JIS Z 2277:2000 Method of tensile testing for metallic materials in liquid helium
Guy Wingate, Ph.D.Validation has a key role to play in providing a high degree of assurance that computer systems supporting drug manufacture are fit for purpose. [1, 2] Those involved in computer validation tend to fall into one of two camps. One group believes in the inherent value of validation as a cost-effective means of quality assurance. The other group sees validation as an ineffective bureaucratic process whose only value is demonstrating regulatory compliance.These two mind-sets can have a big impact on the practical implementation of computer validation. Those with a more positive attitude tend to be more pragmatic,looking at validation as flexible tool that can be tailored to address the individual needs of different computer systems. This group accepts and is willing to make judgment calls on how much validation is enough in different situations. Those with a more negative attitude tend to think of validation like an insurance policy against regulatory censure. They want validation to be an entirely standard process that can be applied without any ambiguity. This group often does not understand and does not want to understand validation rather, it takes a “just tell me what to do and I’ll do it” stance.There are numerous horror stories of regulatory censure for lack of or insufficient validation. Consent decrees can cost pharmaceutical companies many hundreds millions dollars. Although computer validation has not yet triggered a consent decree, it has been a contributory factor. [3] And, of course, there are also horror stories concerning the cost of validation when implemented inappropriately. Not too surprisingly, the two are often connected as a drive to validate too quickly to remediate an adverse regulatory finding. Equally, short-cutting validation to reduce cost is likely to lead to an adverse regulatory finding. Such deficiencies may not be immediately found during initial regulatory inspections because of limited time to review systems. Indeed, it is not always the original validation that can cause problems poor maintenance of the validated state currently accounts for about one third of adverse regulatory computer validation findings. [3] A sense of balance on how much validation is enough must prevail. While it is undeniable that potentially regulatory authorities have extensive powers to financially penalize companies that have critical validation failings, these powers are only executed in extreme circumstances. The expectations of regulatory authorities are founded on common sense and experience. Pharmaceutical companies need to recognize this. Regulatory censure tends to only occur when pharmaceutical companies are seen to take an unreasonable approach to published regulatory requirements.Validation practices are becoming more effective and efficient as the discipline of computer validation matures. Validation costs exceeding 40% or more of project costs should be a distant memory. A survey of published best practices suggests that validation should now account for 10% or less of project budgets. [3] A key development in this maturing process is a much better understanding of the criticality and impact of data and systems supporting business processes and the application of risk management to focus validation activities.The U.S. FDA has been particularly prominent in promoting a risk-based approach [4] although other regulatory authorities have allowed this approach for many years. [5] The ISPE GAMP Forum has published specific guidance on risk management within the context of computer validation [6] and is shortly to publish new guidance on applying a risk-based approach to electronic records and signatures. [7] The International Conference for Harmonisation (ICH) is further embedding a common approach to risk management between U.S., European, and Japanese regulatory authorities within the ICH Q9 initiative.This book imparts some of the latest thinking on computer validation. [url=https://insevent.instrument.com.cn/t/Mp]gc[/url]P, GLP,and GMP regulatory requirements are explained with guidance from seasoned practitioners on how to fulfill them. GAMP guidance plays a central role. This book will be an key resource to IT staff, QA professionals, validation staff, control system engineers, suppliers, and consultants wanting to improve their validation capability. Invaluable suggestions are made throughout dealing with life cycle management, electronic records and signatures, risk management, and regulatory inspections. But, of course, it is not just about doing the right thing, but doing the right thing well, and several chapters deal with specific system examples.
replicate organism detection and counting(RODAC)
开机时听到“劈劈啪啪”的声音数次,可是氚灯没亮,接着面板出现“Lamp lighting failure"的字,现在不知是灯寿命到了还是面板烧了。去买灯或面板要上万银子,求大侠们帮忙分析一下,能有啥好办法。好急啊!!! 看错了,是waters2487 UV检测器。 刚才又开了一下机,灯点亮了,但过几分钟又自动灭掉了,显示”lamping failure“,这又是什么原因啊???
求助澳表标AS2331(Methods of test for metallic and related coating)
[*][作者]:[url=https://link.springer.com/book/10.1007/1-4020-5438-6?page=2#author-1-0]J[font=Archivo, &][size=16px][color=#222222]Xiaoxia Luo, Hua Liu, Zhenwu Lu, and Yao Wang[/color][/size][/font][/url][*][题名]:[b][b][url=https://iopscience.iop.org/book/mono/978-0-7503-3167-8][b]Automated optimization of an aspheric light-emitting diode lens for uniform illumination[/b][/url][/b][/b][list][/list][*][b]【期刊】:optica[/b][*][b]【链接】:[url=https://opg.optica.org/ao/abstract.cfm?uri=ao-50-20-3412#]Automated optimization of an aspheric light-emitting diode lens for uniform illumination (optica.org)[/url][/b]
在看第三方报告时,发现检测机构不同,报告中有的写“MDL”,有的写“REPORTING LIMIT”,有的写“DETECTION LIMIT”,所表达的意思是不是相同的呢?http://simg.instrument.com.cn/bbs/images/default/emyc1010.gif
ASTM E8M 04 Standard Test Methods for Tension Testing of Metallic Materials [Metric]1[~135067~]
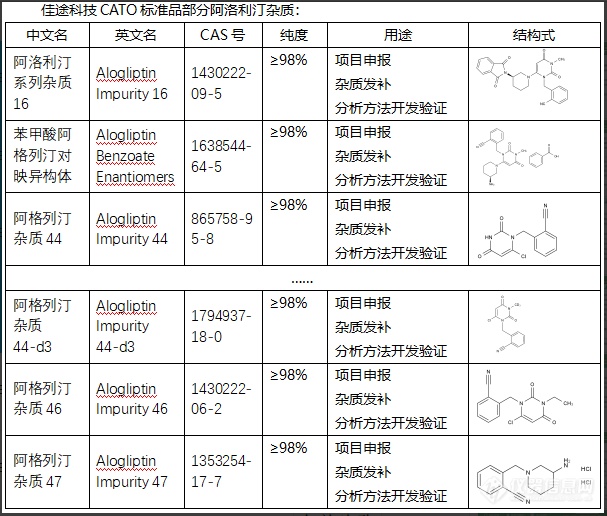
阿洛利汀杂质可以作为标准物质,用于评价阿洛利汀的质量和纯度。通过测量此类杂质的含量,可以对阿洛利汀的生产过程进行控制和优化,以制造出更优质的药物。此外,某些类型的杂质还可能被用作药物的标记物,以跟踪药物在体内的分布和代谢。CATO标准品目前的药品生产技术已经可以有效地降低杂质的含量,保证药品的质量和安全性。任何药物在上市之前,都需要经过严格的质量控制检测,以确保其杂质含量符合规定的标准。此外,药品在上市后也会进行定期的质量监控,以确保其安全性和效力。[img=,607,516]https://ng1.17img.cn/bbsfiles/images/2024/02/202402041447097355_1644_6381668_3.png!w607x516.jpg[/img]
ASTM E290-97aStandard Test Methods for Bend Testing of Material for Ductility1[img]http://www.instrument.com.cn/bbs/images/affix.gif[/img][url=http://www.instrument.com.cn/bbs/download.asp?ID=138746]ASTM E290-97aStandard Test Methods for Bend Testing of Material for Ductility1[/url]
BS EN 1022-1997 Domestic furniture. Seating. Determination of stability
【序号】:1【作者】:Ruokun Jia, LiYing Zhen, YongNan Yan, HaiCheng Gu, LiZhi Fang【题名】:Synthesis of Amphipathic Compound and in the Role of Self-assembled Photonic Film【期刊】:Advances in Intelligent and Soft Computing【年、卷、期、起止页码】: Volume 112, 2012, pp 803-809,【全文链接】 http://link.springer.com/chapter/10.1007/978-3-642-25194-8_94谢谢
Description of the apparatusThe apparatus was designed on the principles of single-beam spectrophotometry. A discharge tube equipped with changeable hollow cathodes, and without the additional circulatory system for re-cycling the argon which fills it, was used as a light source.The graphite crucible is placed in a special chamber, as shown in Fig.2. Brass supports for the graphite contacts between which the graphite crucible is fixed, the mechanism for changing the electrode holding the specimen, and the hinge for the setting of the supplementary heating electrode are mounted on “Texttolite” base.The chamber is hermetically sealed by an aluminium cover and rubber gaskets. Two holes were cut in the cover and equipped with quartz windows to allow the light to pass through the crucible. An additional window was cut in the front for observational purposes, and on the rear wall of the cover is fixed a radiation pyrometer for measuring the temperature of the crucible.……from L’vov
【序号】:1【作者】: Nathaniel D. Mueller, Ethan E. Butler, Karen A. McKinnon, Andrew Rhines, Martin Tingley,N. Michele Holbrook & Peter Huybers【题名】: Cooling of US Midwest summer temperature extremes from cropland intensification【期刊、年、卷、期、起止页码】:Nature Climate Change 6, 317-322 (2016) doi:10.1038/nclimate2825【全文链接】:http://www.nature.com/nclimate/journal/v6/n3/full/nclimate2825.html注明:需要在线版全文,谢谢,包括Methods部分
那位大哥知道Elite-5、Elite-35、Elite-1701这三种柱子的极性的吗,如果测有机磷农残用那种较好?
[font=宋体]【序号】:[/font]1[font=宋体]【作者】:[/font]Xu Y , Ai C , Jiang P , Sun X , Liu Y , Jiang G , Song S ..[font=宋体]【题名】:[/font]Oligosaccharides from Gracilaria lemaneiformis betterattenuated high fat diet-induced metabolic syndrome by promoting theBacteroidales proliferation.[font=宋体]【期刊】:[/font] Food Funct[font=宋体]【年、卷、期、起止页码】:[/font] 2020 Jan29 11(1):1049-1062. [font=宋体]【全文链接】:[/font][font='Times New Roman', serif] doi: 10.1039/c9fo01996k. [/font]
[font=&]【题名】:要出刊版 不要接收稿 A deep-UV light-emitting diode-based absorption detector for benzene, toluene, ethylbenzene, and the xylene compounds[/font][font=&]【全文链接】: https://www.sciencedirect.com/science/article/abs/pii/S0925400516308085[/font]
【作者】:Hao Ming Chen等【题名】:Shape and Particle Size Controlled for Water Splitting【期刊】:书籍Controlled Nanofabrication的第四章【年、卷、期、起止页码】:Pan Stanford Publishing 2012 , Pages 73–102【全文链接】:http://www.crcnetbase.com/doi/abs/10.1201/b13155-5当然如果有全书更好,十分感谢!
【序号】:1【作者】:【题名】:Formation of Volatile Heterocyclic Compounds and Open-Chain Amides of Theanine in Model Systems with Glucose, Tea Leaves, and Tea Extract under Tea-Roasting Conditions【DOI】:10.1021/acs.jafc.2c02039【年、卷、期、起止页码】:【全文链接】:
[font=宋体][back=white]【序号】:[/back][/font][back=white]1[/back][font=宋体][back=white]【作者】:[/back][/font][back=white] XiaoxiaLiu[/back][font=宋体][back=white]【题名】:[/back][/font][back=white]Fucoidan Ameliorated Dextran Sulfate Sodium-Induced Ulcerative Colitis by Modulating GutMicrobiota and Bile Acid Metabolism[/back][font=宋体][back=white]【期刊】:[/back][/font][back=white]J. Agric. FoodChem. [/back][font=宋体][back=white]【年、卷、期、起止页码】:[/back][/font][back=white]2022, 70, 47,14864–14876[/back][font=宋体][back=white]【全文链接】:[/back][/font][font='Segoe UI','sans-serif'][color=#212121][back=white] https://pubs.acs.org/doi/abs/10.1021/acs.jafc.2c06417[/back][/color][/font]
[font=宋体]【序号】:[/font][font=&]1[/font][font=宋体]【作者】:[/font][font='Segoe UI','sans-serif'].[/font][font=&]Rui Li[/font][font=宋体]【题名】:[/font][font=&][b]An ingestible self-orienting system for oral delivery of macromolecules[/b][/font][font=宋体]【期刊】:[/font][font=&]Science[/font][font=宋体]【年、卷、期、起止页码】:[/font][font=&][/font]8 Feb 2019, Vol 363, Issue 6427, pp. 611-615[font=宋体]【全文链接】:[/font][font=&][url=https://doi.org/10.1126/science.aau2277]DOI: 10.1126/science.aau227[/url][/font]
Verification of the Reporting Limit and Validation for Soluble Cadmium in small part metal sample1. Scope The study is to examine the validation of reporting limit of heavy metal soluble element (Cadmium) in small part metal as sample in accordance with test method CPSC-CH-E1004-11(GC051.TP). 2. Reagent and Apparatus 2.1 Inductively Coupled Argon Plasma Spectrometer. 2.2 Electronic balance 1 Div. = 0.001 g. 2.3 Metal Scissors, stainless steel 2.4 Shaking water bath, 37 ± 2°C 2.7 Conc. Hydrochloric acid (A.R.). 2.8 Deionized water.. 2.9 General laboratory apparatus. 3. Experimental In this study of the reporting limit, one trial was performed on non spike metal sample (free of soluble Cd in metal material) and ten trials were performed on spiked metal sample (free of soluble Cd metal material). For small part metal materials by soluble extraction according to CPSC-CH-E1004-11(GC051.TP) 0.2g of the sample was weighed in 50 mL conical flask. Add 50 times the mass of sample ,10ml, of 0.07N hydrochloric acid in conical flask and spike with 0.5mg/l of Cd in sample at 37 ± 2°C and shake continuously for 24 hours.The shaking frequency of the shaker was set to 60 rpm. The bath was cover with lid to avoid light during shaking. After 24 hours, decant an aliquot of acid solution from the flask and save for analysis.The percentage recovery, the mean value and the relative standard deviation for studied sample were reported.4. Result Validation record of reporting limit of soluble Cadmium in small part metal sample according to GC051.TP [font=Time
ASTM E23-02a Standard Test Methods for Notched Bar Impact Testing of Metallic Materials1[~135074~]
ASTM E9-89a (Reapproved 2000) Compression Testing of Metallic Materials at Room Temperature1[~135068~]
原发性肝癌(下称肝癌)是我国第二位的肿瘤死亡原因,其根治性切除术后的高复发率严重影响肝癌总体外科疗效。国内外的临床研究表明:肝癌术后的3年复发率为40%~50%左右,5年复发率为60%~70%乃至更高。高复发率也见于局部微创治疗和肝移植后。探索肝癌术后复发的治疗措施是有效延长患者生存时间的重要课题。从临床病理角度分析,肝癌术后复发转移是指肝癌的原有病灶,虽经“根治性切除”,由于微小原发灶的残留,原发癌在肝内播散形成的微小转移灶,癌细胞经门、体循环播散至重要隐匿部位,形成肝外转移并待机再度侵犯肝脏等原因。而从分子生物学的角度看,肝癌复发转移是一个多步骤、多环节的过程。 其分子机制涉及癌基因、抑癌基因、转移相关基因、生长因子及其受体、黏附分子及细胞外基质、肿瘤血管及机体免疫等多个环节。国产靶向治疗药物利卡汀(碘美妥昔单抗注射液)的靶抗原为肝癌细胞膜抗原HAb18G/CD147是肝癌侵袭、转移过程中重要的信号转导分子,利卡汀结合到靶抗原后,一方面能阻断肝癌的侵袭和转移,同时能杀灭残余的微小病灶和肿瘤细胞。从肝癌的复发机制来看,残余的微小病灶和肿瘤细胞是复发的重要因素。通过现在的治疗方式的综合应用,增强对已有病灶的杀灭能力,可有效的降低复发率。对有乙肝病毒背景的肝癌患者来说,乙肝病毒的慢性感染也能增大术后复发的几率。因此目前临床上肝癌术后(切除术、移植术、消融术)后进行抗病毒治疗、化疗栓塞治疗等已普遍开展,取得了不错的疗效。近年来随着分子靶向药物的出现,肝癌术后联合靶向治疗逐步成为了临床研究和应用的热点。通过已经进行的临床研究已经证实,利卡汀能降低肝癌移植术后、消融术后和TACE术后的降低。

http://ng1.17img.cn/bbsfiles/images/2011/06/201106190739_300436_1641557_3.jpg内地首在药品中检出塑化剂 国家药监局紧急通知要求停用药品“力百汀” 广东展开全面清查 国家食品药品监督管理局昨日在其网站上公布通知,要求立即停止销售和使用葛兰素史克公司生产的阿莫西林克拉维酸钾干混悬剂(商品名为力百汀),已上市的要求召回。经中国食品药品鉴定研究院检验,力百汀中检出邻苯二甲酸二异癸酯(DIDP),即所谓的“塑化剂”。 本报讯 (记者刘俊)昨日,国家药监局官网紧急发出关于停止销售和使用葛兰素史克公司生产的阿莫西林克拉维酸钾干混悬剂的通知,指经中国食品药品鉴定研究院检验,在上述药品中检出DIDP(即“塑化剂”),这也是继食品、保健品后内地首次检出药品“含塑”。 国家药监局通知表示,经中国食品药品鉴定研究院检验,在葛兰素史克公司生产的阿莫西林克拉维酸钾干混悬剂中检出邻苯二甲酸二异癸酯(DIDP)。国家食品药品监督管理局决定立即停止葛兰素史克公司生产的阿莫西林克拉维酸钾干混悬剂产品的销售和使用,已进口上市的由企业召回。 国家药监局这份通报并未同时提供需召回的药品的产地、批次、流向、检出塑化剂含量等更多详情。据记者了解,葛兰素史克公司生产的阿莫西林克拉维酸钾干混悬剂是处方药,在内地的产品名为“力百汀”(Augmentin)。“力百汀”适用于治疗上呼吸道及下呼吸道感染、生殖泌尿道感染、皮肤及软组织感染、骨和关节感染,以及其他敏感菌引起的感染。 省药监局相关负责人昨日告诉记者,该局已在6月17日下午接到国家药监局通知,并于当天将该通知转发各地市局,在全省范围内部署展开葛兰素史克公司生产的阿莫西林克拉维酸钾干混悬剂的检查、封存、登记、监督回收等工作。 昨日,记者走访广州市内多家药房,并未找到“力百汀”的踪影。在昌岗路口附近的大参林,销售员查询了电脑后称,只有儿童服用的阿莫西林克拉维酸钾胶囊,是南京一家药厂生产的;在江南大道的百健医药、岁岁康大药房,记者只看到白云山制药和浙江一家药厂制造的阿莫西林克拉维酸钾药片、胶囊。广东金康大药房、健民等药房均表示,已多年未经营“力百汀”。广东老百姓大药房则表示,该药在香港被曝光“含塑”后,为安全起见已将其下架。
第三届国内LIBS会议通知请看附件,这次或许请了几个具有份量的国外LIBS专家吧,随着越来越多的人开始关注LIBS这一新的分析技术,或许这次会议的人数应超过前两届吧,这次LIBS会议也是再为2014年的国际LIBS会议做铺垫,对LIBS分析技术感兴趣的可以去听一听
最近搞到的,供大家学习参考:无菌检验——隔离器系统验证USP Sterility Testing—Validation of Isolator System/General informance这部分是用于无菌检验隔离器系统的简明验证指南(注意:在这个章节中,“已灭菌”指的是物品或者表面的微生物被清除的状态)在19世纪80年代中期,建立一个无菌检验环境的隔离器就已经开始使用。隔离器可以通过密封的方法或者采用过滤除菌空气保持正压的方法,创造一个无菌的环境。当隔离器处于密闭状态时,仅仅能够在隔离器内部和快速传递仓传递物品;当隔离器打开时,允许通过一个特殊设计的并经过验证可以避免污染物进入的开口递出物品。隔离器采用柔软的塑料(例如聚氯乙烯)、硬塑料、玻璃或不锈钢制造。由于隔离器系统从根本上避免了分析人员与物品的直接接触,因此在无菌检验操作时可以避免物品和辅助设备被污染。当隔离器内部与环境完全隔离时,隔离器内部的物品是无菌条件下的传递。操作者穿着半身衣在隔离器内部进行无菌操作,半身衣是连接在隔离器墙体上的柔软的部分,操作者穿着半身衣有足够的范围在隔离器内部进行操作,操作者也可以通过连接在隔离器墙体上的袖子和手套进行操作。在隔离器中,不要求操作者穿着特殊的无菌衣进行操作,允许操作者穿着标准的实验室服装进行操作。为确保隔离器内部无菌。使用杀孢子剂对隔离器内部进行灭菌处理。 隔离器设计和建造空气处理系统用于无菌检验的隔离器需要配备除菌过滤器(HEPA过滤器是被要求的)。静态时,要求隔离器尘埃粒子符合美国联邦标准209E的100级空气质量要求(看洁净室微生物评价和其他环境控制《1116》)。动态时,不要求隔离器符合100级空气质量要求,不要求隔离器内部的空气流速或者空气交换频率。隔离器系统是要求防止泄漏的,然而,它不是通常意义上的防止隔离器与外界环境进行的空气交换。当与外界环境直接相连的门打开时,隔离器内部的正压保证隔离器内部的无菌环境不被污染。用于无菌检验的隔离器内部空气流可以是单向流或者湍流。传输仓和门隔离器有一个附属的“传递通路”杀菌器,通过传输通道杀菌器可以直接将无菌的培养基、无菌的稀释液、无菌的装备等传递进隔离器系统。一般设计成快速传递仓或门(RTPs),通过快速传递仓或门可以把两个隔离器彼此相连,无菌的物品就能够从一个隔离器传递到另一个隔离器。通过快速传递仓,两个隔离器或者一个隔离器和一个容器就可以在普通环境中连接。通过密封圈或法兰,将传递仓的非无菌表面连接。用垫圈压紧来保证气密性,避免微生物进入。当两个传递仓法兰连接形成一个密封通道时,存在一个狭长的垫圈带,这个部位可能存在微生物污染。因此一旦连接完,在使用传递仓传递物品之前,必须立即用杀孢子剂对垫圈暴露部分进行处理。并且在传递物品时,应当注意无菌技巧的使用,避免物品或手套接触垫圈。将垫圈装配在法兰上时,应当按照传递仓生产商的建议进行预防性的维护和润滑。传递仓垫圈应当按照(生产商的)的要求定期更换并且定期检查,破损的垫圈不能够保证真正的密封。隔离器安装位置的选择用于无菌检验的隔离器不需要安装在洁净区,但是安装在一个限制非授权人员进入的区域仍然是重要的。安装时,应当使隔离器周围有足够的范围,以便移动隔离器,传递物料,以及通常的维护。隔离器所在的房间不要求进行环境监测。隔离器房间温度和湿度对于操作者的安全和舒适是重要的,温湿度对于除菌和净化技术的影响效果也很关键。如果隔离器位于空气补给窗的气流通道中,当隔离器采用蒸汽灭菌时,空气气流会使隔离器个别部位温度较低形成冷凝水。当采用对温度敏感的灭菌方法时,隔离器房间的温度应当是均一的。隔离器系统的验证无菌检验合格与否是产品放行的前提时,在进行无菌检验之前,隔离器系统的验证必须完成。为了核实隔离器及其辅助设备能够用于进行无菌检验,隔离器系统的验证可以分成三个部分:安装验证、性能验证、操作验证。在进行无菌检验隔离器系统验证过程中,应当考虑到下面的观点。在验证程序的特定阶段(例如IQ、OQ、PQ),各个阶段试验的任务是不被确定的,在隔离器用于确定的检测前,隔离器的功能应当被验证并且有文字记录。安装确认IQ阶段包括隔离器系统详细的外观描述,例如隔离器尺寸、内部结构、所用的材料。关于接触面和传递系统,要清晰的画图并标注尺寸。设计的隔离器是否符合使用的规格需要被核实,例如空气补给、真空、外部排气、温湿度控制等。其他与隔离器系统一起使用的设备也要详细描述;任何设计规格的修订都应当详细描述。设备指南和复印件应当编成目录并保存,在需要的时候,操作者可以得到并重新查阅。一旦设计规格符合性被核实,所有的图纸、方法和设备图要编成目录,保存并能够重新得到。所有的文档应重新被审阅,便于核实能够反映出安装系统的关键属性要求。这样就建立了符合隔离器系统设计规格和安装要求通用的基准。在故障模式下或高风险情况下进行分析时,可能导致试验失败的潜在的过程控制问题和设备问题可能被发现,这些问题应当进行鉴定并记录在文档中。如果必要,系统可以被修改,以便将失败的风险降到最低点,并且关键点的控制方法被建立。安装确认的结果被总结成为一个安装确认报告,建议包括下列文档。设备---设备应符合设计规格并被列表记录。IQ报告应核实:符合设计规格的设备被接收,并且按照生产厂商的要求进行安装。结构材质---隔离器系统关键部位的结构材质经检测是符合设计要求的。对隔离器材质的杀菌方法兼容性应当被核实。仪表---仪表被核实符合其精度要求并列表。功能规格---所有操作功能,例如在操作手册、流程、仪器图表中指明的功能,都应当被核实能够执行并且符合规格要求。隔离器系统和其他系统的
[font='Segoe UI','sans-serif'][color=#212121][back=white] [/back][/color][/font][font=宋体][back=white]【序号】:[/back][/font][back=white]1[/back][font=宋体][back=white]【作者】:[/back][/font][back=white]Peiyi Wang[/back][font=宋体][back=white]【题名】:[/back][/font][back=white]Phenolics fromDendrobium officinale Leaf Ameliorate Dextran Sulfate Sodium-Induced ChronicColitis by Regulating Gut Microbiota and Intestinal Barrier[/back][font=宋体][back=white]【期刊】:[/back][/font][back=white] J. Agric.Food Chem. [/back][font=宋体][back=white]【年、卷、期、起止页码】:[/back][/font][font='Segoe UI','sans-serif'][color=#212121][back=white] [/back][/color][/font][back=white]2023, 71, 44, 16630–16646[/back][font=宋体][back=white]【全文链接】:[/back][/font][back=white]https://doi.org/10.1021/acs.jafc.3c05339[/back]
实验室的衬管里面有tenax 填料,然后上面是玻璃棉,这种衬管挺贵的,想问有单卖这种填料的吗?