
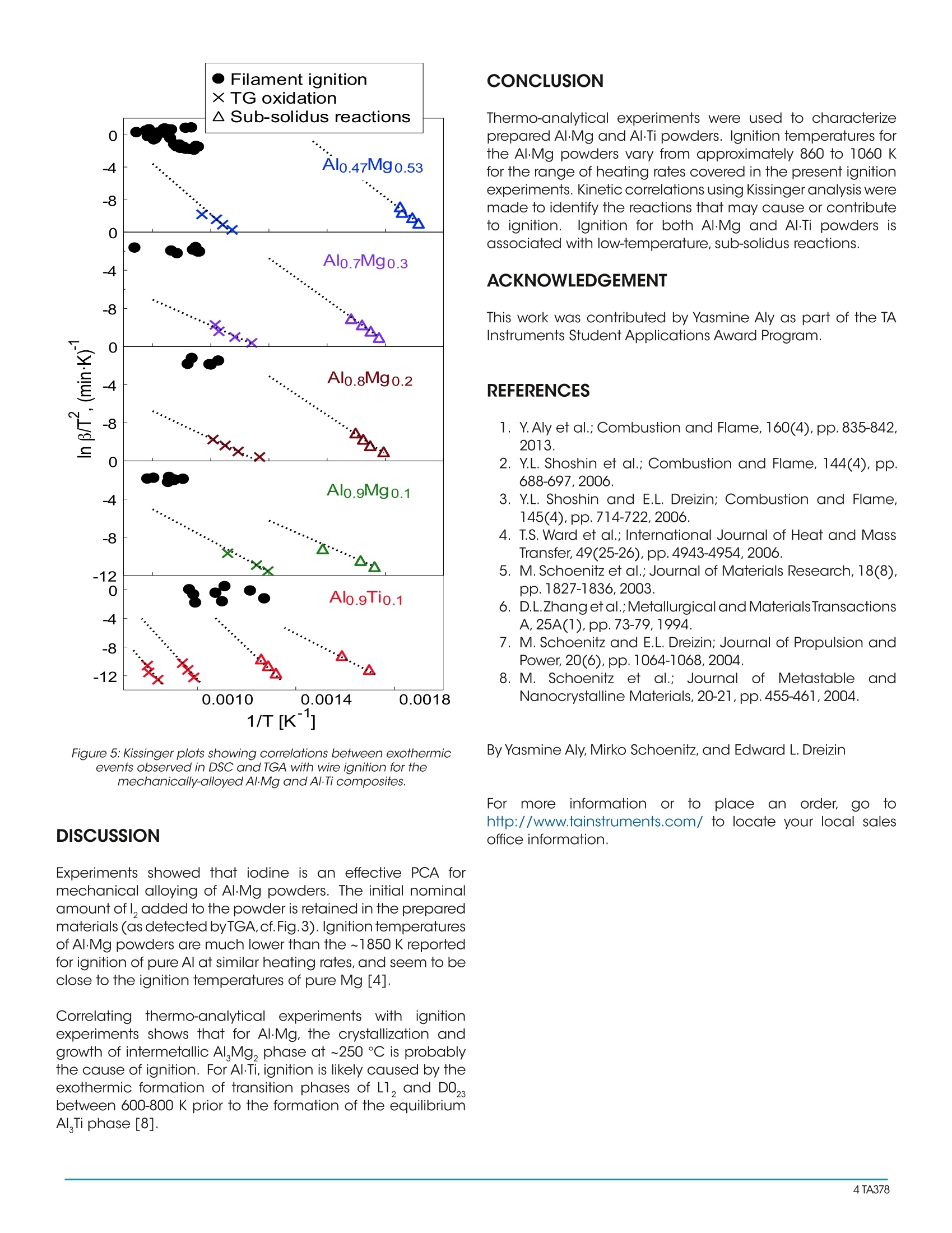
Thermo-analytical experiments were used to characterize prepared Al?Mg and Al?Ti powders. Ignition temperatures for the Al?Mg powders vary from approximately 860 to 1060 K for the range of heating rates covered in the present ignition experiments. Kinetic correlations using Kissinger analysis were made to identify the reactions that may cause or contribute to ignition. Ignition for both Al?Mg and Al?Ti powders is associated with low-temperature, sub-solidus reactions.
方案详情

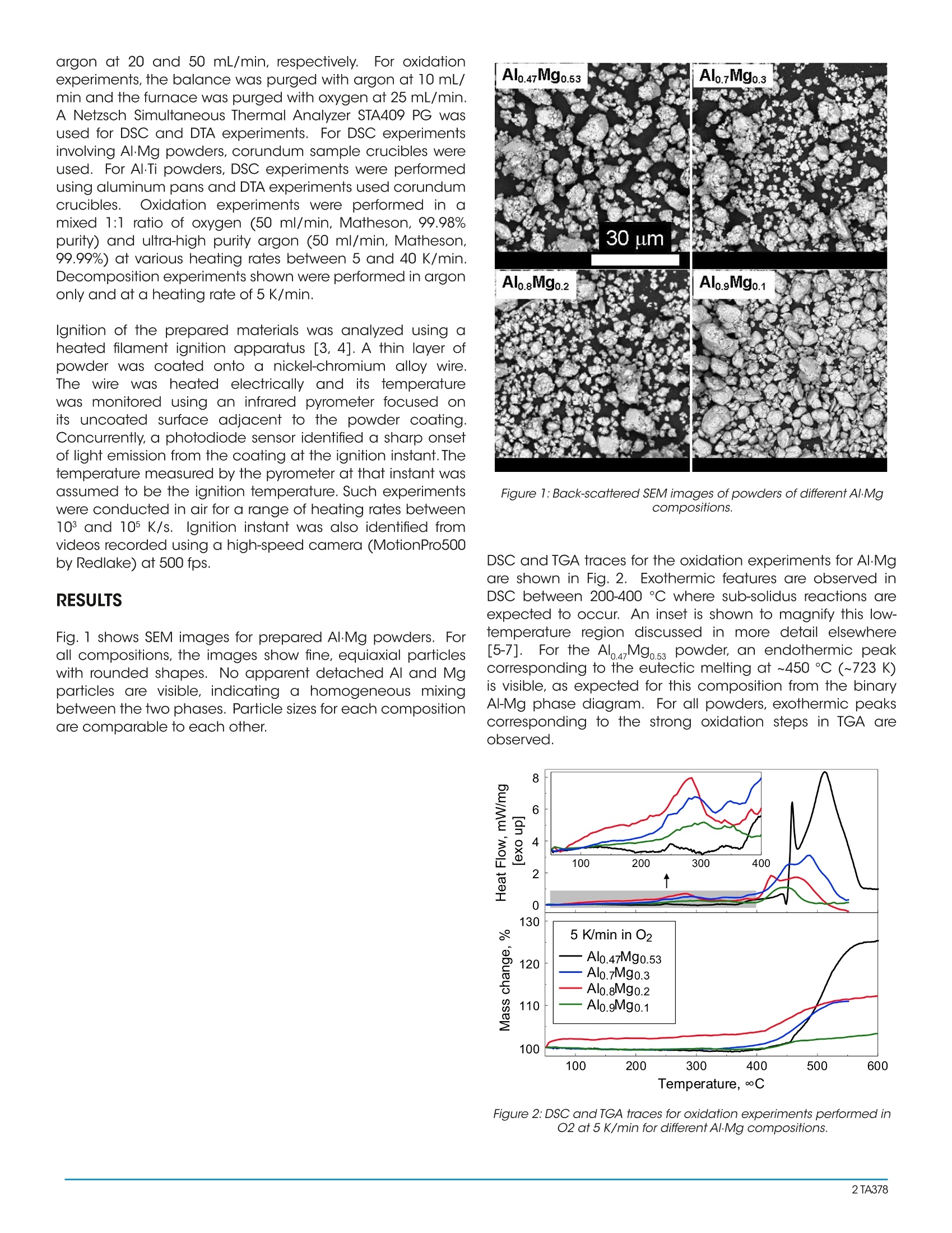
Reactions in Metastable Metal-based ReactivePowders Prepared by Mechanical AlloyingTA378 Keywords: oxidation, ignition, metal combustion, mechanical alloys, reactive powders ABSTRACT Metal powders such as aluminum are commonly used asfuel additives to propellants, explosives, and pyrotechnicsbecause of the improved performance they offer due totheir high combustion enthalpies. However, heterogeneousoxidation of metals may cause long ignition delays, highignition temperatures, low burn rates, and prematureextinction leading to an incomplete combustion. Metal-based,mechanically alloyed materials have been proposedto improve ignition and combustion rates as a result of themetastable phase transitions that occur at low temperaturesbefore, and sometimes during combustion. It is of interestto characterize these materials, and specifically, to explorehow they behave under inert and oxidative environmentsupon heating. In this project,high-energy density, Al-basedmaterials (i.e.,Al·Mg,Al·Ti,etc.) are prepared by mechanicalmilling. Differential scanning calorimetry (DSC) is used toidentify and characterize reactions that occur upon theirheating in different environments, including subsolidusphase changes, eutectic and pure metal melting, andformation of various oxidized products. Thermogravimetricanalysis (TGA) measurements are used to help identify andquantify decomposition and oxidative behaviors. Exothermicreactions observed in DSC are correlated to oxidative weightaains observed in TGA. Kinetic correlations are found usingisoconversion processing to identify the reactions that causeor contribute to ignition of the materials.Results of suchcorrelations will be presented in the paper. INTRODUCTION High combustion enthalpy metal additives to propellants,explosives,o,and ipyrotechnics mayenhance theirperformance. The main challenge with metal additives is thattheir reactions are hindered by slow kinetics that give wayto high ignition temperatures, long ignition delays, slow burnrates, and incomplete combustion. Metal-based reactivematerials, including Al-based alloys have been developed,capable of exothermic reactions preceding combustion,andthus accelerating ignition of these materials. These reactionsinclude sub-solidus, intermetallic phase transformations. Al-based alloys are particularly interesting enabling oneto reduce ignition delays and accelerate burn rates whilestill taking advantage of the high combustion enthalpyof Al. The work presented here involves the preparationand characterization of Al·Mg and Al.Ti powders preparedby mechanical milling.Mechanically alloyed powdersexperience intermetallic, sub-solidus phase changes whenheated, which promote their ignition, e.g., by generating an additional fresh surface. Thermal analysis techniques suchas thermogravimetric analysis (TGA) along with differentialscanning calorimetry (DSC) and differential thermal analysis(DTA) are used to identify and quantify these reactions.Correlations are then made to identify which reactions leadto powder ignition. MATERIALS Starting materials used in the synthesis of the compositesincluded elemental powders of Al (Atlantic EquipmentEngineers,99.8% pure,-325 mesh),Mg (Alfa-Aesar, 99.8% pure,-325 mesh), and Ti (Alfa-Aesar, 99%,-325 mesh). For Al·Mgpowders, the material compositions varied systematicallywith the Al concentration ranging from 47 to 90 at.%.Powderswere ball-milled using a Retsch PM-400 MA planetary mill. Asdescribed elsewhere [1], the first stage of milling involved thepreparation of a coarse, mechanically alloyed AL·Mg powder.Powders of Al and Mg were loaded in steel milling vials inargon. The powder charge was 30 g per vial and the ball topowder mass ratio (BPR) was 10. 9.5 mm-diameter hardenedsteel milling balls were used. A rotational speed was set to350 rpm for 120 min. 50 ml of hexane (C,H) was addedto each milling vial as a process control agent (PCA). Thesecond stage of milling involved the addition of iodine (l,chips,Sigma Aldrich, 99% pure), at 4 wt.% of the initial powderload. The 9.5-mm balls were removed and replaced with thesame mass of 3-mm hardened steel balls, which allowed fora softer milling necessary for particle size reduction. Millingfor the second milling step was continued for 60, 120, and180 min. depending on powder composition. For Al-Ti powders, compositions varied between 75 to 90 at. %Al as described elsewhere [2]. Powders were ball-milled for 15h using an 8000D series SPEX Certiprep shaker mill. Powdersof Al and Ti were loaded in zirconia vials in 5 g batches with aBPR of 10, with 2 wt.% of stearic acid added as PCA. EXPERIMENTAL Scanning electron microscopy (SEM) was used to studypowder morphology using a Phenom Tabletop Microscopeby FEl Technologies Inc. Backscattered electron images weretaken to inspect particle shapes and sizes. Thermal stabilityand temperature-dependent phase transformations werestudied using TGA, DSC, and DTA. A TA Instruments Q5000IRthermogravimetric analyzer (TGA) was used to help identifyand quantify decomposition and oxidative behaviors ofthe composites. The powders were loaded into an aluminacrucible with a sample mass of 2 -4 mg. For decompositionexperiments, the balance and furnace were purged with argon at 20 and 50 mL/min, respectively.FFor oxidationexperiments, the balance was purged with argon at 10 mL/min and the furnace was purged with oxygen at 25 mL/min.A Netzsch Simultaneous Thermal Analyzer STA409 PG wasused for DSC and DTA experiments. For DSC experimentsinvolving Al·Mg powders, corundum sample crucibles wereused. For AlTi powders, DSC experiments were performedusing aluminum pans and DTA experiments used corundumcrucibles. Oxidation experiments were performed in amixed 1:1 ratio of oxygen (50 ml/min, Matheson, 99.98%purity) and ultra-high purity argon (50 ml/min, Matheson,99.99%) at various heating rates between 5 and 40 K/min.Decomposition experiments shown were performed in argononly and at a heating rate of 5 K/min. Ignition of the prepared materials was analyzed using aheated filament ignition apparatus [3, 4]. A thin layer ofpowder was coated onto a nickel-chromium alloy wire.The wire washeated electrically and its temperaturewas monitored using an infrared pyrometer focused onits uncoated surface adjacent to the powder coating.Concurrently, a photodiode sensor identified a sharp onsetof light emission from the coating at the ignition instant. Thetemperature measured by the pyrometer at that instant wasassumed to be the ignition temperature. Such experimentswere conducted in air for a range of heating rates between10 and 105 K/s. Ignition instant was also identified fromvideos recorded using a high-speed camera (MotionPro500by Redlake) at 500 fps. RESULTS Fig. 1 shows SEM images for prepared AI·Mg powders. Forall compositions, the images show fine, equiaxial particleswith rounded shapes. No apparent detached Al and Mgparticles are visible, indicating a homogeneous mixingbetween the two phases. Particle sizes for each compositionare comparable to each other. Figure 1: Back-scattered SEM images of powders of different Al·Mgcompositions. DSC and TGA traces for the oxidation experiments for Al·Mgare shown in Fig. 2. Exothermic features are observed inDSC between 200-400°C where sub-solidus reactions areexpected to occur. An inset is shown to magnify this low-temperature region discussed in more detail elsewhere[5-7].1. For the Alo.47Mgo.53 powder, an endothermic peakcorresponding to the eutectic melting at ~450 ℃ (~723 K)is visible, as expected for this composition from the binaryAI-Mg phase diagram. For all powders, exothermic peakscorresponding to the strong oxidation steps in TGA areobserved. Figure 2: DSC and TGA traces for oxidation experiments performed inO2 at 5 K/min for different AlMg compositions. Fig. 3 shows DSC and TGA traces for the Al·Mg powdersheated in argon. Exothermic peaks at ~250°C are observedin DSC for all powders, corresponding to the crystallizationand growth of Al Mg, phase identified in earlier work [5,7].For the 47/53 and 70/30 compositions, endothermic peakscorresponding to eutectic melting at ~450℃ are also clearlydistinguishable. TGA traces show mass loss indicative ofthe release of 1, which begins at higher temperatures asAl content increases. This trend is consistent for all powdersexcept the 80/20 composition. This may be due to a longermilling time experienced by this powder composition andthus, further stabilization of l, in the metal. Figure 3:DSC and TGA traces for decomposition experimentsperformed in Argon at 5 K/min for different A/-Mg compositions. TGA, DTA, and DSC traces for the oxidation experimentsperformed for AlTi powders are shown in Fi-g1.g 4. Oxidationof AlTi occurs stepwise, starting after Al melting. Largeexothermic peaks corresponding to the two oxidation stepsare visible in DTA. Trace amounts of carbon from the stearicacid leads to precipitation of TiC observed by the endothermin DTA at~900℃. In the low-temperature, sub-solidus region,exothermic peaks corresponding to formation of a series ofless metastable Al Ti phases can be observed in DTA andeven more clearly in DSC. Figure 4: DTA TGA, and DSC experiments for a mechanically alloyedAl0.9Ti0.1 powder at 15 K/min. TGA analysis was conducted for both A·Mg and Al-Ti powdersin the same oxidative environment at different heating ratesin the range of 5 - 40 K/min. Derivatives of TGA traces (DTG)at varying heating rates were used to construct Kissingerplots using the DTG peak maxima. The resulting Kissingerplots (logarithm of heating rate, β, divided by temperaturesquare vs. inverse temperature) are shown in Fig.5. Ignitiontemperatures from the heated filament ignition experimentsare also included. As expected for a thermally activated ignition mechanism,ignition temperaturesiincrease slightlywith increasingheating rates. Ignition temperatures are approximately 860 Kat lower heating rates and 1060 K for higher heating rates forall Al·Mg powders. From Fig. 5, it is apparent that the points corresponding tothe strong oxidation step do not line up with those of filamentignition. However, the points corresponding to sub-solidusreactions seem to correlate with the ignition points. One mustconsider that the measured filament ignition temperaturesrecorded are those of the filament and not of the powdercoating,suggesting that the points seen in Fig. 5 could shiftmore to the right side of the plot, if corrected to represent theactual powder temperature. Considering such a correction,it would be more obvious that the ignition points line up quitewell with low-temperature events (formation of intermetallic,sub-solidus phases). 0.0018 Figure 5: Kissinger plots showing correlations between exothermicevents observed in DSC and TGA with wire ignition for themechanically-alloyed A·Mg and Al-Ti composites. DISCUSSION Experiments showed that iodine is an effective PCA formechanical alloying of Al·Mg powders. The initial nominalamount ofl, added to the powder is retained in the preparedmaterials (as detected byTGA,cf.Fig.3).Ignition temperaturesof AI·Mg powders are much lower than the~1850K reportedfor ignition of pure Al at similar heating rates, and seem to beclose to the ignition temperatures of pure Mg[4]. Correlating thermo-analytical expeerriimmeennttss with igniticnexperiments shows that for Al·Mg, the crystallization andgrowth of intermetallic AlMg, phase at ~250 °C is probablythe cause of ignition. For Al-Ti, ignition is likely caused by theexothermic formation of transition phases of L1, and DO,between 600-800 K prior to the formation of the equilibriumAl Ti phase [8]. CONCLUSION Thermo-analytical experiments were used to characterizeprepared A1·Mg and Al-Ti powders. Ignition temperatures forthe Al·Mg powders vary from approximately 860 to 1060 Kfor the range of heating rates covered in the present ignitionexperiments. Kinetic correlations using Kissinger analysis weremade to identify the reactions that may cause or contributeto ignition. Ignition for both Al·Mg and AlTi powders isassociated with low-temperature, sub-solidus reactions. ACKNOWLEDGEMENT This work was contributed by Yasmine Aly as part of the TAInstruments Student Applications Award Program. REFERENCES 1. Y. Aly et al.; Combustion and Flame, 160(4),pp.835-842,2013. 2. Y.L. Shoshin et al.; Combustion and Flame, 144(4), pp.688-697,2006. 3. Y.L. Shoshin and E.L. Dreizin; Combustion and Flame,145(4),pp.714-722,2006. 4.TT.S. Ward et al.; International Journal of Heat and MassTransfer, 49(25-26),pp.4943-4954,2006. 5. M.Schoenitz et al.; Journal of Materials Research, 18(8),pp. 1827-1836,2003. 6. D.L.Zhang et al.;Metallurgical and Materials TransactionsA,25A(1),pp.73-79,1994. 7. M. Schoenitz and E.L. Dreizin; Journal of Propulsion andPower,20(6),pp.1064-1068,2004. 8. M. Schoenitz et al.;Journal of)fMetastableandNanocrystalline Materials, 20-21, pp.455-461,2004. By Yasmine Aly, Mirko Schoenitz, and Edward L. Dreizin TA ABSTRACTMetal powders such as aluminum are commonly used as fuel additives to propellants, explosives, and pyrotechnics because of the improved performance they offer due to their high combustion enthalpies. However, heterogeneous oxidation of metals may cause long ignition delays, high ignition temperatures, low burn rates, and premature extinction leading to an incomplete combustion. Metalbased, mechanically alloyed materials have been proposed to improve ignition and combustion rates as a result of the metastable phase transitions that occur at low temperatures before, and sometimes during combustion. It is of interest to characterize these materials, and specifically, to explore how they behave under inert and oxidative environments upon heating. In this project, high-energy density, Al-based materials (i.e., Al·Mg, Al∙Ti, etc.) are prepared by mechanical milling. Differential scanning calorimetry (DSC) is used to identify and characterize reactions that occur upon their heating in different environments, including subsolidus phase changes, eutectic and pure metal melting, and formation of various oxidized products. Thermogravimetric analysis (TGA) measurements are used to help identify and quantify decomposition and oxidative behaviors. Exothermic reactions observed in DSC are correlated to oxidative weight gains observed in TGA. Kinetic correlations are found using isoconversion processing to identify the reactions that cause or contribute to ignition of the materials. Results of such correlations will be presented in the paper.
确定


还剩2页未读,是否继续阅读?
TA仪器为您提供《金属中活性检测方案(差示扫描量热)》,该方案主要用于其他中活性检测,参考标准--,《金属中活性检测方案(差示扫描量热)》用到的仪器有TA仪器 Discovery差示扫描量热仪
推荐专场
相关方案
更多















