粒污染的条件下进行,最好在层流柜中进行。使用温热的清洁剂溶液仔细清洗所用的玻璃器皿和过滤设备(膜式过滤器除外),并用大量水冲洗,以去除所有清洁剂痕迹。使用前,用无颗粒水从上到下、从外到内冲洗设备。 Take care not to introduce air bubbles into the preparation to be examined, especially when fractions of the preparation are being transferred to the container in which the determination is to be carried out.注意不要将气泡引入待检查的制剂中,尤其是将制剂的部分转移到要进行测定的容器中时。 In order to check that the environment is suitable for the test, that the glassware is properly cleaned and that the water to be used is particle-free, the following test is carried out: determine the particulate contamination of 5 samples of particle-free water R, each of 5 mL, according to the method described below. If the number of particles of 10 µm or greater size exceeds 25 for the combined 25 mL, the precautions taken for the test are not sufficient. The preparatory steps must be repeated until the environment, glassware and water are suitable for the test。为了检查环境是否适合试验,玻璃器皿是否正确清洁,所使用的水是否无颗粒,应进行以下试验:根据下述方法,测定5个无颗粒水样品的颗粒污染情况,每个样品5 mL。如果10 µm或更大粒径的颗粒数量超过25个,则为试验采取的预防措施是不够的。必须重复准备步骤,直到环境、玻璃器皿和水适合试验 Method方法Mix the contents of the sample by slowly inverting the container 20 times successively. If necessary, cautiously remove the sealing closure. Clean the outer surfaces of the container opening using a jet of particle-free water R and remove the closure, avoiding any contamination of the contents. Eliminate gas bubbles by appropriate measures such as allowing to stand for 2 min or sonicating.通过连续20次缓慢翻转容器,混合样品里的内容物。如有必要,小心地拆下密封盖。使用无颗粒水水流清洁容器开口的外表面,并拆下封盖,避免内容物受到任何污染。通过适当的措施消除气泡,例如静置2分钟或超声波处理。 For large-volume parenterals, single units are tested. For small-volume parenterals less than 25 mL in volume, the contents of 10 or more units are combined in a cleaned container to obtain a volume of not less than 25 mL; where justified and authorised, the test solution may be prepared by mixing the contents of a suitable number of vials and diluting to 25 mL with particle-free water R or with an appropriate solvent without contamination of particles when particle-free water R is not suitable. Small-volume parenterals having a volume of 25 mL or more may be tested individually.对于大容量肠外注射剂,需要测试单个单元。对于体积小于25 mL的小容量肠外注射剂,将10个或更多个的内容物组合在一个清洁的容器中,以获得不小于25 mL的体积的溶液;在合理和授权的情况下,可通过混合适当数量的小瓶中的内容物,并用无颗粒水稀释至25 mL,或在无颗粒水不适用时,用无颗粒污染的适当溶剂稀释至25 mL来制备试验溶液。体积为25 mL或以上的小容量非肠道药物试剂可单独进行试验。 Powders for parenteral administration are reconstituted with particle-free water R or with an appropriate solvent without contamination of particles when particle-free water R is not suitable.用于肠外给药的粉末用无颗粒水重新配制,或当无颗粒水不适用时,使用适当的无颗粒污染的溶剂重新进行配制。 The number of test specimens must be adequate to provide a statistically sound assessment. For large-volume parenterals or for small-volume parenterals having a volume of 25 mL or more, fewer than 10 units may be tested, based on an appropriate sampling plan。测试样本的数量必须足够,以提供统计上合理的评估。根据适当的抽样计划,对于大容量或25 mL以上的小容量肠外静脉,可检测小于10个样品 Remove 4 portions, each of not less than 5 mL, and count the number of particles equal to or greater than 10 µm and 25 µm. Disregard the result obtained for the first portion, and calculate the mean number of particles for the preparation to be examined.取下4份样品,每份不少于5 mL,并计算等于或大于10 µm和25 µm的颗粒数。忽略第一份的结果,并计算待检查制剂的平均颗粒数。 Evaluation评估For preparations supplied in containers with a nominal volume of more than 100 mL, apply the criteria of test 1.A. For preparations supplied in containers with a nominal volume of less than 100 mL, apply the criteria of test 1.B.♦For preparations supplied in containers with a nominal volume of 100 mL, apply the criteria of test 1.B. ♦If the average number of particles exceeds the limits, test the preparation by the microscopic particle count test.对于在标称体积超过100 mL的容器中提供的制剂,应采用试验1A的标准。对于在标称体积小于100 mL的容器中提供的制剂,采用试验1B的标准。对于在标称体积为100 mL的容器中提供的制剂,采用试验1B的标准。如果平均颗粒数超过限值,则通过显微颗粒计数测试对制剂进行测试。 Test 1.A – Solutions for infusion or solutions for injection supplied in containers with a nominal content of more than 100 mLThe preparation complies with the test if the average number of particles present in the units tested does not exceed 25 per millilitre equal to or greater than 10 µm and does not exceed 3 per millilitre equal to or greater than 25 µm.试验1A–输液溶液或注射溶液,装在标称含量超过100 mL的容器中。如果被测单位样品中粒径等于或大于10 µm的颗粒的平均数量不超过每毫升25个,且粒径等于或大于25 µm的颗粒平均数量不超过每毫升3个,则制剂符合试验要求。 Test 1.B – Solutions for infusion or solutions for injection supplied in containers with a nominal content of less than 100 mLThe preparation complies with the test if the average number of particles present in the units tested does not exceed 6000 per container equal to or greater than 10 µm and does not exceed 600 per container equal to or greater than 25 µm.试验1B–输液溶液或注射溶液,装在标称含量小于100 mL的容器中。如果被测单元中粒径等于或大于10 µm的颗粒的平均数量不超过6000个/容器,且粒径等于或大于25 µm的颗粒平均数量不超过600个/容器,则制剂符合试验要求。 METHOD 2. MICROSCOPIC PARTICLE COUNT TEST显微颗粒计数Use a suitable binocular microscope, filter assembly for retaining particulate contamination and membrane filter for examination.使用合适的双目显微镜、过滤器组件(用于保留颗粒污染物)和薄膜过滤器(用于检查)。 The microscope is equipped with an ocular micrometer calibrated with an objective micrometer, a mechanical stage capable of holding and traversing the entire filtration area of the membrane filter, 2 suitable illuminators to provide episcopic illumination in addition to oblique illumination, and is adjusted to 100 ± 10 magnifications.该显微镜配有一个目镜测微计(使用物镜测微计校准)、一个机械工作台(能够容纳并穿过膜过滤器的整个过滤区域)、2个合适的照明器(除了倾斜照明外,还提供反射照明),并将其调整为100±10倍。 The ocular micrometer is a circular diameter graticule (see Figure 2.9.19.-1.) and consists of a large circle divided by crosshairs into quadrants, transparent and black reference circles 10 µm and 25 µm in diameter at 100 magnifications, and a linear scale graduated in 10 µm increments. It is calibrated using a stage micrometer that is certified by either a domestic or international standard institution. A relative error of the linear scale of the graticule within ± 2 per cent is acceptable. The large circle is designated the graticule field of view (GFOV).目镜测微计是一个圆形直径分划器(见图2.9.19.-1)由一个被十字准线分成四个象限的大圆、100倍放大时直径为10 µm和25 µm的透明和黑色参考圆、以及以10 µm增量刻度的线性刻度组成。使用经过国内或国际标准机构认证的测微计对其进行校准。分划线性刻度的相对误差在±2%以内是可以接受的。大圆被指定为网格视场(GFOV)。 2 illuminators are required. One is an episcopic brightfield illuminator internal to the microscope, the other is an external, focusable auxiliary illuminator adjustable to give reflected oblique illumination at an angle of 10-20°.需要2个照明器。一个是显微镜内部的一个反射式亮场照明器,另一个是一个可调的外部可聚焦辅助照明器,以提供10-20°角的反射斜照明。 The filter assembly for retaining particulate contamination consists of a filter holder made of glass or other suitable material, and is equipped with a vacuum source and a suitable membrane filter.用于保留颗粒污染物的过滤器组件包括由玻璃或其他合适材料制成的过滤器支架,并配备有真空源和合适的膜过滤器。 The membrane filter is of suitable size, black or dark grey in colour, non-gridded or gridded, and 1.0 µm or finer in nominal pore size.膜过滤器尺寸合适,颜色为黑色或深灰色,无网格或网格,标称孔径为1.0 µm或更小。 General precautions 一般注意事项The test is carried out under conditions limiting particulate contamination, preferably in a laminar-flow cabinet.Very carefully wash the glassware and filter assembly used, except for the membrane filter, with a warm detergent solution and rinse with abundant amounts of water to remove all traces of detergent. Immediately before use, rinse both sides of the membrane filter and the equipment from top to bottom, outside and then inside, with particle-free water R.试验在限制颗粒污染的条件下进行,最好在层流柜中进行。使用温热的清洁剂溶液仔细清洗所用的玻璃器皿和过滤器组件(膜式过滤器除外),并用大量水冲洗,以去除所有清洁剂痕迹。使用前,用无颗粒水从上到下、从外到内冲洗膜过滤器和设备的两侧。 In order to check that the environment is suitable for the test, that the glassware and the membrane filter are properly cleaned and that the water to be used is particle-free, the following test is carried out: determine the particulate contamination of a 50 mL volume of particle-free water R according to the method described below. If more than 20 particles 10 µm or larger in size or if more than 5 particles 25 µm or larger in size are present within the filtration area, the precautions taken for the test are not sufficient. The preparatory steps must be repeated until the environment, glassware, membrane filter and water are suitable for the test.为了检查环境是否适合试验,玻璃器皿和膜过滤器是否正确清洁,所使用的水是否无颗粒,应进行以下试验:根据下述方法测定50 mL无颗粒水的颗粒污染情况。如果过滤区域内存在20个以上粒径为10 µm或更大的颗粒,或5个以上粒径为25 µm或更大的颗粒,则试验所采取的预防措施是不够的。必须重复准备步骤,直到环境、玻璃器皿、膜过滤器和水适合试验。 Method方法Mix the contents of the samples by slowly inverting the container 20 times successively. If necessary, cautiously remove the sealing closure. Clean the outer surfaces of the container opening using a jet of particle-free water R and remove the closure, avoiding any contamination of the contents.通过将容器连续缓慢翻转20次来混合样品内容物。如有必要,小心地拆下密封盖。使用无颗粒水水流清洁容器开口的外表面,并拆下封盖,避免内容物的任何污染。 For large-volume parenterals, single units are tested. For small-volume parenterals less than 25 mL in volume, the contents of 10 or more units are combined in a cleaned container; where justified and authorised, the test solution may be prepared by mixing the contents of a suitable number of vials and diluting to 25 mL with particle-free water R or with an appropriate solvent without contamination of particles when particle-free water R is not suitable. Small-volume parenterals having a volume of 25 mL or more may be tested individually.对于大容量肠外注射,需要测试单个样品。对于体积小于25 mL的小容量肠外注射剂,将10个或更多个的样品组合在一个清洁的容器中;在合理和授权的情况下,可通过混合适当数量的小瓶中的内容物,并用无颗粒水稀释至25 mL,当无颗粒水不适用时,可用无颗粒污染的适当溶剂稀释至25 mL来制备试验溶液。体积为25 mL或以上的小容量非肠道药物可单独进行试验。 Powders for parenteral administration are constituted with particle-free water R or with an appropriate solvent without contamination of particles when particle-free water R is not suitable.用于肠外给药的粉末由无颗粒水配置,或当无颗粒水不适用时,使用不含污染颗粒的溶剂重新配制。 The number of test specimens must be adequate to provide a statistically sound assessment. For large-volume parenterals or for small-volume parenterals having a volume of 25 mL or more, fewer than 10 units may be tested, based on an appropriate sampling plan.试样数量必须足以提供统计上合理的评估。对于大容量肠外注射剂或体积大于等于25 mL的小容量肠外注射剂,根据适当的取样计划,可测试少于10个样品。 Wet the inside of the filter holder fitted with the membrane filter with several millilitres of particle-free water R. Transfer to the filtration funnel the total volume of a solution pool or of a single unit, and apply vacuum. If needed, add stepwise a portion of the solution until the entire volume is filtered. After the last addition of solution, begin rinsing the inner walls of the filter holder by using a jet of particle-free water R. Maintain the vacuum until the surface of the membrane filter is free from liquid. Place the filter in a Petri dish and allow the filter to air-dry with the cover slightly ajar. After the filter has been dried, place the Petri dish on the stage of the microscope, scan the entire membrane filter under the reflected light from the illuminating device, and count the number of particles that are equal to or greater than 10 µm and the number of particles that are equal to or greater than 25 µm. Alternatively, partial filter count and determination of the total filter count by calculation is allowed. Calculate the mean number of particles for the preparation to be examined.用几毫升无颗粒水湿润装有薄膜过滤器的过滤器支架内部。将溶液或单个样品转移到过滤漏斗中,并施加真空。如果需要,逐步添加一部分溶液,直到整个体积被过滤。最后一次添加溶液后,使用无颗粒水水流开始冲洗过滤器支架的内壁。保持真空,直到过滤膜表面没有液体。将过滤膜放置在有盖培养皿中,让过滤膜风干,盖子稍微半开。过滤膜干燥后,将皮氏培养皿放在显微镜台上,在照明设备的反射光下扫描整个膜过滤器,并计算等于或大于10 µm的颗粒数量和等于或大于25 µm的颗粒数量。或者,如果计算方式允许,可以对部分滤膜上的颗粒计数,并决定总滤膜上的颗粒数量。计算待检查制剂的平均颗粒数。 The particle sizing process with the use of the circular diameter graticule is carried out by transforming mentally the image of each particle into a circle and then comparing it to the 10 µm and 25 µm graticule reference circles. Thereby the particles are not moved from their initial locations within the graticule field of view and are not superimposed on the reference circles for comparison. The inner diameter of the transparent graticule reference circles is used to size white and transparent particles, while dark particles are sized by using the outer diameter of the black opaque graticule reference circles.通过将每个颗粒的图像转换成一个圆,然后将其与10 µm和25 µm的分划参考圆进行比较,使用圆形直径分划的颗粒尺寸来执行颗粒尺寸测定过程。因此,粒子不会从网格视场内的初始位置移动,也不会叠加在参考圆上进行比较。透明分划参考圆的内径用于确定白色和透明粒子的大小,而黑色粒子的大小则使用黑色不透明分划参考圆的外径进行确定。 In performing the microscopic particle count test do not attempt to size or enumerate amorphous, semi-liquid, or otherwise morphologically indistinct materials that have the appearance of a stain or discoloration on the membrane filter. These materials show little or no surface relief and present a gelatinous or film-like appearance. In such cases the interpretation of enumeration may be aided by testing a sample of the solution by the light obscuration particle count test.在进行微观颗粒计数试验时,不要试图测量或列举无定形、半液体或其他形态不明显的材料,这些材料在膜过滤器上出现污渍或变色。这些材料很少或没有表面浮雕,呈现胶状或薄膜状外观。在这种情况下,可通过光阻法粒子计数测试溶液样品来辅助解释计数。 Evaluation评估For preparations supplied in containers with a nominal volume of more than 100 mL, apply the criteria of test 2.A.For preparations supplied in containers with a nominal volume of less than 100 mL, apply the criteria of test 2.B.♦For preparations supplied in containers with a nominal volume of 100 mL, apply the criteria of test 2B.♦ If the average number of particles exceeds the limits, test the preparation by the microscopic particle count test.对于在标称体积大于100 mL的容器中提供的制剂,应采用试验2 A.的标准。对于在标称体积小于100 mL的容器中提供的制剂,采用试验2 B的标准。♦对于在标称体积为100 mL的容器中提供的制剂,采用试验2 B的标准。♦如果平均颗粒数超过限值,则通过显微颗粒计数测试对制剂进行测试。 Test 2.A – Solutions for infusion or solutions for injection supplied in containers with a nominal content of more than 100 mL.The preparation complies with the test if the average number of particles present in the units tested does not exceed 12 per millilitre equal to or greater than 10 µm and does not exceed 2 per millilitre equal to or greater than 25 µm.试验2A–输液溶液或注射溶液,装在标称含量超过100 mL的容器中。如果被测单位中颗粒尺寸等于或大于10 µm的平均数量不超过每毫升12个,且颗粒尺寸等于或大于25 µm的平均数量不超过每毫升2个,则制剂符合试验要求。 Test 2.B – Solutions for infusion or solutions for injection supplied in containers with a nominal content of less than 100 mLThe preparation complies with the test if the average number of particles present in the units tested does not exceed 3000 per container equal to or greater than 10 µm and does not exceed 300 per container equal to or greater than 25 µm.试验2B–在标称含量小于100 mL的容器中提供的输液溶液或注射溶液。如果被测单元中存在的尺寸等于或大于10 µm的颗粒平均数不超过3000个/容器,且尺寸大于等于25 um的颗粒平均数不超过300个/容器,则制剂符合试验要求。 检测结果判定标准总结:方法光阻法显微计数法粒径≥10 μm≥25 μm≥10 μm≥ 25 μm体积≤100 mL6000/容器600/容器3000/容器300/容器体积>100 mL<25/mL<3/mL<12/mL<2/mL
参数原理
2022.03.25
不溶性微粒异物检测 生物药用蛋白制剂 不溶性微粒检测应用案例
生物药用蛋白制剂微粒异物检测探讨 一、案例分析说明首先我们来了解什么是生物药用蛋白制剂:生物药用蛋白制剂是指用于预防、治疗和诊断的蛋白质类物质生物药物,比如胰岛素、内啡肽等等,与小分子药物相比,蛋白质药物具有高活性、特异性强、低毒性、有利于临床应用的特点,具有广阔的应用前景。 生物蛋白制剂同样需要检测澄清度、不溶性微粒、可见异物(参考《中国药典2020版》)。本文通过某生物医药公司药用蛋白制剂的3个检测指标,来进行颗粒物案例分析。 二、检测方法和结果 待检样品:某生物医药公司药用蛋白制剂 检测方法:依据《中国药典2020版》0902澄清度检查;0903不溶性微粒检查;0904可见异物检查。1、0902 澄清度检查检查目的:比较不同批次样品的澄清度检查方法:中国药典0902第1法(目视法)仪器设备:HN-200A澄清度检查专用伞棚灯 测试流程:1、样品准备:不同编号样品(A、B、C、D)于冰箱中取出。2、检测过程:开启HN-200A澄清度检查伞棚灯电源,调节白色光源旋钮,经过标准照度计测量,将灯光强度调节至1000 lx±10%范围,将待测样品置于目视窗口,人眼水平观察。测试结果:图1显示的是不同编号蛋白制剂样品在HN-200A澄清度检查专用伞棚灯下的实物照片,从中可以看到样品A的澄清度最差,样品D具有极好的澄清度。 图1.不同样品在HN-200A澄清度检查专用伞棚灯下的实物照片2、0903 不溶性微粒检查检查目的:检测药用蛋白制剂中的不溶性微粒数量检查方法:中国药典0903第二法(显微计数法)仪器设备:YH-MIP-0103显微计数法不溶性微粒分析仪测试流程:1,样品准备:取0.45μm微孔滤膜 (直径13 mm)置于过滤装置上,将0.4 mL待测样品倒入过滤装置,进行抽离过滤;过滤结束后,将滤膜转移到培养皿中,于低温下烘干。2,检测过程:样品滤膜取出后放入到YH-MIP-0103显微计数法不溶性微粒分析仪的测试台上,调整测试参数,于100倍的放大倍率下进行聚焦扫描,测试结束后得到一张包含样品不溶性微粒的大图以及详细的粒子粒径和数量报告。测试结果: 由于测试样品体积为0.4 mL,故其测试结果判定标准参照《中国药典-0903不溶性微粒检查》章节中小容量体积的标准。表一:《中国药典-0903不溶性微粒检查》结果判定标准总量≥100 mL总量<100 mL尺寸≥10 μm≥25 μm≥ 10 μm≥25 μm数量<12/ mL<2/ mL<3000/容器<300/容器 在此章节中,药典规定:光阻法不适用于黏度过高和易析出结晶的制剂,也不适用于进入传感器时容易产生气泡的注射剂。当光阻法测定结果不符合规定或者供试品不适于光阻法测定时,应采用显微计数法进行测定,并以显微计数法的测定结果作为判定依据。测试数据:表二:不同样品的不同尺寸范围微粒的数量及判定结果样品编号粒子数N判定结果尺寸≥10μm尺寸≥25μmON/NGA480137ONB59172ONC56366OND33224ON 以上A、B、C、D样品不溶性微粒的测试结果表明该系列样品中的不溶性微粒检测符合《中国药典-0903不溶性微粒检测》章节中的检测标准。从不同样品的不溶性微粒的测试结果来看,可以看到样品A中尺寸≥25 μm的粒子数量最多,而D样品中尺寸≥25 μm的粒子数量最少,这与澄清度检查章节中的测试结果具有很好的关联性。图2为样品A的显微计数法不溶性微粒扫描的全局图以及部分图,从图2 (b)、(c)中可以清晰地观察到样品制剂里面的不溶性微粒的形状,表明该测试结果可以作为辅助手段鉴别不溶性微粒的来源。图2.样品A显微计数法不溶性微粒检测全局图片(a),部分图片 (b) (c)。3、0904 可见异物检查检查目的:观察样品制剂中的可见异物检查方法:中国药典0904第1法(灯检法)仪器设备:Lu -200A可见异物检查专用伞棚灯测试流程:1、仪器准备:进行可见异物检测时应避免引入可见异物,测试环境应为无菌无尘环境。检测前应对容器外壁进行擦拭,必要时将药液转移至洁净透明的适宜容器中进行观察。2、检测过程:打开Lu -200A可见异物检查专用伞棚灯,调节光源的光亮强度,配用照度计,将仪器的光强调节至1000 lx-1500 lx,将样品置于遮光板边缘处,手持容器颈部,轻轻反转以避免产生气泡,随后进行观察。测试结果:可见异物系指存在于注射剂、眼用液体制剂和无菌原料药中,在规定条件下目视可以观测到的不溶性物质,其尺寸大于50 μm。下表为《中国药典》0904章节中对于注射液无菌制剂中不溶性微粒数量的判定要求。表三:注射用无菌制剂结果判定 图3为不同样品在Lu-200A可见异物检查专用伞棚灯下的实物图,可以清晰地观察到样品溶液中存在可见异物,并且其中的可见异物数量超过药典中的规定标准,其中样品A中的可见异物最多,这与该系列样品的不溶性微粒测试结果相一致。 图3. 不同样品在Lu-200A可见异物检查专用伞棚灯下的实物图 三、 检测总结果经过对上述生物蛋白制剂微粒异物检测分析可以得出以下结论:该生物蛋白质剂经过澄清度检查、不溶性微粒检查以及可见异物检查之后,虽然该生物制剂的不溶性微粒检查结果已经达到了《中国药典》中相关章节的检测标准,但是其可见异物检查观察到的粒子数量已超过《中国药典》中的标准规范。以上的测试结果表明HN-200A澄清度检查专用伞棚灯、YH-MIP-0103显微计数法不溶性微粒分析仪、Lu-200A可见异物检查专用伞棚灯均可对生物药用蛋白制剂中的微粒异物进行有效的检测,并且三项测试得到的测试结果具有很好的一致性,表明以上仪器均符合《中国药典》中相关检查的仪器要求。
应用实例
2022.03.21
日本药典2.65、9.23溶液颜色检查规范
日本药典2.65、9.23溶液颜色检查规范日本药典在2.65和9.23章节中对溶液颜色检查规范作出了描述。 在日本药典的溶液颜色检查的介绍中,提到了两个系列的比色液,分别为A-T系列比色液和B\BY\Y\GY\R系列比色液,并介绍了两种溶液颜色检查方法,均为目视法:方法1:将供试品溶液和参考液体(如水、溶剂或专论中规定颜色的比色液)各2.0 mL分别置于外径为12 mm的透明和无色玻璃试管中,并在散射光下、在白色背景下横向观察,比较颜色。方法2:将供试品溶液和参考液体(如水、溶剂或专论中规定颜色的比色液)分别放入内径为15–25 mm的透明无色平底试管中,使该层的深度为40 mm,在散射光下、在白色背景下垂直观察,比较颜色。 溶液颜色评判标准:当使用A-T系列比色液进行颜色比较时,除非另有规定,否则应将供试品溶液和比色液放入纳氏管中,并在白色背景下横向观察。当使用B\BY\Y\GY\R系列比色液时,可以使用以上两种方法比较颜色,并在专论中说明使用的方法编号。如果溶液具有水或溶剂的外观,其颜色没有比色液B9深,则溶液为无色。 日本药典中溶液颜色检查提及的两个系列的比色液均由以下的比色原液配置而成:氯化钴(II)比色原液:称取65 g六水合氯化钴溶解于盐酸(25 mL)和水的混合液中,制成1000 mL的溶液。精确测量10 mL该溶液,并加水至精确250 mL。精确量取25 mL溶液,添加75mL水和0.05g多氯联苯-氯化钠指示剂,并逐滴添加稀释氨溶液(1/10),直到溶液颜色从红紫色变为黄色。在终点附近添加0.2 mL稀释氨溶液(1/10)后,用0.01 mol/L二氢乙二胺四乙酸二钠进行滴定,直至溶液颜色发生变化,从黄色变为红紫色。每1mL的0.01 mol/L二氢乙二胺四乙酸二钠溶液相当于2.379 mg的 CoCl2·6H2O。根据滴定值,加入稀盐酸(1/40)制成每毫升含有59.5 mg六水合氯化钴(CoCl2·6H2O)的溶液,并将该溶液用作比色原液。硫酸铜(II)比色原液:称取65 g五水合硫酸铜,溶解于盐酸(25 mL)和水的混合液中,制成1000 mL的溶液。精确量取10 mL该溶液,并加水至精确250 mL。精确量取25 mL该溶液,添加75 mL水、10 mL氯化铵溶液(3/50)、2 mL稀氨溶液(1/10)和0.05 g多氯联苯-氯化钠指示剂。用0.01 mol/L二氢乙二胺四乙酸二钠溶液进行滴定,直至溶液颜色从绿色变为紫色。每1mL的0.01 mol/L二氢乙二胺四乙酸二钠溶液相当于2.497 mg的CuSO4·5H2O。根据滴定值,加入稀盐酸(1/40)制成每毫升含有62.4 mg五水硫酸铜(CuSO4·5H2O)的溶液,并将该溶液用作比色原液。三氯化铁(III)比色原液:称取55g六水合三氯化铁,溶解于盐酸(25 mL)和水的混合液中,制成1000 mL的溶液。精确测量10 mL该溶液,移至碘量瓶中,加入15ml水和3g碘化钾,塞紧,在黑暗的地方静置15分钟。向混合物中加入100 mL水,并用0.1 mol/L硫代硫酸钠指示剂(相当于1 mL的淀粉指示剂)滴定其中的游离碘。每1 mL的0.1mol/L硫代硫酸钠溶液相当于27.03mg的六水合三氯化铁(FeCl3·6H2O)。根据滴定值,加入稀盐酸(1/40)制成每毫升含45.0 mg六水合氯化铁(FeCl3.6H2O)的溶液,并将该溶液用作比色原液。比色原液制备好后应储存在具塞玻璃瓶中。 A-T系列比色液的制备:用刻度小于0.1mL的滴定管或移液管精确量取如下表所示体积的比色原液和水,并混合,将溶液储存在具塞玻璃瓶中。颜色比色液氯化钴比色原液体积 (mL)三氯化铁比色原液体积(mL)硫酸铜比色原液体积 (mL)水体积 (mL)A0.10.40.14.4B0.30.90.33.5C0.10.60.14.2D0.30.60.43.7E0.41.20.33.1F0.31.2—3.5G0.51.20.23.1H0.21.5—3.3I0.42.20.12.3J0.43.50.11.0K0.54.5——L0.83.80.10.3M0.12.00.12.8N—4.90.1—O0.14.80.1—P0.20.40.14.3Q0.20.30.14.4R0.30.40.24.1S0.20.1—4.7T0.50.50.43.6 B\BY\Y\GY\R系列比色液的配置方法如下:1.不同色系的初始比色液的配置方法如下:单个颜色的初始比色液体积( mL)三氯化铁比色原液氯化钴比色原液硫酸铜比色原液稀盐酸(1/10)棕色3.03.02.41.6棕黄色2.41.00.46.2黄色2.40.60.07.0黄绿色9.60.20.20.0红色1.02.00.07.0 2.不同色系的比色液的配置方法如下:比色液体积 (mL)单个颜色的初始比色液稀盐酸 (1/10)棕色比色液B175.025.0B250.050.0B337.562.5B425.075.0B512.587.5B65.095.0B72.597.5B81.598.5B91.099.0棕黄色比色液BY1100.00.0BY275.025.0BY350.050.0BY425.075.0BY512.587.5BY65.095.0BY72.597.5黄色比色液Y1100.00.0Y275.025.0Y350.050.0Y425.075.0Y512.587.5Y65.095.0Y72.597.5黄绿色比色液GY125.075.0GY215.085.0GY38.591.5GY45.095.0GY53.097.0GY61.598.5GY70.7599.25红色比色液R1100.00.0R275.025.0R350.050.0R437.562.5R525.075.0R612.587.5R75.095.0胤煌科技提供相应解决方案:上海胤煌科技针对药剂的溶液颜色检查推出了下列产品:1、Qz-82a溶液颜色检查专用伞棚灯此溶液颜色检查专用伞棚灯是专门针对于目视法溶液颜色检查推出的设备,拥有日光型COB集成LED光源,在完成溶液颜色检查的同时,可以减少光源对眼睛的刺激。另外,此仪器可以同时实现水平角度和垂直角度的溶液观察,能有效简化操作,更快得到对比结果。 2、YH-COS-0101溶液颜色检查分析仪此仪器采用D65组合LED光源对液体溶剂进行透射式测量,仪器不仅具有传统的色度色差测量,同时内置中国药典标准比色液库,可以选配欧洲药典或美国药典比色数据库,可以直接测量出待测溶液的色号。
参数原理
2022.03.15
欧洲药典2.2.2溶液颜色检查规定
欧洲药典2.2.2溶液颜色检查规定 在欧洲药典10.0版本的2.2.2章节中提出了两种方法对褐-黄-红色范围内的液体着色程度进行检查:方法1使用外径为12 mm的无色透明中性玻璃管,将2.0 mL待检液体与2.0 mL对照溶液(见参考溶液表)进行比较。比较漫射日光下的颜色,在白色背景下水平观察。方法2使用相同的无色、透明、中性玻璃管,底部平坦,内径为15 mm至25 mm,将待检测液体与对照溶液(见参考溶液表)进行比较,层深为40 mm。比较漫射日光下的颜色,在白色背景下垂直观察。 此药典中指出:如果溶液的外观为水,或颜色不比参考溶液B9深,则溶液为无色。其中所涉及到的溶液配置方法如下:初级溶液黄色溶液:在约900 mL的盐酸和975 mL的水的混合物中加入4.6 g FeCl3•6H2O,并用相同的混合物稀释至1000.0 mL。通过添加相同的酸性混合物,滴定并调整溶液,使其每毫升含有45.0 mg FeCl3•6H2O。溶液避免光照。滴定:将10.0 mL黄色溶液、15mL水、5 mL盐酸和4 g碘化钾放入装有磨砂玻璃塞的250 mL锥形烧瓶中,盖上烧瓶,让其在黑暗中静置15分钟,并添加100mL水。用0.1 mL硫代硫酸钠滴定释放的碘。在滴定结束时加入5 mL淀粉溶液作为指示剂。1 mL的0.1 M硫代硫酸钠相当于27.03 mg FeCl3•6H2O。 红色溶液:将60 g六水合氯化钴溶解在25 mL盐酸和975 mL水的混合物中,并用相同的混合物稀释至1000.0 mL。通过添加相同的酸性混合物,滴定并调整溶液,使其每毫升含有59.5 mg CoCl2•6H2O滴定:将5.0 mL的红色溶液,5 mL稀释过的氧化氢溶液和10 mL 300 g/L氢氧化钠溶液放入装有圆形玻璃塞的250 mL锥形烧瓶中。轻轻煮沸10 min,冷却后加入60 mL稀硫酸和2 g碘化钾。盖上烧瓶,轻轻摇动溶解沉淀物。使用0.1 M的硫代硫酸钠溶液对游离碘进行滴定,在滴定结束时添加淀粉溶液作为指示剂。当溶液变成粉红色时,即达到终点。1mL的0.1M硫代硫酸钠相当于23.79mg的CoCl2•6H2O。 蓝色溶液:将63 g五水硫酸铜溶解在约25 mL盐酸和975 mL水的混合物中,并用相同的混合物稀释至1000.0 mL。通过添加相同的酸性混合物,滴定并调整溶液,使其每毫升含有62.4 mg CuSO4•5H2O。滴定:将10.0 mL蓝色溶液、50毫升水、1.2毫升稀醋酸和3克碘化钾放入装有磨砂玻璃塞的250 mL锥形烧瓶中。用0.1M硫代硫酸钠溶液滴定释放的碘,在滴定结束时添加0.5毫升淀粉溶液作为指示剂。当溶液呈现轻微的淡棕色时,即达到终点。1 mL 的0.1M硫代硫酸钠溶液相当于24.97 mg CuSO4•5H2O。 标准溶液使用3种初始溶液制备5种标准溶液,如下所示(表2.2.2.-1): 表2.2.2.-1标准溶液体积(毫升)黄色溶液红色溶液蓝色溶液盐酸溶液(10 g/L)B(棕色)3.03.02.41.6BY(棕黄色)2.41.00.46.2Y(黄色)2.40.60.07.0GY(黄绿色)9.60.20.20.0R(红色)1.02.00.07.0 方法1和2的参考溶液 表2.2.2.-2参考溶液-棕色溶液参考溶液体积(毫升)棕色溶液盐酸溶液(10 g/L)B175.025.0B250.050.0B337.562.5B425.075.0B512.587.5B65.095.0B72.597.5B81.598.5B91.099.0 表2.2.2.-3参考溶液-棕黄色溶液参考溶液体积(毫升)棕黄色溶液盐酸溶液(10 g/L)BY1100.00.0BY275.025.0BY350.050.0BY425.075.0BY512.587.5BY65.095.0BY72.597.5 表2.2.2.-4参考溶液-黄色溶液参考溶液体积(毫升)黄色溶液盐酸溶液(10 g/L)Y1100.00.0Y275.025.0Y350.050.0Y425.075.0Y512.587.5Y65.095.0Y72.597.5 表2.2.2.-5参考溶液-黄绿色溶液参考溶液体积(毫升)黄绿色溶液盐酸溶液(10 g/L)GY125.075.0GY215.085.0GY38.591.5GY45.095.0GY53.097.0GY61.598.5GY70.7599.25 表2.2.2.-6参考溶液-红色溶液参考溶液体积(毫升)红色溶液盐酸溶液(10 g/L)R1100.00.0R275.025.0R350.050.0R437.562.5R525.075.0R612.587.5R75.095.0 溶液的储存对于方法I,参考溶液可储存在无色、透明、外径为12mm的中性玻璃密封管中,避免光线照射。对于方法II,使用前立即从标准溶液中制备参考溶液。 在这项检查方法中我们可以看到,欧洲药典对于溶液颜色检查方法为目视法。其比色液的色调主要包含五个,分别为:棕色、棕黄色、黄色、黄绿色和红色。标准溶液按照一定标准调配成标准比色液(参考溶液),用于溶液的颜色检查。 解决方案:上海胤煌科技针对药剂的溶液颜色检查推出了下列产品:1、Qz-82a溶液颜色检查专用伞棚灯 此溶液颜色检查专用伞棚灯是专门针对于目视法溶液颜色检查推出的设备,拥有日光型COB集成LED光源,在完成溶液颜色检查的同时,可以减少光源对眼睛的刺激。另外,此仪器可以同时实现水平角度和垂直角度的溶液观察,能有效简化操作,更快得到对比结果。2、YH-COS-0101溶液颜色检查分析仪此仪器采用D65组合LED光源对液体溶剂进行透射式测量,仪器不仅具有传统的色度色差测量,同时内置中国药典标准比色液库,可以选配欧洲药典或美国药典比色数据库,可以直接测量出待测溶液的色号。
参数原理
2022.03.09
延期通知| 2022·第二届mRNA药物技术创新峰会
延期通知| 2022·第二届mRNA药物技术创新峰会 由于上海疫情影响,原定于2022年3月10日-11日在上海张江海科雅乐轩酒店举行的已经延期举行,下面来看看官方通知吧。 青山不改,阵容不变!待疫情散去,胤煌科技期待与您早日相约!
企业动态
2022.03.09
胤煌科技/邀您参加2022•第二届mRNA药物技术创新峰会
胤煌科技/邀您参加2022•第二届mRNA药物技术创新峰会2021年,国内的mRNA领域蓬勃发展,经过国内mRNA赛道上这不平凡的2021年,大家有了一些宝贵的经验,一些可喜的成果。第二届mRNA药物技术创新峰会即将于2022年3月10日在上海张江举行,峰会将在序列、递送、工艺、应用四个板块,聊一聊2021年mRNA领域发生的事情,展示成果,分享经验,探讨难题,展望未来!此次会议邀请到了上海主要的mRNA研发生产企业,如复星、斯微、蓝鹊、康希诺等;以复旦大学林金钟为代表的高校mRNA科研力量;mRNA上下游产业链的知名企业;奉贤、宝山两个核酸产业园;以及一些生物医药主管部门,可以说是目前上海最强的mRNA产业阵容,不容错过!上海胤煌科技有限公司在此次会议的的展位号为B1,胤煌科技诚挚地欢迎您的到来! 峰会目标:构建中国mRNA药物产业生态体系 促进中国mRNA 药物事业发展 期待国产mRNA 疫苗早日上市主要议题:本次议会将围绕mRNA技术应用场景、序列合成、递送系统、生产工艺四大方面展开讨论:mRNA药物技术最前沿进展;mRNA疫苗的研发与应用;mRNA疫苗的临床评价;mRNA疫苗的生产与储存;mRNA技术的关键问题和应用前景;mRNA技术的特点、优势与价值;不同mRNA技术平台比较;mRNA疫苗上游开发的策略;mRNA序列的合成、优化;mRNA药物递送系统的开发;mRNA大规模纯化的挑战与解决方案;国内外mRNA技术产业布局及挑战;mRNA药物行业投资热点与机会;mRNA技术研究与成果转化;mRNA技术的知识产权保护;.....日程安排:此次胤煌科技展出的产品有:
企业动态
2022.03.03
【放假通知】胤煌科技2022年春节放假通知
胤煌科技2022年春节放假通知 2021年即将过去,上海胤煌科技有限公司全体员工感谢各位一直以来的信任和支持!希望在新的一年里胤煌科技能与您携手共进,共创辉煌!我们会继续坚持公司“优质产品,业技术,完善服务,让检测分析更业”的服务宗旨,为客户提供全面准确的检测分析解决方案,优质的检测服务,让客户拥有业,优化的设备方案。 2022年春节即将到来,在此胤煌科技向各位新老客户和广大朋友拜个早年,祝大家新春愉快!以下为本公司此次春节假日安排: 放假日期为2022年1月29日(星期六)至2022年2月13日(星期日),公司成员将于2022年2月14日(星期一)正式上班。由此带来的不便,敬请谅解! 再次感谢大家对胤煌科技的支持及关注!胤煌科技在此恭祝各位新老顾客新春吉祥,阖家欢乐!新年新气象,更上一层楼!
新品
2022.01.25
【VacCon 2022第四届新型疫苗研发与产业化论坛】 圆满落幕
【VacCon 2022第四届新型疫苗研发与产业化论坛】圆满落幕 VacCon 2022第四届新型疫苗研发与产业化论坛于2022年1月7日-8日在成都富力丽思卡尔顿酒店成功召开。 本次论坛以“后新冠时代,聚焦下一代新型疫苗的技术壁垒突破”为主题,邀请了60多位疫苗/中和抗体/小分子新冠药物领域政府监管机构专家、科研专家科学家及领军企业负责人国内专家以及医药疫苗相关企业的专业代表,作出了精彩的汇报。 论坛汇报分为三个专题进行,分别是:核酸疫苗(mRNA与DNA)、其他新型疫苗、新冠疫苗及药物。大会的目的在于切实推进中国新型疫苗的转化与产业化,以期早日解除新冠疫情带来的威胁。 上海胤煌科技有限公司受邀参加本次论坛,介绍了胤煌科技的主打产品:显微计数法不溶性微粒分析仪、CHDF高精度纳米粒度分析仪、动态图像法粒度粒形分析仪等产品,并得到参会老师的关注和肯定。
企业动态
2022.01.11
胤煌科技 | 邀您参加2022年第四届新型疫苗论坛-成都站
大会亮点* 应对突变株及第一代疫苗保护效力下降挑战,解析新一代疫苗/中和抗体/小分子药物政 策监管、研发立项(广谱 性、长效性)、临床开发策略及商业化前景* 追踪现有大规模/上市后疫苗临床安全性与有效性,寻找增强免疫最优临床开发策略* 深度探讨mRNA技术升级与创新,突破递送技术壁垒,开创疫苗药物新天地;聆听mRNA 疫苗及药物CMC工艺生产、质控、产业链建设等最优解决方案* 获取前沿的新型疫苗(重组疫苗、病毒载体疫苗)创新技术资讯,展望其在众多适应症下 的广阔应用前景及转化潜力产品介绍显微计数法不溶性微粒分析仪测试范围:1μm-500μm1-全滤膜自动扫描,操作简单,节约时间;2-颗粒自动计数,保护眼睛,减少人为操作误差;3-符合各国药典/国标法规,一键自动出具对应报告;4-符合21CFR Part11及GMP对数据完整性的要求;应用领域应用范围:乳剂、脂质体、滴眼剂、混悬剂、易产生气泡剂型、粘度大制剂、无菌粉末、注射液、疫苗等会议流程
企业动态
2021.12.30
显微计数法不溶性微粒分析仪产品升级
仪器型号: YH-MIP-0103工作原理: 显微计数法检测范围: 1μm-500μm中国药典规定为什么要选择显微计数法? 第1:当光阻法测定结果不符合规定或供试品 不适于用光阻法测定时,应采用显微计数法进行测 定,并以显微计数法的测定结果作为判定依据 。第二:光阻法不适用对于易产生气泡、高粘度 的制剂产品,在检测不溶性微粒时要采用第二法 (显微计数法)来检测。设备构成√膜处理装置; √显微镜主机; √全自动电动样品台; √图像处理及操作软件;技术优势√全滤膜自动扫描,操作简单,节约时间;√颗粒自动计数,保护眼睛,减少人为操作误差;√符合各国药典/国标法规,一键自动出具对应报告;√符合21CFR Part11及GMP对数据完整性的要求;技术参数符合各国药典规定,自动出报告(中英双译)广泛应用于:乳剂,脂质体,混悬剂,滴眼液,疫苗,蛋白质注射液,有色注射液,医疗器械等方面。
新品
2021.12.14
苏州站【2021年-CMC化学制药产业大会】圆满落幕
【2021年-CMC化学制药产业大会】于2021年9月29 - 30日在苏州国际会展中心召开。本次大会宗旨:本次大会宗旨在打破产业链中各板块的界限,让不同板块之间的专家倾听和分享经验和看法,真正实现“知己知彼”。本次大会以“链接医药全产业链,解决符合国情的未满足用药需求”为主题,从原料药中间体起步研讨,途径制剂及改良创新,再到源头创新新药的全方位探路技术,政策及解决方案,会议现场上海胤煌科技有限公司受邀参加本次CMC化学制药产业大会,并于会议上展示胤煌科技的主打产品:粒度及Zeta电位分析仪,高分辨纳米粒度仪,显微计数法不溶性微粒分析仪,伞棚灯,单颗粒纳米粒度仪等产品,助力注射剂一致性评价,并得到参会老师的关注和肯定。
企业动态
2021.10.09
胤煌科技 | 中国国际生物&化学制药产业大会-苏州站
胤煌科技 | 中国国际生物&化学制药产业大会-苏州站展位号:产品介绍:上海胤煌科技有限公司公司目前经营的产品有伞棚灯、纳米粒度仪、Zeta电位分析仪、原液纳米粒度及Zeta电位分析仪、显微计数法不溶性微粒分析仪、接触角、薄膜相变测量仪、等检测分析设备、可以为半导体、面板、医药及材料等相关领域提供专业的检测分析设备。
企业动态
2021.09.24