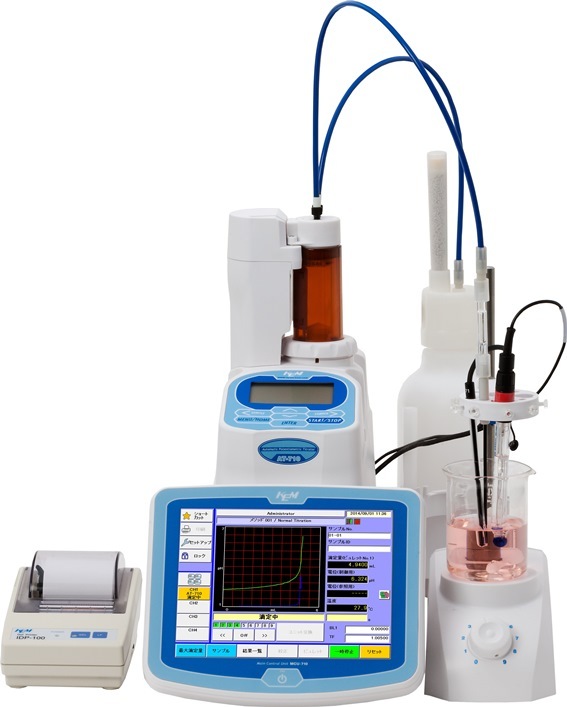
水溶性酸,碱,盐溶液表面统一的分子图像
The molecular structure of the interfacial regions of aqueous electrolytes is poorly understood, despite itscrucial importance in many biological, technological, and atmospheric processes. A long-term controversypertains between the standard picture of an ion-free surface layer and the strongly ion specific behaviorindicating in many cases significant propensities of simple inorganic ions for the interface. Here, we presenta unified and consistent view of the structure of the air/solution interface of aqueous electrolytes containingmonovalent inorganic ions. Molecular dynamics calculations show that in salt solutions and bases the positivelycharged ions, such as alkali cations, are repelled from the interface, whereas the anions, such as halides orhydroxide, exhibit a varying surface propensity, correlated primarily with the ion polarizability and size. Thebehavior of acids is different due to a significant propensity of hydronium cations for the air/solution interface.Therefore, both cations and anions exhibit enhanced concentrations at the surface and, consequently, theseacids (unlike bases and salts) reduce the surface tension of water. The results of the simulations are supportedby surface selective nonlinear vibrational spectroscopy(VSFG), which reveals among other things that the hydroniumcations are present at the air/solution interface. The ion specific propensities for the air/solution interfacehave important implications for a whole range of heterogeneous physical and chemical processes, includingatmospheric chemistry of aerosols, corrosion processes, and bubble coalescence.