面议
TA 仪器
暂无样本
Affinity ITC/ ITC Auto
--
美洲
仪器种类: 微量热仪
产地类别: 进口
温度范围(°C): 2~80
为您推荐相似的差示扫描量热仪(DSC/DTA)
Affinity ITC和Affinity ITC Auto是专为极具挑战性的生命科学实验室所设计的,满足了需要高灵敏度、高生产力和业界领先ITC技术的需求。Affinity ITC的先进工艺考量了所有测试关键因素,能确保获得高质量的ITC数据。
特点与优势
AccuShot能够将滴定样品导入正确位置,以实现最佳混合。
FlexSpin提供了创新的低速搅拌,实现最佳的混合效率和最高的灵敏度
全自动化及用户可选择的系统清理程序,可排除不同实验间造成的交叉污染
智能定位装置实现精准可靠的注射
通过主动式固态加热及冷却系统,可排除不同实验间造成的交叉污染
智能定位装置实现精准可靠的注射
通过主动式固态加热及冷却系统,实现真正的恒温控制
可选择标准体积(1.0 mL)或小体积(190 μL)的量测池
业内公认的具备温控功能的96位液体处理自动进样系统
自动进样器可以在最初购买时配置,也可以在后续使用中添加
强大的ITC Run及Nano Analyze为方法优化、模型拟合、批量分析、绘图以及数据导出提供了最为全面的工具
TA仪器完善了用户的需求。Affinity ITC是一款测量分子间相互作用的强大工具,无论您是有经验的还是没经验的ITC用户,我们都有足够的信心让用户得到优异的ITC数据。
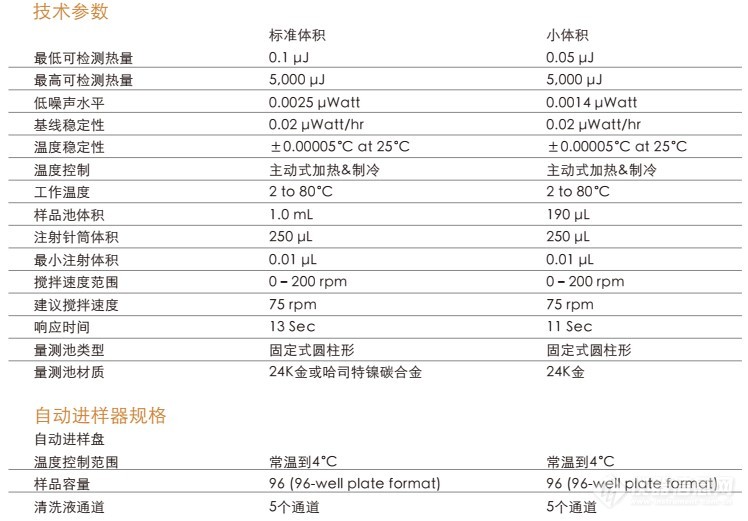
TA 仪器差示扫描量热仪Affinity ITC/ ITC Auto的工作原理介绍
差示扫描量热仪Affinity ITC/ ITC Auto的使用方法?
TA 仪器Affinity ITC/ ITC Auto多少钱一台?
差示扫描量热仪Affinity ITC/ ITC Auto可以检测什么?
差示扫描量热仪Affinity ITC/ ITC Auto使用的注意事项?
TA 仪器Affinity ITC/ ITC Auto的说明书有吗?
TA 仪器差示扫描量热仪Affinity ITC/ ITC Auto的操作规程有吗?
TA 仪器差示扫描量热仪Affinity ITC/ ITC Auto报价含票含运吗?
TA 仪器Affinity ITC/ ITC Auto有现货吗?
最多添加5台
